Search
- Page Path
- HOME > Search
- Surveillance for metachronous cancers after endoscopic resection of esophageal squamous cell carcinoma
- Ryu Ishihara
- Received October 10, 2023 Accepted December 17, 2023 Published online May 10, 2024
- DOI: https://doi.org/10.5946/ce.2023.263 [Epub ahead of print]

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - The literature pertaining to surveillance following treatment for esophageal squamous cell carcinoma (SCC) was reviewed and summarized, encompassing the current status and future perspectives. Analysis of the standardized mortality and incidence ratios for these cancers indicates an elevated risk of cancer in the oral cavity, pharynx, larynx, and lungs among patients with esophageal SCC compared to the general population. To enhance the efficacy of surveillance for these metachronous cancers, risk stratification is needed. Various factors, including multiple Lugol-voiding lesions, multiple foci of dilated vascular areas, young age, and high mean corpuscular volume, have been identified as predictors of metachronous SCCs. Current practice involves stratifying the risk of metachronous esophageal and head/neck SCCs based on the presence of multiple Lugol-voiding lesions. Endoscopic surveillance, scheduled 6–12 months post-endoscopic resection, has demonstrated effectiveness, with over 90% of metachronous esophageal SCCs treatable through minimally invasive modalities. Narrow-band imaging emerges as the preferred surveillance method for esophageal and head/neck SCC based on comparative studies of various imaging techniques. Innovative approaches, such as artificial intelligence-assisted detection systems and radiofrequency ablation of high-risk background mucosa, may improve outcomes in patients following endoscopic resection.
- 1,535 View
- 35 Download

- Complications of endoscopic resection in the upper gastrointestinal tract
- Takeshi Uozumi, Seiichiro Abe, Mai Ego Makiguchi, Satoru Nonaka, Haruhisa Suzuki, Shigetaka Yoshinaga, Yutaka Saito
- Clin Endosc 2023;56(4):409-422. Published online June 21, 2023
- DOI: https://doi.org/10.5946/ce.2023.024
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
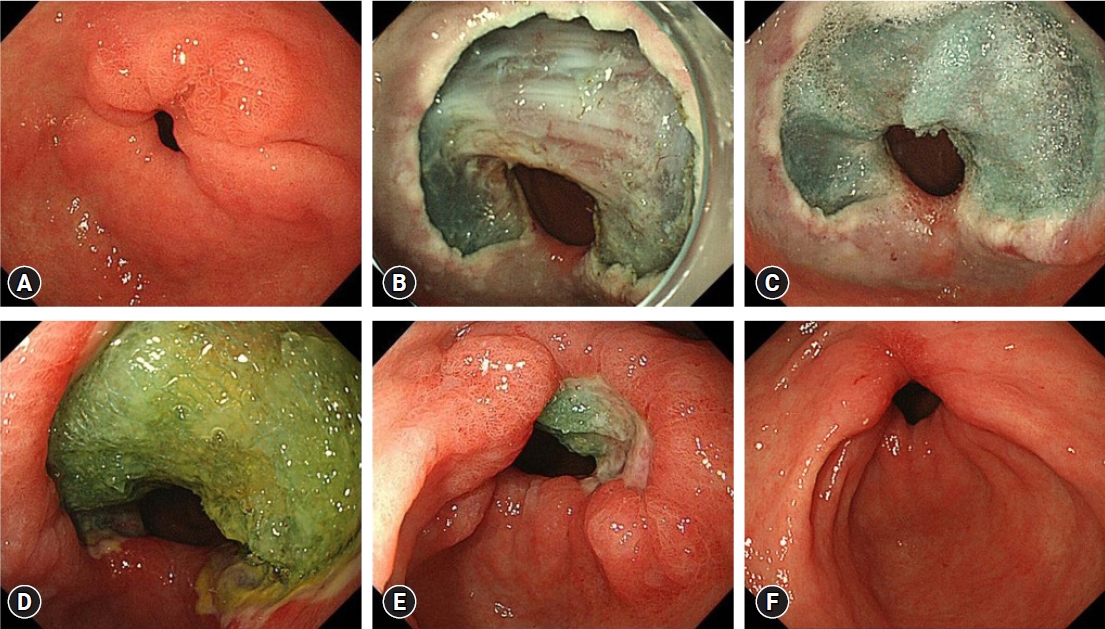
ePub - Endoscopic resection (ER) is widely utilized as a minimally invasive treatment for upper gastrointestinal tumors; however, complications could occur during and after the procedure. Post-ER mucosal defect leads to delayed perforation and bleeding; therefore, endoscopic closure methods (endoscopic hand-suturing, the endoloop and endoclip closure method, and over-the-scope clip method) and tissue shielding methods (polyglycolic acid sheets and fibrin glue) are developed to prevent these complications. During duodenal ER, complete closure of the mucosal defect significantly reduces delayed bleeding and should be performed. An extensive mucosal defect that comprises three-quarters of the circumference in the esophagus, gastric antrum, or cardia is a significant risk factor for post-ER stricture. Steroid therapy is considered the first-line option for the prevention of esophageal stricture, but its efficacy for gastric stricture remains unclear. Methods for the prevention and management of ER-related complications in the esophagus, stomach, and duodenum differ according to the organ; therefore, endoscopists should be familiar with ways of preventing and managing organ-specific complications.
-
Citations
Citations to this article as recorded by- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
Seung Joo Kang, Chung Hyun Tae, Chang Seok Bang, Cheol Min Shin, Young-Hoon Jeong, Miyoung Choi, Joo Ha Hwang, Yutaka Saito, Philip Wai Yan Chiu, Rungsun Rerknimitr, Christopher Khor, Vu Van Khien, Kee Don Choi, Ki-Nam Shim, Geun Am Song, Oh Young Lee
Clinical Endoscopy.2024; 57(2): 141. CrossRef - Risk factors for intraoperative and delayed perforation related with gastric endoscopic submucosal dissection
Takuya Mimura, Yoshinobu Yamamoto, Haruhisa Suzuki, Kohei Takizawa, Toshiaki Hirasawa, Yoji Takeuchi, Kenji Ishido, Shu Hoteya, Tomonori Yano, Shinji Tanaka, Norihiko Kudara, Masahiro Nakagawa, Yumi Mashimo, Masahiro Ishigooka, Kazutoshi Fukase, Taichi Sh
Journal of Gastroenterology and Hepatology.2024;[Epub] CrossRef - Endoscopic submucosal dissection for early gastric cancer: It is time to consider the quality of its outcomes
Gwang Ha Kim
World Journal of Gastroenterology.2023; 29(43): 5800. CrossRef
- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
- 3,253 View
- 165 Download
- 3 Web of Science
- 3 Crossref

- Accuracy of administrative claim data for gastric adenoma after endoscopic resection
- Ga-Yeong Shin, Hyun Ho Choi, Jae Myung Park, Sang Yoon Kim, Jun Young Park, Donghoon Kang, Yu Kyung Cho, Sung Soo Kim, Myung-Gyu Choi
- Clin Endosc 2023;56(3):325-332. Published online March 21, 2023
- DOI: https://doi.org/10.5946/ce.2022.147

-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: Administrative databases provide valuable information for large-cohort studies. This study aimed to evaluate the diagnostic accuracy of an administrative database for resected gastric adenomas.
Methods
Data of patients who underwent endoscopic resection for benign gastric lesions were collected from three hospitals. Gastric adenoma cases were identified in the hospital database using International Classification of Diseases (ICD) 10-codes. The non-adenoma group included patients without gastric adenoma codes. The diagnostic accuracy for gastric adenoma was analyzed based on the pathological reports of the resected specimen.
Results
Among 5,095 endoscopic resections with codes for benign gastric lesions, 3,909 patients were included in the analysis. Among them, 2,831 and 1,078 patients were allocated to the adenoma and non-adenoma groups, respectively. Regarding the overall diagnosis of gastric adenoma with ICD-10 codes, the sensitivity, specificity, positive predictive value, and negative predictive value were 98.7%, 88.5%, 95.2%, and 96.8%, respectively. There were no significant differences in these parameters between the tertiary and secondary centers.
Conclusions
Administrative codes of gastric adenoma, according to ICD-10 codes, showed good accuracy and can serve as a useful tool to study prognosis of these patients in real-world data studies in the future. -
Citations
Citations to this article as recorded by- Gastric Cancer Incidence and Mortality After Endoscopic Resection of Gastric Adenoma: A Nationwide Cohort Study
Jae Myung Park, Songhee Cho, Ga-Yeong Shin, Jayoun Lee, Minjee Kim, Hyeon Woo Yim
American Journal of Gastroenterology.2023; 118(12): 2166. CrossRef
- Gastric Cancer Incidence and Mortality After Endoscopic Resection of Gastric Adenoma: A Nationwide Cohort Study
- 2,272 View
- 119 Download
- 1 Web of Science
- 1 Crossref

-
Application of a traction metal clip with a fishhook-like device in wound sutures after endoscopic resection

- Wang Fangjun, Leng Xia, Gao Yi, Shen Xiuyun, Wang Wenping, Liu Huamin, Liu Pengfei
- Clin Endosc 2022;55(4):525-531. Published online July 28, 2022
- DOI: https://doi.org/10.5946/ce.2021.241
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
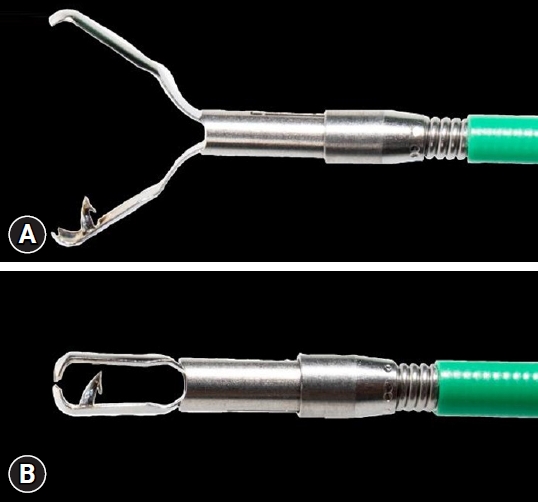
ePub - Background
/Aims: Endoscopic wound suturing is an important factor that affects the ability to remove large and full-thickness lesions during endoscopic resection. We aimed to evaluate the effect of a traction metal clip with a fishhook-like device on wound sutures after endoscopic resection.
Methods
From July 2020 to April 2021, patients who met the enrollment criteria were treated with a fishhook-like device during the operation to suture the postoperative wound (group A). Patients with similar conditions and similar size wounds who were treated with a “purse-string suture” to suture the wounds were retrospectively analyzed as the control group (group B). Difference in the suture rate, adverse events, time required for suturing, and number of metal clips were compared between the two groups.
Results
The time required for suturing was 7.72±0.51 minutes in group A and 11.50±0.91 minutes in group B. This difference was statistically significant (F=13.071, p=0.001). The number of metal clamps used in group A averaged 8.1 pieces/case, and the number of metal clamps used in group B averaged 7.3 pieces/case. This difference was not statistically significant (F=0.971, p>0.05).
Conclusions
The traction metal clip with the fishhook-like device is ingeniously designed and easy to operate. It has a good suture effect on the wound after endoscopic submucosal dissection and effectively prevents postoperative adverse events.
- 2,421 View
- 116 Download

- Endoscopic treatment for rectal neuroendocrine tumor: which method is better?
- Seung Min Hong, Dong Hoon Baek
- Clin Endosc 2022;55(4):496-506. Published online July 11, 2022
- DOI: https://doi.org/10.5946/ce.2022.115

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Recently, research on rectal neuroendocrine tumors (NETs) has increased during the last few decades. Rectal NETs measuring <10 mm without atypical features and confined to the submucosal layer have only 1% risk of metastasis, and the long-term survival probability of patients without metastasis at the time of diagnosis is approximately 100%. Therefore, the current guidelines suggest endoscopic resection of rectal NETs of <10 mm is regarded as a safe therapeutic option. However, there are currently no clear recommendations for technique selection for endoscopic resection. The choice of treatment modality for rectal NETs should be based on the lesion size, endoscopic characteristics, grade of differentiation, depth of vertical involvement, lymphovascular invasion, and risk of metastasis. Moreover, the complete resection rate, complications, and experience at the center should be considered. Modified endoscopic mucosal resection is the most suitable resection method for rectal NETs of <10 mm, because it is an effective and safe technique that is relatively simple and less time-consuming compared with endoscopic submucosal dissection. Endoscopic submucosal dissection should be considered when the tumor size is >10 mm, suctioning is not possible due to fibrosis in the lesion, or when the snaring for modified endoscopic mucosal resection does not work well.
-
Citations
Citations to this article as recorded by- Treatment strategy and post‐treatment management of colorectal neuroendocrine tumor
Masau Sekiguchi, Takahisa Matsuda, Yutaka Saito
DEN Open.2024;[Epub] CrossRef - Comparison of endoscopic resection therapies for rectal neuroendocrine tumors
Meijiao Lu, Hongxia Cui, Mingjie Qian, Yating Shen, Jianhong Zhu
Minimally Invasive Therapy & Allied Technologies.2024; : 1. CrossRef - A Review of Colonoscopy in Intestinal Diseases
Seung Hong, Dong Baek
Diagnostics.2023; 13(7): 1262. CrossRef - Treatment of localized well-differentiated rectal neuroendocrine tumors: A focused review
Shigenobu Emoto, Hiroaki Nozawa, Kazuhito Sasaki, Koji Murono, Hiroyuki Matsuzaki, Yuichiro Yokoyama, Shinya Abe, Yuzo Nagai, Yuichiro Yoshioka, Takahide Shinagawa, Hirofumi Sonoda, Soichiro Ishihara
Formosan Journal of Surgery.2023; 56(3): 73. CrossRef - Clinical application of endoscopic ultrasonography in the management of rectal neuroendocrine tumors
Soo-Young Na, Seong Jung Kim, Hyoun Woo Kang
International Journal of Gastrointestinal Intervention.2023; 12(3): 105. CrossRef - Endoscopic submucosal dissection coupled with �modified clip coupled with elastic ring� traction removing rectal neuroendocrine tumor
Jing Zhou, Li-Sheng Wang, De-Feng Li, Rui-Yue Shi
Revista Española de Enfermedades Digestivas.2023;[Epub] CrossRef
- Treatment strategy and post‐treatment management of colorectal neuroendocrine tumor
- 3,992 View
- 285 Download
- 6 Web of Science
- 6 Crossref

- Current Treatment Strategy for Superficial Nonampullary Duodenal Epithelial Tumors
- Tetsuya Suwa, Kohei Takizawa, Noboru Kawata, Masao Yoshida, Yohei Yabuuchi, Yoichi Yamamoto, Hiroyuki Ono
- Clin Endosc 2022;55(1):15-21. Published online September 29, 2021
- DOI: https://doi.org/10.5946/ce.2021.141

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic submucosal dissection (ESD) is the standard treatment method for esophageal, gastric, and colorectal cancers. However, it has not been standardized for duodenal lesions because of its high complication rates. Recently, minimally invasive and simple methods such as cold snare polypectomy and underwater endoscopic mucosal resection have been utilized more for superficial nonampullary duodenal epithelial tumors (SNADETs). Although the rate of complications associated with duodenal ESD has been gradually decreasing because of technical advancements, performing ESD for all SNADETs is unnecessary. As such, the appropriate treatment plan for SNADETs should be chosen according to the lesion type, patient condition, and endoscopist’s skill.
-
Citations
Citations to this article as recorded by- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
Seung Joo Kang, Chung Hyun Tae, Chang Seok Bang, Cheol Min Shin, Young-Hoon Jeong, Miyoung Choi, Joo Ha Hwang, Yutaka Saito, Philip Wai Yan Chiu, Rungsun Rerknimitr, Christopher Khor, Vu Van Khien, Kee Don Choi, Ki-Nam Shim, Geun Am Song, Oh Young Lee
Clinical Endoscopy.2024; 57(2): 141. CrossRef - Underwater Endoscopic Mucosal Resection Versus Conventional Endoscopic Mucosal Resection for Superficial Non-ampullary Duodenal Epithelial Tumors ≤20 mm
Zhikun Yin, Ji Li, Weilin Yang, Weifeng Huang, Dong Xu, Xiaoyi Lei, Jinyan Zhang
Journal of Clinical Gastroenterology.2023; 57(9): 928. CrossRef - Long-term outcomes of endoscopic resection for duodenal neuroendocrine tumors
Kiyoun Yi, Gwang Ha Kim, Su Jin Kim, Cheol Woong Choi, Moon Won Lee, Bong Eun Lee, Geun Am Song
Scientific Reports.2023;[Epub] CrossRef - Endoscopic management of NADTs
Enrique Pérez-Cuadrado-Robles, Pierre H. Deprez
Endoscopy International Open.2022; 10(06): E733. CrossRef - Duodenaladenome und -karzinome: chirurgische Therapiekonzepte
Michael Ghadimi, Jochen Gaedcke
Allgemein- und Viszeralchirurgie up2date.2022; 16(03): 257. CrossRef - Issues and Prospects of Current Endoscopic Treatment Strategy for Superficial Non-Ampullary Duodenal Epithelial Tumors
Tetsuya Suwa, Masao Yoshida, Hiroyuki Ono
Current Oncology.2022; 29(10): 6816. CrossRef - Duodenaladenome und -karzinome: chirurgische Therapiekonzepte
Michael Ghadimi, Jochen Gaedcke
Onkologie up2date.2022; 4(04): 325. CrossRef
- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
- 5,012 View
- 352 Download
- 4 Web of Science
- 7 Crossref

- Efficacy of Underwater Endoscopic Mucosal Resection for Superficial Non-Ampullary Duodenal Epithelial Tumor
- Masanori Furukawa, Akira Mitoro, Takahiro Ozutumi, Yukihisa Fujinaga, Keisuke Nakanishi, Koh Kitagawa, Soichiro Saikawa, Sinya Sato, Yasuhiko Sawada, Hiroaki Takaya, Kosuke Kaji, Hideto Kawaratani, Tadashi Namisaki, Kei Moriya, Takemi Akahane, Junichi Yamao, Hitoshi Yoshiji
- Clin Endosc 2021;54(3):371-378. Published online February 18, 2021
- DOI: https://doi.org/10.5946/ce.2020.147

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic resection (ER) for superficial non-ampullary duodenal epithelial tumors (SNADETs) is challenging. Conventional endoscopic mucosal resection (CEMR) is also problematic due to the anatomical features of the duodenum. We compared the safety and efficacy of underwater endoscopic mucosal resection (UEMR) with those of CEMR through a retrospective analysis.
Methods
Altogether, 44 consecutive patients with 46 SNADETs underwent ER (18 CEMR cases and 28 UEMR cases) between January 2016 and October 2019. We investigated the proportions of en bloc resection, R0 resection, complications, resection time, and total procedure time and compared the outcomes of patients from the CEMR group with those of patients from the UEMR group.
Results
The median tumor size was 8.0 mm (range, 2.0–20.0 mm). The UEMR group showed a higher proportion of en bloc resection (96.4% vs. 72.2%, p<0.05) and significantly lower median resection time and total procedure time (4 min vs. 9.5 min, p<0.05 and 13 min vs. 19 min, p<0.05; respectively) than the CEMR group. No complications were observed. However, two patients treated with piecemeal resection in the CEMR group had residual tumors.
Conclusions
UEMR is a feasible therapeutic option for SNADETs. It can be recommended as a standard treatment. -
Citations
Citations to this article as recorded by- Conventional versus underwater endoscopic resection for superficial non-ampullary duodenal epithelial tumours
Hajime Miyazaki, Osamu Dohi, Tsugitaka Ishida, Mayuko Seya, Katsuma Yamauchi, Hayato Fukui, Takeshi Yasuda, Takuma Yoshida, Naoto Iwai, Toshifumi Doi, Ryohei Hirose, Ken Inoue, Akihito Harusato, Naohisa Yoshida, Kazuhiko Uchiyama, Tomohisa Takagi, Takeshi
Japanese Journal of Clinical Oncology.2024; 54(2): 137. CrossRef - Can underwater endoscopic mucosal resection be an alternative to conventional endoscopic mucosal resection for superficial non‐ampullary duodenal epithelial tumors?
Hidenori Tanaka, Yuji Urabe, Hiroki Takemoto, Kazuki Ishibashi, Hirona Konishi, Yuka Matsubara, Yudai Takehara, Shin Morimoto, Fumiaki Tanino, Noriko Yamamoto, Hajime Teshima, Junichi Mizuno, Issei Hirata, Hirosato Tamari, Akiyoshi Tsuboi, Ken Yamashita,
DEN Open.2024;[Epub] CrossRef - Underwater Endoscopic Mucosal Resection Vs Conventional EMR for Superficial Nonampullary Duodenal Epithelial Tumors in the Western Setting
Rui Morais, José Amorim, Renato Medas, Bernardo Sousa-Pinto, João Santos-Antunes, Romain Legros, Jérémie Albouys, Frédéric Moll, Margarida Marques, Filipe Vilas-Boas, Eduardo Rodrigues-Pinto, Irene Gullo, Fátima Carneiro, Elisa Gravito Soares, Pedro Amaro
Clinical Gastroenterology and Hepatology.2024;[Epub] CrossRef - Underwater versus conventional endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumors ≤20mm: A systematic review and meta-analysis
Xiu-He Lv, Rong Luo, Qing Lu, Kai Deng, Jin-Lin Yang
Digestive and Liver Disease.2023; 55(6): 714. CrossRef - Underwater Versus Conventional Endoscopic Mucosal Resection for Superficial Non-ampullary Duodenal Epithelial Tumors: A Systematic Review and Meta-Analysis
Jae Gon Lee, Sang Pyo Lee, Hyun Joo Jang, Sea Hyub Kae
Digestive Diseases and Sciences.2023; 68(4): 1482. CrossRef - A feasibility study comparing gel immersion endoscopic resection and underwater endoscopic mucosal resection for superficial nonampullary duodenal epithelial tumors
Akihiro Miyakawa, Toshio Kuwai, Yukie Sakuma, Manabu Kubota, Akira Nakamura, Ei Itobayashi, Haruhisa Shimura, Yoshio Suzuki, Kenji Shimura
Endoscopy.2023; 55(03): 261. CrossRef - Underwater Endoscopic Mucosal Resection Versus Conventional Endoscopic Mucosal Resection for Superficial Non-ampullary Duodenal Epithelial Tumors ≤20 mm
Zhikun Yin, Ji Li, Weilin Yang, Weifeng Huang, Dong Xu, Xiaoyi Lei, Jinyan Zhang
Journal of Clinical Gastroenterology.2023; 57(9): 928. CrossRef - Efficacy and safety of underwater endoscopic mucosal resection for ≤20 mm superficial non-ampullary duodenal epithelial tumors: Systematic review and meta-analysis
Jixiang Liu, Shaojie Duan, Yichong Wang, Hongye Peng, Youjia Kong, Shukun Yao
Frontiers in Medicine.2023;[Epub] CrossRef - Underwater versus conventional EMR for nonpedunculated colorectal lesions: a randomized clinical trial
Luciano Lenz, Bruno Martins, Gustavo Andrade de Paulo, Fabio Shiguehissa Kawaguti, Elisa Ryoka Baba, Ricardo Sato Uemura, Carla Cristina Gusmon, Sebastian Naschold Geiger, Renata Nobre Moura, Caterina Pennacchi, Marcelo Simas de Lima, Adriana Vaz Safatle-
Gastrointestinal Endoscopy.2023; 97(3): 549. CrossRef - Endoscopic resection of superficial non‐ampullary duodenal epithelial tumor
Motohiko Kato, Takanori Kanai, Naohisa Yahagi
DEN Open.2022;[Epub] CrossRef - The Application of Underwater Endoscopic Mucosal Resection for Nonampullary Duodenal Adenomas
Xiu-He Lv, Jin-Lin Yang
Clinical Gastroenterology and Hepatology.2022; 20(8): 1884. CrossRef - Utility of underwater EMR for nonpolypoid superficial nonampullary duodenal epithelial tumors ≤20 mm
Kenichiro Okimoto, Daisuke Maruoka, Tomoaki Matsumura, Kengo Kanayama, Naoki Akizue, Yuki Ohta, Takashi Taida, Keiko Saito, Yosuke Inaba, Yohei Kawasaki, Makoto Arai, Jun Kato, Naoya Kato
Gastrointestinal Endoscopy.2022; 95(1): 140. CrossRef - Reply
Yasushi Yamasaki, Noriya Uedo
Clinical Gastroenterology and Hepatology.2022; 20(8): 1884. CrossRef - Appropriate selection of endoscopic resection for superficial nonampullary duodenal adenomas in association with recurrence
Kenichiro Okimoto, Daisuke Maruoka, Tomoaki Matsumura, Kengo Kanayama, Naoki Akizue, Yuki Ohta, Takashi Taida, Keiko Saito, Yosuke Inaba, Yohei Kawasaki, Jun Kato, Naoya Kato
Gastrointestinal Endoscopy.2022; 95(5): 939. CrossRef - Reply to Lv and Yang
Motohiko Kato, Yoji Takeuchi, Shu Hoteya, Tsuneo Oyama, Satoru Nonaka, Shoichi Yoshimizu, Naomi Kakushima, Ken Ohata, Hironori Yamamoto, Yuko Hara, Hisashi Doyama, Osamu Dohi, Yasushi Yamasaki, Hiroya Ueyama, Kengo Takimoto, Koichi Kurahara, Tomoaki Tashi
Endoscopy.2022; 54(05): 523. CrossRef - Endoscopic treatment selection for superficial duodenal tumors: pay attention to small lesions
Xiu-He Lv, Jin-Lin Yang
Endoscopy.2022; 54(05): 522. CrossRef - Resectability of underwater endoscopic mucosal resection for duodenal tumor: A single‐center, retrospective pilot study
Yosuke Toya, Masaki Endo, Masanao Yamazato, Shun Yamada, Tomo Kumei, Minami Hirai, Makoto Eizuka, Toshifumi Morishita, Risaburo Akasaka, Shunichi Yanai, Noriyuki Uesugi, Tamotsu Sugai, Takayuki Matsumoto
Journal of Gastroenterology and Hepatology.2021; 36(11): 3191. CrossRef
- Conventional versus underwater endoscopic resection for superficial non-ampullary duodenal epithelial tumours
- 4,277 View
- 144 Download
- 16 Web of Science
- 17 Crossref

- The Use of Endoscopic Clipping in Preventing Delayed Complications after Endoscopic Resection for Superficial Non-Ampullary Duodenal Tumors
- Jee Young An, Byung-Wook Kim, Joon Sung Kim, Jae-Myung Park, Tae Ho Kim, Jaesin Lee
- Clin Endosc 2021;54(4):563-569. Published online November 24, 2020
- DOI: https://doi.org/10.5946/ce.2020.109
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
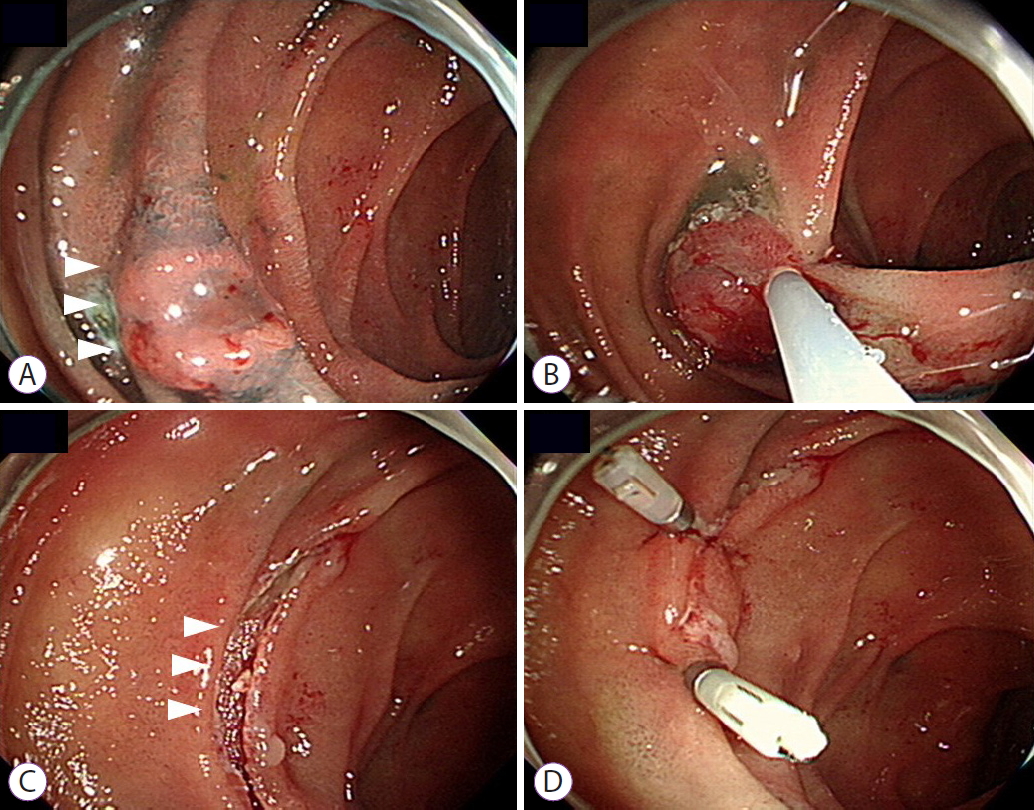
ePub - Background
/Aims: Endoscopic resection (ER) has recently been accepted as the standard treatment modality for superficial nonampullary duodenal tumors (SNADTs). However, the procedure can cause adverse events such as perforation and bleeding. This study aimed to investigate the efficacy of prophylactic clipping in the prevention of delayed complications.
Methods
A retrospective review of the medical records of patients who underwent ER for SNADT from 3 centers was performed. Patients were divided into 2 groups: the immediate clipping group (ICG) and the no clipping group (NCG). Various baseline characteristics and factors associated with the appearance of delayed complications, such as size of the lesion, tumor location, histologic type, and co-morbidities, were compared between the two groups.
Results
A total of 99 lesions from 99 patients were included in this study. Fifty-two patients were allocated into ICG and 47 patients were allocated into NCG. Delayed bleeding occurred in 1 patient from ICG and in 8 patients from NCG. Delayed perforation occurred in 1 patient from ICG and in 3 patients from NCG. There were no procedure-related deaths in both groups.
Conclusions
Although the use of endoscopic clipping seemed to reduce the risk of developing delayed complications, further studies using a prospective design is required. -
Citations
Citations to this article as recorded by- The Effect of Tegoprazan on the Treatment of Endoscopic Resection-Induced Artificial Ulcers: A Multicenter, Randomized, Active-Controlled Study
Byung-Wook Kim, Jong Jae Park, Hee Seok Moon, Wan Sik Lee, Ki-Nam Shim, Gwang Ho Baik, Yun Jeong Lim, Hang Lak Lee, Young Hoon Youn, Jun Chul Park, In-Kyung Sung, Hyunsoo Chung, Jeong Seop Moon, Gwang Ha Kim, Su Jin Hong, Hyuk Soon Choi
Gut and Liver.2024; 18(2): 257. CrossRef - Endoscopic diagnosis and treatment of superficial non-ampullary duodenal epithelial tumors: A review
Zheng Zhao, Yue Jiao, Shuyue Yang, Anni Zhou, Guiping Zhao, Shuilong Guo, Peng Li, Shutian Zhang
Journal of Translational Internal Medicine.2023; 11(3): 206. CrossRef - Long-term outcomes of endoscopic resection for duodenal neuroendocrine tumors
Kiyoun Yi, Gwang Ha Kim, Su Jin Kim, Cheol Woong Choi, Moon Won Lee, Bong Eun Lee, Geun Am Song
Scientific Reports.2023;[Epub] CrossRef - Endoscopic clipping in non-variceal upper gastrointestinal bleeding treatment
Giuseppe Galloro, Angelo Zullo, Gaetano Luglio, Alessia Chini, Donato Alessandro Telesca, Rosa Maione, Matteo Pollastro, Giovanni Domenico De Palma, Raffaele Manta
Clinical Endoscopy.2022; 55(3): 339. CrossRef - Endoscopic Closure After Endoscopic Resection for Superficial Non-Ampullary Duodenal Tumors
Satoshi Tanabe, Takuya Wada
Clinical Endoscopy.2021; 54(4): 453. CrossRef
- The Effect of Tegoprazan on the Treatment of Endoscopic Resection-Induced Artificial Ulcers: A Multicenter, Randomized, Active-Controlled Study
- 3,330 View
- 73 Download
- 4 Web of Science
- 5 Crossref

- Successful Endoscopic Resection of Gastric Mucosa-Associated Lymphoid Tissue Lymphoma Unresponsive to Helicobacter pylori Eradication Therapy
- Jeongmin Choi
- Clin Endosc 2022;55(1):136-140. Published online November 16, 2020
- DOI: https://doi.org/10.5946/ce.2020.232

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Eradication of Helicobacter pylori is the first-line treatment for gastric mucosa-associated lymphoid tissue (MALT) lymphomas; however, lesions may persist in 20% of patients after initial treatment, thereby necessitating the use of an additional therapeutic approach. Other treatment options include radiation therapy, chemotherapy, endoscopic resection, rituximab therapy, or watchful waiting. We present a case of localized gastric MALT lymphoma that did not respond to H. pylori eradication therapy. The patient waited for 12 months but the tumor showed no signs of regression endoscopically. Histologic examination revealed residual MALT lymphoma. The tumor was then successfully treated using endoscopic submucosal dissection and the patient remained disease-free for 4 years. To our knowledge, this is the first case in which a gastric MALT lymphoma was treated with endoscopic submucosal dissection. In conclusion, endoscopic resection may be recommended as second-line therapy for properly selected patients with gastric MALT lymphoma as it is effective and minimally invasive.
-
Citations
Citations to this article as recorded by- A Common Symptom With an Uncommon Diagnosis: A Case of Primary Esophageal Diffuse Large B-cell Lymphoma
Shruthi Narasimha, Rasiq Zackria, Jonathan Hughes, Vijay Jayaraman
Cureus.2024;[Epub] CrossRef - A Case of Esophageal MALT Lymphoma Mimicking a Subepithelial Tumor
Ha Eun Lee, Gwang Ha Kim, Min Ji Kim, Kyung Bin Kim, Dong Chan Joo, Hye Kyung Jeon, Moon Won Lee, Bong Eun Lee
The Korean Journal of Gastroenterology.2024; 83(4): 157. CrossRef - Clinical Management of Patients with Gastric MALT Lymphoma: A Gastroenterologist’s Point of View
Tamara Matysiak-Budnik, Kateryna Priadko, Céline Bossard, Nicolas Chapelle, Agnès Ruskoné-Fourmestraux
Cancers.2023; 15(15): 3811. CrossRef - Endoscopic Submucosal Dissection for Treatment of Localized Gastric Mucosa-associated Lymphoid Tissue Lymphoma: A Case Series
Jun-young Seo, Kee Don Choi, In Hye Song, Young Soo Park, Hee Kyong Na, Ji Yong Ahn, Jeong Hoon Lee, Kee Wook Jung, Do Hoon Kim, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2023; 23(3): 188. CrossRef - Bacteria-Mediated Oncogenesis and the Underlying Molecular Intricacies: What We Know So Far
Shashanka K. Prasad, Smitha Bhat, Dharini Shashank, Akshatha C. R., Sindhu R., Pornchai Rachtanapun, Devananda Devegowda, Prasanna K. Santhekadur, Sarana Rose Sommano
Frontiers in Oncology.2022;[Epub] CrossRef - Primary Esophageal Lymphoma: Clinical Experience in Diagnosis and Treatment
Junchi Qu, Yanyan Zhuang, Dandan Zheng, Fengting Huang, Shineng Zhang
Cureus.2021;[Epub] CrossRef
- A Common Symptom With an Uncommon Diagnosis: A Case of Primary Esophageal Diffuse Large B-cell Lymphoma
- 4,302 View
- 207 Download
- 5 Web of Science
- 6 Crossref

- Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer
- Chan Hyuk Park, Dong-Hoon Yang, Jong Wook Kim, Jie-Hyun Kim, Ji Hyun Kim, Yang Won Min, Si Hyung Lee, Jung Ho Bae, Hyunsoo Chung, Kee Don Choi, Jun Chul Park, Hyuk Lee, Min-Seob Kwak, Bun Kim, Hyun Jung Lee, Hye Seung Lee, Miyoung Choi, Dong-Ah Park, Jong Yeul Lee, Jeong-Sik Byeon, Chan Guk Park, Joo Young Cho, Soo Teik Lee, Hoon Jai Chun
- Clin Endosc 2020;53(2):142-166. Published online March 30, 2020
- DOI: https://doi.org/10.5946/ce.2020.032
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
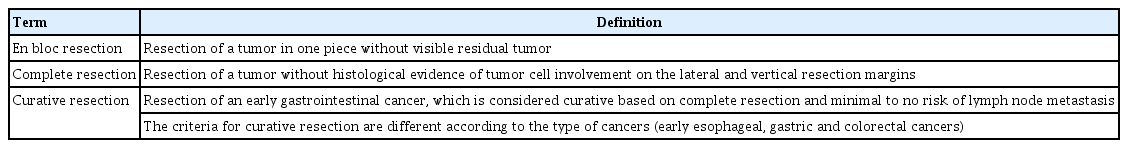
ePub - Although surgery was the standard treatment for early gastrointestinal cancers, endoscopic resection is now a standard treatment for early gastrointestinal cancers without regional lymph node metastasis. High-definition white light endoscopy, chromoendoscopy, and image-enhanced endoscopy such as narrow band imaging are performed to assess the edge and depth of early gastrointestinal cancers for delineation of resection boundaries and prediction of the possibility of lymph node metastasis before the decision of endoscopic resection. Endoscopic mucosal resection and/or endoscopic submucosal dissection can be performed to remove early gastrointestinal cancers completely by en bloc fashion. Histopathological evaluation should be carefully made to investigate the presence of risk factors for lymph node metastasis such as depth of cancer invasion and lymphovascular invasion. Additional treatment such as radical surgery with regional lymphadenectomy should be considered if the endoscopically resected specimen shows risk factors for lymph node metastasis. This is the first Korean clinical practice guideline for endoscopic resection of early gastrointestinal cancer. This guideline was developed by using mainly de novo methods and encompasses endoscopic management of superficial esophageal squamous cell carcinoma, early gastric cancer, and early colorectal cancer. This guideline will be revised as new data on early gastrointestinal cancer are collected.
-
Citations
Citations to this article as recorded by- Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study
Hae Won Yoo, Su Jin Hong, Shin Hee Kim
Gastroenterology.2024; 166(2): 313. CrossRef - A Modified eCura System to Stratify the Risk of Lymph Node Metastasis in Undifferentiated-Type Early Gastric Cancer After Endoscopic Resection
Hyo-Joon Yang, Hyuk Lee, Tae Jun Kim, Da Hyun Jung, Kee Don Choi, Ji Yong Ahn, Wan Sik Lee, Seong Woo Jeon, Jie-Hyun Kim, Gwang Ha Kim, Jae Myung Park, Sang Gyun Kim, Woon Geon Shin, Young-Il Kim, Il Ju Choi
Journal of Gastric Cancer.2024; 24(2): 172. CrossRef - Management after non-curative endoscopic resection of T1 rectal cancer
Hao Dang, Daan A. Verhoeven, Jurjen J. Boonstra, Monique E. van Leerdam
Best Practice & Research Clinical Gastroenterology.2024; 68: 101895. CrossRef - Tumor size discrepancy between endoscopic and pathological evaluations in colorectal endoscopic submucosal dissection
Takeshi Onda, Osamu Goto, Toshiaki Otsuka, Yoshiaki Hayasaka, Shun Nakagome, Tsugumi Habu, Yumiko Ishikawa, Kumiko Kirita, Eriko Koizumi, Hiroto Noda, Kazutoshi Higuchi, Jun Omori, Naohiko Akimoto, Katsuhiko Iwakiri
World Journal of Gastrointestinal Endoscopy.2024; 16(3): 136. CrossRef - Nomograms and prognosis for superficial esophageal squamous cell carcinoma
Hong Tao Lin, Ahmed Abdelbaki, Somashekar G Krishna
World Journal of Gastroenterology.2024; 30(10): 1291. CrossRef - A new clinical model for predicting lymph node metastasis in T1 colorectal cancer
Kai Wang, Hui He, Yanyun Lin, Yanhong Zhang, Junguo Chen, Jiancong Hu, Xiaosheng He
International Journal of Colorectal Disease.2024;[Epub] CrossRef - The role of endoluminal surgery in a colorectal surgical practice. A global view
Ilker Ozgur, Fevzi Cengiz
Seminars in Colon and Rectal Surgery.2024; 35(2): 101023. CrossRef - Enhanced multi-class pathology lesion detection in gastric neoplasms using deep learning-based approach and validation
Byeong Soo Kim, Bokyung Kim, Minwoo Cho, Hyunsoo Chung, Ji Kon Ryu, Sungwan Kim
Scientific Reports.2024;[Epub] CrossRef - Innovations in dedicated PET instrumentation: from the operating room to specimen imaging
Hossein Arabi, Abdollah Saberi Manesh, Habib Zaidi
Physics in Medicine & Biology.2024; 69(11): 11TR03. CrossRef - Venous invasion and lymphatic invasion are correlated with the postoperative prognosis of pancreatic neuroendocrine neoplasm
Sho Kiritani, Junichi Arita, Yuichiro Mihara, Rihito Nagata, Akihiko Ichida, Yoshikuni Kawaguchi, Takeaki Ishizawa, Nobuhisa Akamatsu, Junichi Kaneko, Kiyoshi Hasegawa
Surgery.2023; 173(2): 365. CrossRef - Long-term outcomes after endoscopic versus surgical resection of T1 colorectal carcinoma
Hyun Jin Bae, Hoyeon Ju, Han Hee Lee, Jinsu Kim, Bo-In Lee, Sung Hak Lee, Daeyoun David Won, Yoon Suk Lee, In Kyu Lee, Young-Seok Cho
Surgical Endoscopy.2023; 37(2): 1231. CrossRef - Resection speed of endoscopic submucosal dissection according to the location of gastric neoplasia: a learning curve using cumulative sum analysis
Jun-Hyung Cho, So-Young Jin, Suyeon Park
Surgical Endoscopy.2023; 37(4): 2969. CrossRef - Endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma: long-term follow-up in a Western center
Andreas Probst, Alanna Ebigbo, Stefan Eser, Carola Fleischmann, Tina Schaller, Bruno Märkl, Stefan Schiele, Bernd Geissler, Gernot Müller, Helmut Messmann
Clinical Endoscopy.2023; 56(1): 55. CrossRef - A Randomized Controlled Trial of Fibrin Glue to Prevent Bleeding After Gastric Endoscopic Submucosal Dissection
Hyun Deok Lee, Eunwoo Lee, Sang Gyun Kim, Cheol Min Shin, Jun Chul Park, Kee Don Choi, Seokyung Hahn, Soo-Jeong Cho
American Journal of Gastroenterology.2023; 118(5): 892. CrossRef - Endoscopic Resection of Undifferentiated Early Gastric Cancer
Yuichiro Hirai, Seiichiro Abe, Mai Ego Makiguchi, Masau Sekiguchi, Satoru Nonaka, Haruhisa Suzuki, Shigetaka Yoshinaga, Yutaka Saito
Journal of Gastric Cancer.2023; 23(1): 146. CrossRef - Pre-procedure oral administration of pronase improves efficacy of lugol chromoendoscopy in esophageal squamous cell carcinoma screening: a prospective, double-blinded, randomized, controlled trial
Xin Zhao, Meng Guo, Shaohua Zhu, Linhui Zhang, Tao Dong, Hui Luo, Weihua Yu, Jiangyi Zhu, Xiaotong Fan, Ying Han, Zhiguo Liu
Surgical Endoscopy.2023; 37(6): 4421. CrossRef - Endoscopic advances in the management of gastric cancer and premalignant gastric conditions
Erica Park, Makoto Nishimura, Priya Simoes
World Journal of Gastrointestinal Endoscopy.2023; 15(3): 114. CrossRef - Comparative Cost Analysis Between Endoscopic Resection and Surgery for Submucosal Colorectal Cancer
Soo Min Noh, Sung Wook Hwang, Sang Hyoung Park, Dong-Hoon Yang, Byong Duk Ye, In Ja Park, Seok-Byung Lim, Jeong-Sik Byeon
Diseases of the Colon & Rectum.2023; 66(5): 723. CrossRef - Descriptive Analysis of Gastric Cancer Mortality in Korea, 2000-2020
Tung Hoang, Hyeongtaek Woo, Sooyoung Cho, Jeeyoo Lee, Sayada Zartasha Kazmi, Aesun Shin
Cancer Research and Treatment.2023; 55(2): 603. CrossRef - Endoscopically injectable and self‐crosslinkable hydrogel‐mediated stem cell transplantation for alleviating esophageal stricture after endoscopic submucosal dissection
Hyunsoo Chung, Soohwan An, Seung Yeop Han, Jihoon Jeon, Seung‐Woo Cho, Yong Chan Lee
Bioengineering & Translational Medicine.2023;[Epub] CrossRef - Deep learning-based clinical decision support system for gastric neoplasms in real-time endoscopy: development and validation study
Eun Jeong Gong, Chang Seok Bang, Jae Jun Lee, Gwang Ho Baik, Hyun Lim, Jae Hoon Jeong, Sung Won Choi, Joonhee Cho, Deok Yeol Kim, Kang Bin Lee, Seung-Il Shin, Dick Sigmund, Byeong In Moon, Sung Chul Park, Sang Hoon Lee, Ki Bae Bang, Dae-Soon Son
Endoscopy.2023; 55(08): 701. CrossRef - A 6-year nationwide population-based study on the current status of gastric endoscopic resection in Korea using administrative data
Jae Yong Park, Mi-Sook Kim, Beom Jin Kim, Jae Gyu Kim
Scientific Reports.2023;[Epub] CrossRef - Efficacy and Safety of ClearCut™ Knife H-type in Endoscopic Submucosal Dissection for Gastric Neoplasms: A Multicenter, Randomized Trial
Eun Jeong Gong, Hyun Lim, Sang Jin Lee, Do Hoon Kim
Journal of Gastric Cancer.2023; 23(3): 451. CrossRef - Endoscopic resection for local residual or recurrent cancer after definitive chemoradiotherapy or radiotherapy for esophageal squamous cell carcinoma
Yasuhiro Tani, Ryu Ishihara, Noriko Matsuura, Yuki Okubo, Yushi Kawakami, Hirohisa Sakurai, Takahiko Nakamura, Katsunori Matsueda, Muneaki Miyake, Satoki Shichijo, Akira Maekawa, Takashi Kanesaka, Sachiko Yamamoto, Yoji Takeuchi, Koji Higashino, Noriya Ue
Scientific Reports.2023;[Epub] CrossRef - Modified underwater endoscopic mucosal resection for intermediate-sized sessile colorectal polyps
Dong Hyun Kim, Seon-Young Park, Hye-Su You, Yong-Wook Jung, Young-Eun Joo, Dae-Seong Myung, Hyun-Soo Kim, Nah Ihm Kim, Seong-Jung Kim, Jae Kyun Ju
Frontiers in Medicine.2023;[Epub] CrossRef - Clinical Application of the Kyoto Classification of Gastritis
Gwang Ha Kim
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2023; 23(2): 89. CrossRef - Endoscopic Resection for Gastric Adenocarcinoma of the Fundic Gland Type: A Case Series
Hwa Jin Lee, Gwang Ha Kim, Dong Chan Joo, Moon Won Lee, Bong Eun Lee, Kyungbin Kim
The Korean Journal of Gastroenterology.2023; 81(6): 259. CrossRef - The optimal interval of surveillance gastroscopy after endoscopic resection for gastric neoplasia: a multicenter cohort study
Younghee Choe, Byung-Wook Kim, Tae Ho Kim, Jun-Won Chung, Jongwon Kim, Soo-Young Na, Joon Sung Kim
Surgical Endoscopy.2023; 37(10): 7556. CrossRef - External Validation of the eCura System for Undifferentiated-Type Early Gastric Cancer with Noncurative Endoscopic Resection
Hyo-Joon Yang, Young-Il Kim, Ji Yong Ahn, Kee Don Choi, Sang Gyun Kim, Seong Woo Jeon, Jie-Hyun Kim, Sung Kwan Shin, Hyuk Lee, Wan Sik Lee, Gwang Ha Kim, Jae Myung Park, Woon Geon Shin, Il Ju Choi
Gut and Liver.2023; 17(4): 537. CrossRef - Outcomes of the Conventional versus Pocket-Creation Method for Endoscopic Submucosal Dissection of Gastric Body Tumors Using a Dual Knife: A Retrospective Study
Sang Pyo Lee, Hyun Joo Jang, Sea Hyub Kae, Jae Gon Lee
Gut and Liver.2023; 17(4): 547. CrossRef - LEARNING CURVE IN ESOPHAGEAL ENDOSCOPIC SUBMUCOSAL DISSECTION BY WESTERN ENDOSCOSPISTS TRAINED IN JAPAN: EXPERIENCE IN LATIN AMERICA
Josué ALIAGA RAMOS, Naohisa YOSHIDA, Rafiz ABDUL RANI, Vitor N ARANTES
Arquivos de Gastroenterologia.2023; 60(2): 208. CrossRef - Diagnostic Performance of Endoscopic Ultrasonography with Water-Filled Balloon Method for Superficial Esophageal Squamous Cell Carcinoma
Yugo Suzuki, Kosuke Nomura, Daisuke Kikuchi, Toshiro Iizuka, Mako Koseki, Yusuke Kawai, Takayuki Okamura, Yorinari Ochiai, Junnosuke Hayasaka, Yutaka Mitsunaga, Hiroyuki Odagiri, Satoshi Yamashita, Akira Matsui, Kenichi Ohashi, Shu Hoteya
Digestive Diseases and Sciences.2023; 68(10): 3974. CrossRef - Should All Undifferentiated Early Gastric Cancer Patients Undergoing Noncurative Endoscopic Resection Be Sent to the Operating Room?
Jung-Wook Kim, Albert C. Kim
Gut and Liver.2023; 17(5): 665. CrossRef - Comparing endoscopic mucosal resection with endoscopic submucosal dissection in colorectal adenoma and tumors: Meta-analysis and system review
Nian Wang, Lei Shu, Song Liu, Lin Yang, Tao Bai, Zhaohong Shi, Xinghuang Liu, Paolo Aurello
PLOS ONE.2023; 18(9): e0291916. CrossRef - Weighing the benefits of lymphadenectomy in early-stage colorectal cancer
Seung Min Baik, Ryung-Ah Lee
Annals of Surgical Treatment and Research.2023; 105(5): 245. CrossRef - Endoscopic treatment of colorectal polyps and early colorectal cancer
Yunho Jung
Journal of the Korean Medical Association.2023; 66(11): 642. CrossRef - Endoscopic submucosal dissection for early gastric cancer: It is time to consider the quality of its outcomes
Gwang Ha Kim
World Journal of Gastroenterology.2023; 29(43): 5800. CrossRef - A Retrospective Multicenter Study of Risk Factors, Stratification, and Prognosis of Lymph Node Metastasis in T1 and T2 Colorectal Cancer
Eui Myung Kim, Il Tae Son, Byung Chun Kim, Jun Ho Park, Byung Mo Kang, Jong Wan Kim
Journal of Clinical Medicine.2023; 12(24): 7744. CrossRef - A nomogram for predicting the risk of postoperative fever in elderly patients undergoing endoscopic submucosal dissection of the upper gastrointestinal tract
Zhixiang Xu, Jing Zhuang, Xin Zhu, Jun Yao
Medicine.2023; 102(50): e36438. CrossRef - Usage trends of colorectal endoscopic submucosal dissection according to hospital types based on nationwide claims data
Ji Eun Na, Bohyoung Kim, Sung Hoon Jung, Arum Choi, Sukil Kim, Tae-Oh Kim
Medicine.2023; 102(43): e35514. CrossRef - Long-term outcomes of endoscopic resection followed by additional surgery after non-curative resection in undifferentiated-type early gastric cancer: a nationwide multi-center study
Jie-Hyun Kim, Young-Il Kim, Ji Yong Ahn, Woon Geon Shin, Hyo-Joon Yang, Su Youn Nam, Byung-Hoon Min, Jae-Young Jang, Joo Hyun Lim, Wan Sik Lee, Bong Eun Lee, Moon Kyung Joo, Jae Myung Park, Hang Lak Lee, Tae-Geun Gweon, Moo In Park, Jeongmin Choi, Chung H
Surgical Endoscopy.2022; 36(3): 1847. CrossRef - A Simple Risk Scoring System for Predicting the Occurrence of Aspiration Pneumonia After Gastric Endoscopic Submucosal Dissection
Kyemyung Park, Na Young Kim, Ki Jun Kim, Chaerim Oh, Dongwoo Chae, So Yeon Kim
Anesthesia & Analgesia.2022; 134(1): 114. CrossRef - Long-term outcomes of endoscopic mucosal resection for early-stage esophageal adenocarcinoma
Kesha Oza, Tejasvi Peesay, Benjamin Greenspun, John E. Carroll, Shervin Shafa, Jay C. Zeck, Nadim G. Haddad, Marc Margolis, Puja Gaur Khaitan
Surgical Endoscopy.2022; 36(7): 5136. CrossRef - Long-Term Outcomes and Prognostic Factors of Superficial Esophageal Cancer in Patients Aged ≥ 65 Years
Jin Won Chang, Da Hyun Jung, Cheal Wung Huh, Jun Chul Park, Sung Kwan Shin, Sang Kil Lee, Yong Chan Lee
Frontiers in Medicine.2022;[Epub] CrossRef - Tumor Location as a Prognostic Factor in T1 Colorectal Cancer
Katsuro Ichimasa, Shin-ei Kudo, Yuta Kouyama, Kenichi Mochizuki, Yuki Takashina, Masashi Misawa, Yuichi Mori, Takemasa Hayashi, Kunihiko Wakamura, Hideyuki Miyachi
Journal of the Anus, Rectum and Colon.2022; 6(1): 9. CrossRef - Artificial Intelligence for Detecting and Delineating Margins of Early ESCC Under WLI Endoscopy
Wei Liu, Xianglei Yuan, Linjie Guo, Feng Pan, Chuncheng Wu, Zhongshang Sun, Feng Tian, Cong Yuan, Wanhong Zhang, Shuai Bai, Jing Feng, Yanxing Hu, Bing Hu
Clinical and Translational Gastroenterology.2022; 13(1): e00433. CrossRef - Machine Learning Model to Stratify the Risk of Lymph Node Metastasis for Early Gastric Cancer: A Single-Center Cohort Study
Ji-Eun Na, Yeong-Chan Lee, Tae-Jun Kim, Hyuk Lee, Hong-Hee Won, Yang-Won Min, Byung-Hoon Min, Jun-Haeng Lee, Poong-Lyul Rhee, Jae J. Kim
Cancers.2022; 14(5): 1121. CrossRef - Chinese consensus on prevention of colorectal neoplasia (2021, Shanghai)
Journal of Digestive Diseases.2022; 23(2): 58. CrossRef - Advances in the application of regenerative medicine in prevention of post-endoscopic submucosal dissection for esophageal stenosis
Jiaxin Wang, Yan Zhao, Peng Li, Shutian Zhang
Journal of Translational Internal Medicine.2022; 10(1): 28. CrossRef - Prolonged ischemia of the ileum and colon after surgical mucosectomy explains contraction and failure of “mucus free” bladder augmentation
Dániel Urbán, Gabriella Varga, Dániel Érces, Mahmoud Marei Marei, Raimondo Cervellione, David Keene, Anju Goyal, Tamás Cserni
Journal of Pediatric Urology.2022; 18(4): 500.e1. CrossRef - Safety and efficacy of prophylactic gastric open peroral endoscopic myotomy for prevention of post‐ESD stenosis: A case series (with video)
Won Dong Lee, Jae Sun Song, Byung Sun Kim, Min A. Yang, Young Jae Lee, Gum Mo Jung, Ji Woong Kim, Yong Keun Cho, Jin Woong Cho
Journal of Digestive Diseases.2022; 23(4): 220. CrossRef - Therapeutic approach to non-curative resection after endoscopic treatment in early gastric cancer
Eun Jeong Gong, Chang Seok Bang
Journal of the Korean Medical Association.2022; 65(5): 284. CrossRef - Endoscopic treatment for early gastric cancer
Ji Yong Ahn
Journal of the Korean Medical Association.2022; 65(5): 276. CrossRef - Endoscopic diagnosis of early gastric cancer
Dong Chan Joo, Gwang Ha Kim
Journal of the Korean Medical Association.2022; 65(5): 267. CrossRef - Current status of the gastric cancer screening program in Korea
Young-Il Kim, Il Ju Choi
Journal of the Korean Medical Association.2022; 65(5): 250. CrossRef - Colorectális polypok ellátása
Szabolcs Ábrahám, Illés Tóth, Dániel Váczi, György Lázár
Magyar Sebészet.2022; 75(2): 155. CrossRef - Utility of a deep learning model and a clinical model for predicting bleeding after endoscopic submucosal dissection in patients with early gastric cancer
Ji Eun Na, Yeong Chan Lee, Tae Jun Kim, Hyuk Lee, Hong-Hee Won, Yang Won Min, Byung-Hoon Min, Jun Haeng Lee, Poong-Lyul Rhee, Jae J Kim
World Journal of Gastroenterology.2022; 28(24): 2721. CrossRef - Rare primary rectal mucosa-associated lymphoid tissue lymphoma with curative resection by endoscopic submucosal dissection: A case report and review of literature
Yan Tao, Qiong Nan, Zi Lei, Ying-Lei Miao, Jun-Kun Niu
World Journal of Clinical Cases.2022; 10(21): 7599. CrossRef - Prevention of stricture after endoscopic submucosal dissection for esophageal cancer: intralesional steroid infusion using a spray tube
Jong Yeul Lee
Clinical Endoscopy.2022; 55(4): 516. CrossRef - Paneth Cell Carcinoma of the Stomach
Jun Wan Kim, Gwang Ha Kim, Kyung Bin Kim
The Korean Journal of Gastroenterology.2022; 80(1): 34. CrossRef - Composite scoring system and optimal tumor budding cut-off number for estimating lymph node metastasis in submucosal colorectal cancer
Jeong-ki Kim, Ye-Young Rhee, Jeong Mo Bae, Jung Ho Kim, Seong-Joon Koh, Hyun Jung Lee, Jong Pil Im, Min Jung Kim, Seung-Bum Ryoo, Seung-Yong Jeong, Kyu Joo Park, Ji Won Park, Gyeong Hoon Kang
BMC Cancer.2022;[Epub] CrossRef - Multidisciplinary Treatment Strategy for Early Colon Cancer: A Review-An English Version
Gyung Mo Son, Su Bum Park, Tae Un Kim, Byung-Soo Park, In Young Lee, Joo-Young Na, Dong Hoon Shin, Sang Bo Oh, Sung Hwan Cho, Hyun Sung Kim, Hyung Wook Kim
Journal of the Anus, Rectum and Colon.2022; 6(4): 203. CrossRef - Extragastric Metastasis of Early Gastric Cancer After Endoscopic Submucosal Dissection With Lymphovascular Invasion and Negative Resected Margins
Han Myung Lee, Yoonjin Kwak, Hyunsoo Chung, Sang Gyun Kim, Soo-Jeong Cho
Journal of Gastric Cancer.2022; 22(4): 339. CrossRef - Comparison between a novel core knife and the conventional IT knife 2 for endoscopic submucosal dissection of gastric mucosal lesions
Myeongsoon Park, Jin Wook Lee, Dong Woo Shin, Jungseok Kim, Yoo Jin Lee, Ju Yup Lee, Kwang Bum Cho
Clinical Endoscopy.2022; 55(6): 767. CrossRef - Need for careful endoscopic evaluation of large gastric neoplasms before endoscopic submucosal dissection
Seung Woo Lee
Clinical Endoscopy.2022; 55(6): 753. CrossRef - Regression of gastric endoscopic submucosal dissection induced polypoid nodular scar after Helicobacter pylori eradication: A case report
Byung Chul Jin, Ae Ri Ahn, Seong-Hun Kim, Seung Young Seo
World Journal of Clinical Cases.2022; 10(34): 12793. CrossRef - Simultaneous analysis of tumor-infiltrating immune cells density, tumor budding status, and presence of lymphoid follicles in CRC tissue
Adam R. Markowski, Anna J. Markowska, Wiktoria Ustymowicz, Anna Pryczynicz, Katarzyna Guzińska-Ustymowicz
Scientific Reports.2022;[Epub] CrossRef - Role of Endoscopy in Management of Upper Gastrointestinal Cancers
Jeff Liang, Yi Jiang, Yazan Abboud, Srinivas Gaddam
Diseases.2022; 11(1): 3. CrossRef - Association between severe hepatic steatosis examined by Fibroscan and the risk of high-risk colorectal neoplasia
Kwang Woo Kim, Hyoun Woo Kang, Hosun Yoo, Yukyung Jun, Hyun Jung Lee, Jong Pil Im, Ji Won Kim, Joo Sung Kim, Seong-Joon Koh, Yong Jin Jung, Atsushi Hosui
PLOS ONE.2022; 17(12): e0279242. CrossRef - Long-term outcomes and clinical safety of expanded indication early gastric cancer treated with endoscopic submucosal dissection versus surgical resection: a meta-analysis
Xing Xu, Guoliang Zheng, Na Gao, Zhichao Zheng
BMJ Open.2022; 12(12): e055406. CrossRef - Long-Term Safety of Delayed Surgery After Upfront Endoscopic Resection for Early Gastric Cancer: A Propensity Matched Study
Ji Eun Na, Yeong Gi Kim, Tae Jun Kim, Hyuk Lee, Yang Won Min, Byung-Hoon Min, Jun Haeng Lee, Seon Yeong Baek, Min Su Park, Poong-Lyul Rhee, Jae J. Kim
Annals of Surgical Oncology.2021; 28(1): 106. CrossRef - Incidence rates, risk factors, and outcomes of aspiration pneumonia after gastric endoscopic submucosal dissection: A systematic review and meta‐analysis
Dong Tang, Fuxiang Yuan, Xiaoying Ma, Haixia Qu, Yuan Li, Weiwei Zhang, Huan Ma, Haiping Liu, Yan Yang, Lin Xu, Yuqiang Gao, Shuhui Zhan
Journal of Gastroenterology and Hepatology.2021; 36(6): 1457. CrossRef - Clinical feasibility and oncologic safety of primary endoscopic submucosal dissection for clinical submucosal invasive early gastric cancer
Ji Eun Na, Hyuk Lee, Yang Won Min, Byung-Hoon Min, Jun Haeng Lee, Poong-Lyul Rhee, Kyoung-Mee Kim, Jae J. Kim
Journal of Cancer Research and Clinical Oncology.2021; 147(10): 3051. CrossRef - Endoscopic resections for superficial esophageal squamous cell epithelial neoplasia: focus on histological discrepancies between biopsy and resected specimens
Lang Yang, Hua Jin, Xiao-li Xie, Yang-tian Cao, Zhen-hua Liu, Na Li, Peng Jin, Yu-qi He, Jian-qiu Sheng
BMC Gastroenterology.2021;[Epub] CrossRef - Atypical Scar Patterns after Gastric Endoscopic Submucosal Dissection
Bomin Kim, Beom Jin Kim, Hong Jip Yoon, Hyunsuk Lee, Jae Yong Park, Chang Hwan Choi, Jae Gyu Kim
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2021; 21(1): 72. CrossRef - Endoscopic Submucosal Dissection versus Surgery for Undifferentiated-Type Early Gastric Cancer: A Systematic Review and Meta-Analysis
Cheal-Wung Huh, Dae Won Ma, Byung-Wook Kim, Joon Sung Kim, Seung Jae Lee
Clinical Endoscopy.2021; 54(2): 202. CrossRef - Role of Endoscopic Ultrasound in Selecting Superficial Esophageal Cancers for Endoscopic Resection
Jinju Choi, Hyunsoo Chung, Ayoung Lee, Jue Lie Kim, Soo-Jeong Cho, Sang Gyun Kim
The Annals of Thoracic Surgery.2021; 111(5): 1689. CrossRef - Recent advances in early esophageal cancer: diagnosis and treatment based on endoscopy
Hang Yang, Bing Hu
Postgraduate Medicine.2021; 133(6): 665. CrossRef - Endoscopic Resection of Gastric Cancer
Ga Hee Kim, Hwoon-Yong Jung
Gastrointestinal Endoscopy Clinics of North America.2021; 31(3): 563. CrossRef - Gastric Mucosa-Associated Lymphoid Tissue Lymphomas Diagnosed by Jumbo Biopsy Using Endoscopic Submucosal Dissection: A Case Report
Jian Han, Jun Wang, Hua-ping Xie
Frontiers in Medicine.2021;[Epub] CrossRef - Considerations for Endoscopic Treatment of Undifferentiated-type Early Gastric Cancer
Kyoungwon Jung
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2021; 21(2): 103. CrossRef - Papillary Adenocarcinoma
Tae-Se Kim, Byung-Hoon Min
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2021; 21(2): 122. CrossRef - Early gastrointestinal cancer: The application of artificial intelligence
Hang Yang, Bing Hu
Artificial Intelligence in Gastrointestinal Endoscopy.2021; 2(4): 185. CrossRef - Successful Endoscopic Resection of Primary Rectal Mucosa-Associated Lymphoid Tissue Lymphoma by Endoscopic Submucosal Dissection: A Case Report
Jian Han, Zhe Zhu, Chao Zhang, Hua-ping Xie
Frontiers in Medicine.2021;[Epub] CrossRef - Assessment of the Diagnostic Performance of Endoscopic Ultrasonography After Conventional Endoscopy for the Evaluation of Esophageal Squamous Cell Carcinoma Invasion Depth
Ryu Ishihara, Junki Mizusawa, Ryoji Kushima, Noriko Matsuura, Tomonori Yano, Tomoko Kataoka, Haruhiko Fukuda, Noboru Hanaoka, Toshiyuki Yoshio, Seiichiro Abe, Yoshinobu Yamamoto, Shinji Nagata, Hiroyuki Ono, Masashi Tamaoki, Naohiro Yoshida, Kohei Takizaw
JAMA Network Open.2021; 4(9): e2125317. CrossRef - Variation in Diagnosis, Treatment, and Outcome of Esophageal Cancer in a Regionalized Care System in Ontario, Canada
Steven Habbous, Olga Yermakhanova, Katharina Forster, Claire M. B. Holloway, Gail Darling
JAMA Network Open.2021; 4(9): e2126090. CrossRef - Risk Stratification of T1 Colorectal Cancer Metastasis to Lymph Nodes: Current Status and Perspective
Katsuro Ichimasa, Shin-ei Kudo, Hideyuki Miyachi, Yuta Kouyama, Masashi Misawa, Yuichi Mori
Gut and Liver.2021; 15(6): 818. CrossRef - Close Observation versus Additional Surgery after Noncurative Endoscopic Resection of Esophageal Squamous Cell Carcinoma
Byeong Geun Song, Ga Hee Kim, Charles J. Cho, Hyeong Ryul Kim, Yang Won Min, Hyuk Lee, Byung-Hoon Min, Ho June Song, Yong-Hee Kim, Jun Haeng Lee, Hwoon-Yong Jung, Jae Ill Zo, Young Mog Shim
Digestive Surgery.2021; 38(3): 247. CrossRef
- Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study
- 15,874 View
- 1,123 Download
- 88 Web of Science
- 88 Crossref

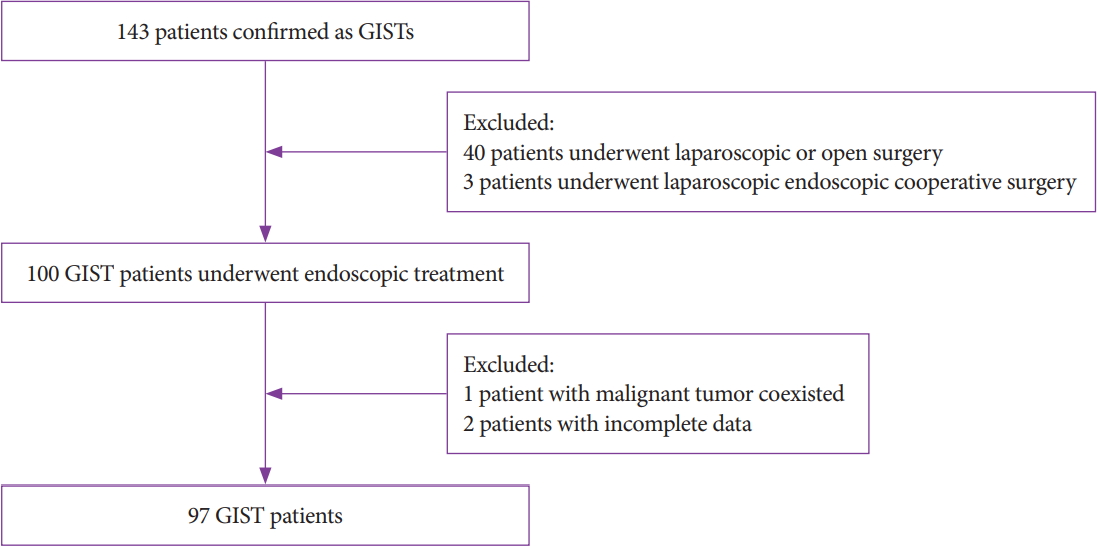
- Efficacy and Safety of Endoscopic Treatment for Gastrointestinal Stromal Tumors in the Upper Gastrointestinal Tract
- Cicilia Marcella, Shakeel Sarwar, Hui Ye, Rui Hua Shi
- Clin Endosc 2020;53(4):458-465. Published online March 17, 2020
- DOI: https://doi.org/10.5946/ce.2019.121
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic treatment (ET) has been applied for decades to treat subepithelial tumors, including gastrointestinal stromal tumors (GISTs). However, the efficacy of ET remains debatable. In this study, we evaluated the efficacy and safety of ET for GISTs in the upper gastrointestinal tract.
Methods
This retrospective single-center study included 97 patients who underwent ET. All patients were enrolled from July 2014 to July 2018. Parameters such as demographics, size, resection margin, complications, pathological features, procedure time, total cost, and follow-up were investigated and analyzed.
Results
Our study achieved 100% en bloc resection and 77.4% (72/93) R0 resection. The most common location was the fundus with a mean tumor size of 2.1±1.43 cm. The mean age, procedure time, hospital stay, and cost were 59.7±11.29 years, 64.7±35.23 minutes, 6.8 days, and 5,337 dollars, respectively. According to National Institutes of Health classification, 63 (64.9%), 26 (26.8%), 5 (5.2%), and 3 (3.1%) patients belonged to the very low, low, intermediate, and high risk classification, respectively. Immunohistochemistry results showed a 100% positive rate of CD34, DOG-1, CD117, and Ki67. A mean follow-up of 21.3±13.0 months showed no recurrence or metastasis.
Conclusions
ET is effective and safe for curative removal of GISTs in the upper gastrointestinal tract, and it can be a treatment of choice for patients with no metastasis. -
Citations
Citations to this article as recorded by- Comparison of endoscopic full-thickness resection and cap-assisted endoscopic full-thickness resection in the treatment of small (≤1.5 cm) gastric GI stromal tumors
Jinping Yang, Muhan Ni, Jingwei Jiang, Ximei Ren, Tingting Zhu, Shouli Cao, Shahzeb Hassan, Ying Lv, Xiaoqi Zhang, Yongyue Wei, Lei Wang, Guifang Xu
Gastrointestinal Endoscopy.2022; 95(4): 660. CrossRef - The necessarity of treatment for small gastric subepithelial tumors (1–2 cm) originating from muscularis propria: an analysis of 972 tumors
Jinlong Hu, Xinzhu Sun, Nan Ge, Sheng Wang, Jintao Guo, Xiang Liu, Guoxin Wang, Siyu Sun
BMC Gastroenterology.2022;[Epub] CrossRef - Natural History of Asymptomatic Esophageal Subepithelial Tumors of 30 mm or Less in Size
Seokin Kang, Do Hoon Kim, Yuri Kim, Dongsub Jeon, Hee Kyong Na, Jeong Hoon Lee, Ji Yong Ahn, Kee Wook Jung, Kee Don Choi, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung
Journal of Korean Medical Science.2022;[Epub] CrossRef - Massive Digestive Hemorrhagia Revealing a Gastro-Intestinal Stromal Tumor of the Jejunum
Yasmine Cherouaqi, Fatima zahra Belabbes, Hanane Delsa, Anass Nadi, Fedoua Rouibaa
Cureus.2021;[Epub] CrossRef - Endoscopic Treatment for Gastrointestinal Stromal Tumors in the Upper Gastrointestinal Tract
In Kyung Yoo, Joo Young Cho
Clinical Endoscopy.2020; 53(4): 383. CrossRef - Recent advances in the management of gastrointestinal stromal tumor
Monjur Ahmed
World Journal of Clinical Cases.2020; 8(15): 3142. CrossRef
- Comparison of endoscopic full-thickness resection and cap-assisted endoscopic full-thickness resection in the treatment of small (≤1.5 cm) gastric GI stromal tumors
- 4,654 View
- 150 Download
- 6 Web of Science
- 6 Crossref

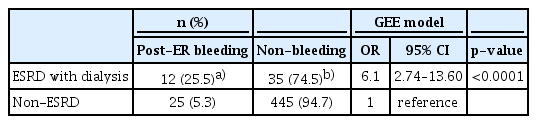
- Bleeding after Endoscopic Resection in Patients with End-Stage Renal Disease on Dialysis: A Multicenter Propensity Score-Matched Analysis
- In Kyung Yoo, Chan Gyoo Kim, Young Ju Suh, Younkyung Oh, Gwang Ho Baik, Sun Moon Kim, Young Dae Kim, Chul-Hyun Lim, Jung Won Jeon, Su Jin Hong, Byoung Wook Bang, Joon Sung Kim, Jun-Won Chung
- Clin Endosc 2020;53(4):452-457. Published online October 25, 2019
- DOI: https://doi.org/10.5946/ce.2019.107
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Frequent bleeding after endoscopic resection (ER) has been reported in patients with end-stage renal disease (ESRD). We aimed to evaluate the association and clinical significance of bleeding with ER in ESRD patients on dialysis.
Methods
Between February 2008 and December 2018, 7,571 patients, including 47 ESRD patients on dialysis who underwent ER for gastric neoplasia, were enrolled. A total of 47 ESRDpatients on dialysis were propensity score-matched 1:10 to 470 non-ESRD patients, to adjust for between-group differences in variables such as age, sex, comorbidities, anticoagulation use, tumor characteristics, and ER method. Matching was performed using an optimal matching algorithm. For the matched data, clustered comparisons were performed using the generalized estimating equation method. Medical records were retrospectively reviewed. Frequency and outcomes of post-ER bleeding were evaluated.
Results
Bleeding was more frequent in the ESRD with dialysis group than in the non-ESRD group. ESRD with dialysis conferred a significant risk of post-ER bleeding (odds ratio, 6.1; 95% confidence interval, 2.7–13.6; p<0.0001). All post-ER bleeding events were controlled using endoscopic hemostasis except in 1 non-ESRD case that needed surgery.
Conclusions
ESRD with dialysis confers a bleeding risk after ER. However, all bleeding events could be managed endoscopically without sequelae. Concern about bleeding should not stop endoscopists from performing ER in ESRD patients on dialysis. -
Citations
Citations to this article as recorded by- Effect of renal insufficiency on the short‐ and long‐term outcomes of endoscopic submucosal dissection for early gastric cancer: Propensity score‐matched analysis
Tae‐Se Kim, Byung‐Hoon Min, Sun‐Young Baek, Kyunga Kim, Yang Won Min, Hyuk Lee, Poong‐Lyul Rhee, Jae J. Kim, Jun Haeng Lee
Digestive Endoscopy.2023; 35(7): 869. CrossRef - Safeness of Endoscopic Resection in Patients with End-Stage Renal Disease on Dialysis
Sun-Jin Boo
Clinical Endoscopy.2020; 53(4): 381. CrossRef
- Effect of renal insufficiency on the short‐ and long‐term outcomes of endoscopic submucosal dissection for early gastric cancer: Propensity score‐matched analysis
- 4,590 View
- 133 Download
- 2 Web of Science
- 2 Crossref

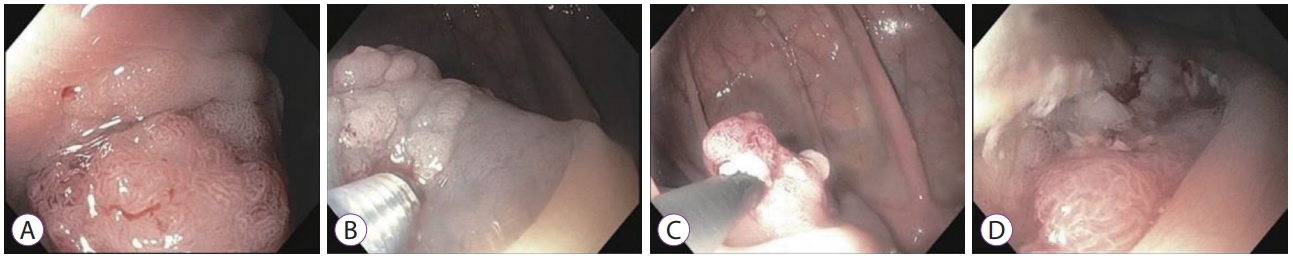
- Colonic Intramucosal Cancer in the Interposed Colon Treated with Endoscopic Mucosal Resection: A Case Report and Review of Literature
- Seung-Ho Baek, Jang-Ho Lee, Dong Ryeol Yoo, Hye Yeong Kim, Meihua Jin, Ah-reum Jang, Dong-Hoon Yang, Jeong-Sik Byeon
- Clin Endosc 2019;52(4):377-381. Published online July 30, 2019
- DOI: https://doi.org/10.5946/ce.2018.129
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Colon interposition is a surgical procedure used for maintenance of luminal conduit after esophagectomy. Although epithelial neoplasia, such as adenoma and adenocarcinoma, may develop in the interposed colon, there are only few case reports on the condition. Due to the rarity of this condition, there is no definite consensus on recommending screening endoscopy for the early detection of neoplasia in the interposed colons. Here, we report a case of intramucosal adenocarcinoma in an interposed colon. Initial endoscopic resection for this tumor failed to accomplish complete resection. A subsequent endoscopic resection was performed 1 month later and complete resection was achieved. Based on our experience and recommendation on screening endoscopy for gastric cancer in Korea, we suggest that regular screening esophagogastroduodenoscopies should be performed following esophagectomy to detect early neoplasia in the stomach and interposed colon and avoid adverse results induced by delayed detection.
-
Citations
Citations to this article as recorded by- The presence of adenocarcinoma of the right colon and polyp in colonic graft in a female patient with colon interposition due to caustic stricture of the esophagus in childhood
Stojan Latincic, Maja Pavlov, Jovica Vasiljevic, Dragan Vasin, Milena Papovic
Srpski arhiv za celokupno lekarstvo.2024; 152(1-2): 71. CrossRef
- The presence of adenocarcinoma of the right colon and polyp in colonic graft in a female patient with colon interposition due to caustic stricture of the esophagus in childhood
- 6,650 View
- 73 Download
- 1 Web of Science
- 1 Crossref

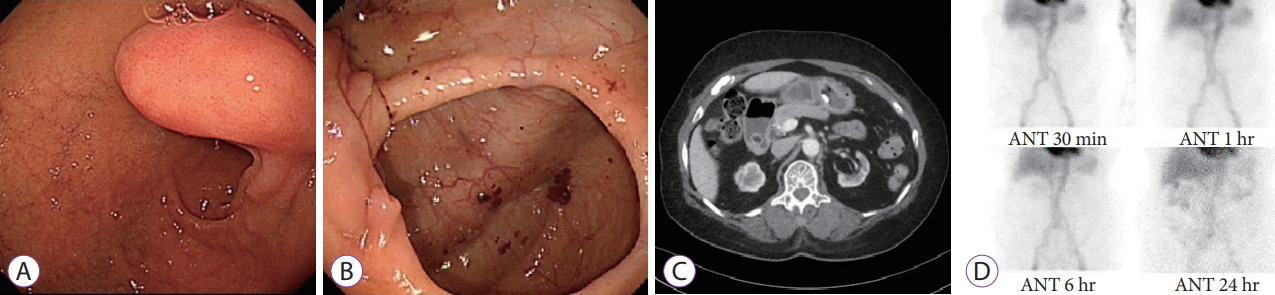
- A Case of a Bleeding Duodenal Lipoma Successfully Controlled by Endoscopic Resection
- Seo Yeon Gwak, Mi Kyung Lee, Yong Kang Lee
- Clin Endosc 2020;53(2):236-240. Published online July 24, 2019
- DOI: https://doi.org/10.5946/ce.2019.035
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - This is a case report of successful endoscopic resection (ER) of a bleeding duodenal lipoma. An 85-year-old woman who was diagnosed with asymptomatic subepithelial tumor of the duodenum 3 years ago visited the emergency room with hematemesis and was admitted to our hospital. Emergent esophagogastroduodenoscopy revealed bleeding from an ulcer on the superior aspect of a subepithelial tumor measuring about 20 mm in diameter, at the superior duodenal angle. The ulcer was in the active stage (A1), with a visible vessel. The bleeding was controlled by ER of the tumor using a snare. The final pathological diagnosis was duodenal lipoma with mucosal ulceration. The patient showed no signs of bleeding for 10 days after the procedure; subsequently, she was discharged and followed up for regular checkups.
-
Citations
Citations to this article as recorded by- Endoscopically resected duodenal lipoma as an uncommon cause of upper gastrointestinal bleeding: a case report
Dong Chan Joo, Gwang Ha Kim, Bong Eun Lee, Moon Won Lee, Cheolung Kim
The Ewha Medical Journal.2024;[Epub] CrossRef
- Endoscopically resected duodenal lipoma as an uncommon cause of upper gastrointestinal bleeding: a case report
- 6,653 View
- 131 Download
- 3 Web of Science
- 1 Crossref

- A Rare Case of Lymph Node Metastasis from Early Gastric Cancer
- Takaaki Yoshikawa, Yoshio Kadokawa, Masaya Ohana, Akihisa Fukuda, Hiroshi Seno
- Clin Endosc 2019;52(4):369-372. Published online October 5, 2018
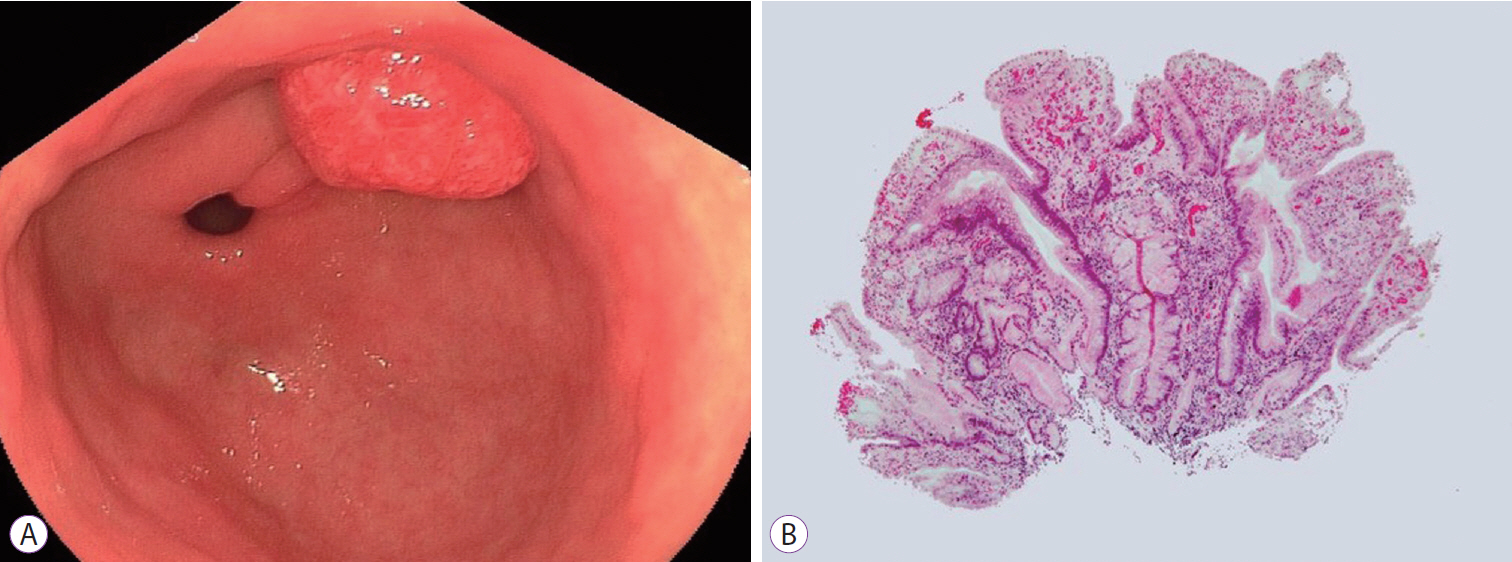
- DOI: https://doi.org/10.5946/ce.2018.130
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Gastric cancers that fulfill the Japanese criteria for curative endoscopic resection show a low risk of lymph node (LN) metastasis. Here, we report a case of LN metastasis from early gastric cancer that fulfilled the curative criteria. A 74-year-old Japanese woman was referred to our hospital for treatment of early gastric cancer identified at the site of a hyperplastic polyp that had been diagnosed 10 years prior to presentation. Contrast-enhanced computed tomography did not show any lymphadenopathy and laparoscopy-assisted distal gastrectomy was performed. Histopathological examination revealed a predominantly moderately differentiated adenocarcinoma that measured 15 mm in size and was confined to the mucosa. However, a single metastatic regional LN was observed. A few cancer cells showed positive staining for alpha-fetoprotein. It should be noted that early gastric cancer can be accompanied by LN metastasis even if it fulfills the criteria for curative endoscopic resection.
- 4,689 View
- 125 Download

- Long-Term Survival and Tumor Recurrence in Patients with Superficial Esophageal Cancer after Complete Non-Curative Endoscopic Resection: A Single-Center Case Series
- Ji Wan Lee, Charles J. Cho, Do Hoon Kim, Ji Yong Ahn, Jeong Hoon Lee, Kee Don Choi, Ho June Song, Sook Ryun Park, Hyun Joo Lee, Yong Hee Kim, Gin Hyug Lee, Hwoon-Yong Jung, Sung-Bae Kim, Jong Hoon Kim, Seung-Il Park
- Clin Endosc 2018;51(5):470-477. Published online June 1, 2018
- DOI: https://doi.org/10.5946/ce.2018.025

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: To report the long-term survival and tumor recurrence outcomes in patients with superficial esophageal cancer (SEC) after complete non-curative endoscopic resection (ER).
Methods
We retrieved ER data for 24 patients with non-curatively resected SEC. Non-curative resection was defined as the presence of submucosal and/or lymphovascular invasion on ER pathology. Relevant clinical and tumor-specific parameters were reviewed.
Results
The mean age of the 24 study patients was 66.3±8.3 years. Ten patients were closely followed up without treatment, while 14 received additional treatment. During a mean follow-up of 59.0±33.2 months, the 3- and 5-year survival rates of all cases were 90.7% and 77.6%, respectively. The 5-year overall survival rates were 72.9% in the close observation group and 82.1% in the additional treatment group (p=0.958). The 5-year cumulative incidences of all cases of recurrence (25.0% vs. 43.3%, p=0.388), primary EC recurrence (10.0% vs. 16.4%, p=0.558), and metachronous EC recurrence (16.7% vs. 26.7%, p=0.667) were similar between the two groups.
Conclusions
Patients with non-curatively resected SEC showed good long-term survival outcomes. Given the similar oncologic outcomes, close observation may be an option with appropriate caution taken for patients who are medically unfit to receive additional therapy. -
Citations
Citations to this article as recorded by- Close Observation versus Additional Surgery after Noncurative Endoscopic Resection of Esophageal Squamous Cell Carcinoma
Byeong Geun Song, Ga Hee Kim, Charles J. Cho, Hyeong Ryul Kim, Yang Won Min, Hyuk Lee, Byung-Hoon Min, Ho June Song, Yong-Hee Kim, Jun Haeng Lee, Hwoon-Yong Jung, Jae Ill Zo, Young Mog Shim
Digestive Surgery.2021; 38(3): 247. CrossRef - Non-Curative Endoscopic Resection for Superficial Esophageal Cancer
Eun Hye Kim, Jun Chul Park
Clinical Endoscopy.2018; 51(5): 399. CrossRef
- Close Observation versus Additional Surgery after Noncurative Endoscopic Resection of Esophageal Squamous Cell Carcinoma
- 5,702 View
- 132 Download
- 2 Web of Science
- 2 Crossref

- Endoscopic Treatment of Subepithelial Tumors
- Su Young Kim, Kyoung-Oh Kim
- Clin Endosc 2018;51(1):19-27. Published online January 31, 2018
- DOI: https://doi.org/10.5946/ce.2018.020
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Gastrointestinal subepithelial tumors (SETs) are generally found during endoscopy and their incidence has gradually increased. Although the indications for the endoscopic treatment of patients with SETs remain to be established, the feasibility and safety of endoscopic dissection, including the advantages of this method compared with surgical treatment, have been validated in many studies. The development of endoscopic techniques, such as endoscopic submucosal dissection, endoscopic enucleation, endoscopic excavation, endoscopic submucosal tunnel dissection, submucosal tunnel endoscopic resection, and endoscopic full-thickness resection has enabled the removal of SETs while reducing the occurrence of complications. Here, we discuss the endoscopic treatment of patients with SETs, outcomes for endoscopic treatment, and procedure-related complications. We also consider the advantages and disadvantages of the various endoscopic techniques.
-
Citations
Citations to this article as recorded by- Present situation of minimally invasive surgical treatment for early gastric cancer
Chun-Yan Li, Yi-Feng Wang, Li-Kang Luo, Xiao-Jun Yang
World Journal of Gastrointestinal Oncology.2024; 16(4): 1154. CrossRef - Cold snare endoscopic resection for subepithelial tumors of the upper third of the esophagus
Xiaosan Hu, Lifeng Zhou, Jian Chen, Yunlin Yue
Revista Española de Enfermedades Digestivas.2023;[Epub] CrossRef - An Atypical Presentation of a Colonic Lipoma: Avoiding Surgery with a Deeper Endoscopic Look
Mafalda João, Inês Cunha, Elisa Gravito-Soares, Marta Gravito-Soares, Pedro Amaro, Pedro Figueiredo
GE - Portuguese Journal of Gastroenterology.2022; 29(1): 45. CrossRef - Endoscopic Resection of Upper Gastrointestinal Subepithelial Tumours: Our Clinical Experience and Results
Mehmet Zeki Buldanlı, Oktay Yener
Prague Medical Report.2022; 123(1): 20. CrossRef - Natural History of Asymptomatic Esophageal Subepithelial Tumors of 30 mm or Less in Size
Seokin Kang, Do Hoon Kim, Yuri Kim, Dongsub Jeon, Hee Kyong Na, Jeong Hoon Lee, Ji Yong Ahn, Kee Wook Jung, Kee Don Choi, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung
Journal of Korean Medical Science.2022;[Epub] CrossRef - Risk stratification in patients with upper gastrointestinal submucosal tumors undergoing submucosal tunnel endoscopic resection
Yong Lv, Shaohua Li, Xiuhe Lv, Qing Liu, Yu Zheng, Yang Su, Changbin Yang, Yanglin Pan, Liping Yao, Huahong Xie
Frontiers in Medicine.2022;[Epub] CrossRef - Endoscopic versus surgical resection in the management of gastric schwannomas
Ya-qi Zhai, Ning-li Chai, Wen-gang Zhang, Hui-kai Li, Zhong-sheng Lu, Xiu-xue Feng, Sheng-zhen Liu, En-qiang Linghu
Surgical Endoscopy.2021; 35(11): 6132. CrossRef - Endoscopic Full-Thickness Resection for Gastric Subepithelial Lesions Arising from the Muscularis Propria
Ah Lon Jung, Sang Wook Park, Gun Young Hong, Hyeong Chul Moon, Seo Joon Eun
Clinical Endoscopy.2021; 54(1): 131. CrossRef - A Review of Endoscopic Full-thickness Resection, Submucosal Tunneling Endoscopic Resection, and Endoscopic Submucosal Dissection for Resection of Subepithelial Lesions
Vicky H. Bhagat, Marina Kim, Michel Kahaleh
Journal of Clinical Gastroenterology.2021; 55(4): 309. CrossRef - A modified endoscopic full thickness resection for gastric subepithelial tumors from muscularis propria layer: Novel method
Jung Min Lee, In Kyung Yoo, Sung Pyo Hong, Joo Young Cho, Young Kwan Cho
Journal of Gastroenterology and Hepatology.2021; 36(9): 2558. CrossRef - Endoscopic resection of esophageal and gastric submucosal tumors from the muscularis propria layer: submucosal tunneling endoscopic resection versus endoscopic submucosal excavation: A systematic review and meta-analysis
Fernando Lopes Ponte Neto, Diogo Turiani Hourneaux de Moura, Vitor Massaro Takamatsu Sagae, Igor Braga Ribeiro, Fabio Catache Mancini, Mateus Bond Boghossian, Thomas R. McCarty, Nelson Tomio Miyajima, Edson Ide, Wanderley Marques Bernardo, Eduardo Guimarã
Surgical Endoscopy.2021; 35(12): 6413. CrossRef - The retrospective comparison between submucosal tunneling endoscopic resection and endoscopic submucosal excavation for managing esophageal submucosal tumors originating from the muscularis propria layer
Yingtong Chen, Min Wang, Lili Zhao, He Chen, Li Liu, Xiang Wang, Zhining Fan
Surgical Endoscopy.2020; 34(1): 417. CrossRef - Ligation-assisted endoscopic mucosal resection for esophageal granular cell tumors is safe and effective
Shria Kumar, Vinay Chandrasekhara, Michael L Kochman, Nuzhat Ahmad, Sara Attalla, Immanuel K Ho, David L Jaffe, Peter J Lee, Kashyap V Panganamamula, Monica Saumoy, Danielle Fortuna, Gregory G Ginsberg
Diseases of the Esophagus.2020;[Epub] CrossRef - Endoscopic Full Thickness Resection for Gastrointestinal Tumors - Challenges and Solutions
Hung Leng Kaan, Khek Yu Ho
Clinical Endoscopy.2020; 53(5): 541. CrossRef - Gestielter submuköser Tumor im Jejunum
Tanja Miltner
Der Gastroenterologe.2019; 14(6): 470. CrossRef - Submucosal Tunnel Endoscopic Resection for Esophageal Submucosal Tumors: A Multicenter Study
Sufang Tu, Silin Huang, Guohua Li, Xiaowei Tang, Haitao Qing, Qiaoping Gao, Jingwen Fu, Guoping Du, Wei Gong
Gastroenterology Research and Practice.2018; 2018: 1. CrossRef
- Present situation of minimally invasive surgical treatment for early gastric cancer
- 7,531 View
- 272 Download
- 16 Web of Science
- 16 Crossref

- Endoscopic Therapeutic Approach for Dysplasia in Inflammatory Bowel Disease
- Sung Noh Hong
- Clin Endosc 2017;50(5):437-445. Published online September 29, 2017
- DOI: https://doi.org/10.5946/ce.2017.132
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Long-standing intestinal inflammation in patients with inflammatory bowel disease (IBD) induces dysplastic change in the intestinal mucosa and increases the risk of subsequent colorectal cancer. The evolving endoscopic techniques and technologies, including dye spraying methods and high-definition images, have been replacing random biopsies and have been revealed as more practical and efficient for detection of dysplasia in IBD patients. In addition, they have potential usefulness in detailed characterization of lesions and in the assessment of endoscopic resectability. Most dysplastic lesions without an unclear margin, definite ulceration, non-lifting sign, and high index of malignant change with suspicion for lymph node or distant metastases can be removed endoscopically. However, endoscopic resection of dysplasia in chronic IBD patients is usually difficult because it is often complicated by submucosal fibrosis. In patients with dysplasias that demonstrate submucosa fibrosis or a large size (≥20 mm), endoscopic submucosal dissection (ESD) or ESD with snaring (simplified or hybrid ESD) is an alternative option and may avoid a colectomy. However, a standardized endoscopic therapeutic approach for dysplasia in IBD has not been established yet, and dedicated specialized endoscopists with interest in IBD are needed to fully investigate recent emerging techniques and technologies.
-
Citations
Citations to this article as recorded by- Considerations for Colorectal Neoplasia Detection in Inflammatory Bowel Disease Clinical Trials
Mira M. Yang, Keith Usiskin, Harris A. Ahmad, Shabana Ather, Antoine Sreih, James B. Canavan, Francis A. Farraye, Christopher Ma
Digestive Diseases.2024; 42(1): 12. CrossRef - Beyond the SCENIC route: updates in chromoendoscopy and dysplasia screening in patients with inflammatory bowel disease
Loren Galler Rabinowitz, Nikhil A. Kumta, James F. Marion
Gastrointestinal Endoscopy.2022; 95(1): 30. CrossRef - Elevating the Technique: Resecting Complex Dysplastic Lesions of the Colon in Patients with Inflammatory Bowel Disease
Eshandeep S. Boparai, Fernando S. Velayos, Abhik Roy, Carolyn Li, Ahmed S. Alkoraishi, Craig A. Munroe
Digestive Diseases and Sciences.2020; 65(1): 78. CrossRef - Role of interventional inflammatory bowel disease in the era of biologic therapy: a position statement from the Global Interventional IBD Group
Bo Shen, Gursimran Kochhar, Udayakumar Navaneethan, Xiuli Liu, Francis A. Farraye, Yago Gonzalez-Lama, David Bruining, Darrell S. Pardi, Martin Lukas, Martin Bortlik, Kaicun Wu, Ajit Sood, David A. Schwartz, William J. Sandborn, Roger Charles, Yan Chen, M
Gastrointestinal Endoscopy.2019; 89(2): 215. CrossRef - Management of Inflammatory Bowel Disease–Associated Dysplasia in the Modern Era
Shailja C. Shah, Steven H. Itzkowitz
Gastrointestinal Endoscopy Clinics of North America.2019; 29(3): 531. CrossRef
- Considerations for Colorectal Neoplasia Detection in Inflammatory Bowel Disease Clinical Trials
- 7,286 View
- 181 Download
- 4 Web of Science
- 5 Crossref

- Metachronous Gastric Cancer Following Curative Endoscopic Resection of Early Gastric Cancer
- Seiichiro Abe, Ichiro Oda, Takeyoshi Minagawa, Masau Sekiguchi, Satoru Nonaka, Haruhisa Suzuki, Shigetaka Yoshinaga, Amit Bhatt, Yutaka Saito
- Clin Endosc 2018;51(3):253-259. Published online September 18, 2017
- DOI: https://doi.org/10.5946/ce.2017.104
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - This review article summarizes knowledge about metachronous gastric cancer (MGC) occurring after curative endoscopic resection (ER) of early gastric cancer (EGC), treatment outcomes of patients who developed MGC, and efficacy of Helicobacter pylori eradication to prevent MGC. The incidence of MGC following curative ER increases over time and is higher than in patients undergoing gastrectomy. Increasing age and multifocal EGC are independent risk factors for developing MGC. An MGC following curative ER is usually a small (<20 mm) and differentiated intramucosal cancer. Most MGC lesions are found at an early stage on semiannual or annual surveillance endoscopy and are successfully treated by further ER, with excellent long-term outcomes. Eradication of H. pylori may reduce the risk of MGC following ER of EGC, but further prospective studies with long-term outcomes are required. Surveillance endoscopy following gastric ER should be continued indefinitely, due to the risk of MGC even after successful H. pylori eradication. Risk stratification and tailored endoscopic surveillance schedules need to be developed.
-
Citations
Citations to this article as recorded by- Impact of endoscopy intervals on metachronous gastric cancer after endoscopic submucosal dissection: Comparison between 1 year and half‐a‐year
Yuichiro Ozeki, Kingo Hirasawa, Atsushi Sawada, Ryosuke Ikeda, Masafumi Nishio, Takehide Fukuchi, Ryosuke Kobayashi, Chiko Sato, Shin Maeda
Digestive Endoscopy.2024; 36(3): 332. CrossRef - High risk of multiple gastric cancers in Japanese individuals with Lynch syndrome
Nobuhiko Kanaya, Thijs A. van Schaik, Hideki Aoki, Yumiko Sato, Fumitaka Taniguchi, Kunitoshi Shigeyasu, Kokichi Sugano, Kiwamu Akagi, Hideyuki Ishida, Kohji Tanakaya
Annals of Gastroenterological Surgery.2024;[Epub] CrossRef - Risk assessment of metachronous gastric cancer development using OLGA and OLGIM systems after endoscopic submucosal dissection for early gastric cancer: a long-term follow-up study
Yun Suk Na, Sang Gyun Kim, Soo-Jeong Cho
Gastric Cancer.2023; 26(2): 298. CrossRef - Metachronous lesions after gastric endoscopic submucosal dissection: first assessment of the FAMISH prediction score
Andreia Rei, Raquel Ortigão, Mariana Pais, Luís P. Afonso, Pedro Pimentel-Nunes, Mário Dinis-Ribeiro, Diogo Libânio
Endoscopy.2023; 55(10): 909. CrossRef - Variation in the rate of detection of minute and small early gastric cancers at diagnostic endoscopy may reflect the performance of individual endoscopists
Daisuke Murakami, Masayuki Yamato, Yuji Amano, Takayoshi Nishino, Makoto Arai
BMJ Open Gastroenterology.2023; 10(1): e001143. CrossRef - Decision to perform additional surgery after non-curative endoscopic submucosal dissection for gastric cancer based on the risk of lymph node metastasis: a long-term follow-up study
Seunghan Lee, Sang Gyun Kim, Soo-Jeong Cho
Surgical Endoscopy.2023; 37(10): 7738. CrossRef - Helicobacter pylori Eradication Can Reverse Rho GTPase Expression in Gastric Carcinogenesis
Jue Lie Kim, Sang Gyun Kim, Enerelt Natsagdorj, Hyunsoo Chung, Soo-Jeong Cho
Gut and Liver.2023; 17(5): 741. CrossRef - Risk assessment of metachronous gastric cancer after endoscopic submucosal dissection based on endoscopic intestinal metaplasia
Chino Iizuka, Soichiro Sue, Sho Onodera, Aya Ikeda, Ryosuke Ikeda, Yoshihiro Goda, Kuniyasu Irie, Hiroaki Kaneko, Shin Maeda
JGH Open.2023; 7(11): 783. CrossRef - Second gastric cancer after curative endoscopic resection of differentiated-type early gastric cancer: post-hoc analysis of a single-arm confirmatory trial
Masao Yoshida, Kohei Takizawa, Noriaki Hasuike, Hiroyuki Ono, Narikazu Boku, Tomohiro Kadota, Junki Mizusawa, Ichiro Oda, Naohiro Yoshida, Yusuke Horiuchi, Kingo Hirasawa, Yoshinori Morita, Yoshinobu Yamamoto, Manabu Muto, Masao Yoshida, Kohei Takizawa, H
Gastrointestinal Endoscopy.2022; 95(4): 650. CrossRef - Therapeutic approach to non-curative resection after endoscopic treatment in early gastric cancer
Eun Jeong Gong, Chang Seok Bang
Journal of the Korean Medical Association.2022; 65(5): 284. CrossRef - Assessment of Outcomes From 1-Year Surveillance After Detection of Early Gastric Cancer Among Patients at High Risk in Japan
Yoshinobu Yamamoto, Naohiro Yoshida, Tomonori Yano, Takahiro Horimatsu, Noriya Uedo, Noboru Kawata, Hiromitsu Kanzaki, Shinichiro Hori, Kenshi Yao, Seiichiro Abe, Chikatoshi Katada, Chizu Yokoi, Ken Ohata, Hisashi Doyama, Kenichi Yoshimura, Hideki Ishikaw
JAMA Network Open.2022; 5(8): e2227667. CrossRef - Management of patients with multiple primary сancer in the practice of a modern oncologist. Case report and literature review
D. A. Khlanta, G. P. Gens
Siberian journal of oncology.2022; 21(4): 147. CrossRef - Assigning a different endoscopist for each annual follow-up may contribute to improved gastric cancer detection rates
Shuhei Unno, Kimihiro Igarashi, Hiroaki Saito, Dai Hirasawa, Toru Okuzono, Yukari Tanaka, Masato Nakahori, Tomoki Matsuda
Endoscopy International Open.2022; 10(10): E1333. CrossRef - Statistical proof of Helicobacter pylori eradication in preventing metachronous gastric cancer after endoscopic resection in an East Asian population
Mohsen Karbalaei, Masoud Keikha
World Journal of Gastrointestinal Surgery.2022; 14(8): 867. CrossRef - Follow-up after endoscopic resection for early gastric cancer in 3 French referral centers
Bernadette de Rauglaudre, Mathieu Pioche, Fabrice Caillol, Jean-Philippe Ratone, Anna Pellat, Romain Coriat, Jerôme Rivory, Thomas Lambin, Laetitia Dahan, Marc Giovanini, Maximilien Barret
iGIE.2022; 1(1): 49. CrossRef - Documento de posicionamiento de la AEG, la SEED y la SEAP sobre cribado de cáncer gástrico en poblaciones con baja incidencia
Joaquín Cubiella, Ángeles Pérez Aisa, Miriam Cuatrecasas, Pilar Díez Redondo, Gloria Fernández Esparrach, José Carlos Marín-Gabriel, Leticia Moreira, Henar Núñez, M. Luisa Pardo López, Enrique Rodríguez de Santiago, Pedro Rosón, José Miguel Sanz Anquela,
Gastroenterología y Hepatología.2021; 44(1): 67. CrossRef - Incidence of metachronous cancer after endoscopic submucosal dissection: a comparison between undifferentiated-type and differentiated-type early gastric cancer
Mitsuaki Ishioka, Toshiyuki Yoshio, Yuji Miyamoto, Ken Namikawa, Yoshitaka Tokai, Shoichi Yoshimizu, Yusuke Horiuchi, Akiyoshi Ishiyama, Toshiaki Hirasawa, Tomohiro Tsuchida, Junko Fujisaki
Gastrointestinal Endoscopy.2021; 93(3): 557. CrossRef - Gastric cancer screening in low incidence populations: Position statement of AEG, SEED and SEAP
Joaquin Cubiella, Ángeles Pérez Aisa, Miriam Cuatrecasas, Pilar Díez Redondo, Gloria Fernández Esparrach, José Carlos Marín-Gabriel, Leticia Moreira, Henar Núñez, M. Luisa Pardo López, Enrique Rodríguez de Santiago, Pedro Rosón, José Miguel Sanz Anquela,
Gastroenterología y Hepatología (English Edition).2021; 44(1): 67. CrossRef - Molecular risk markers related to local tumor recurrence at histological margin-free endoscopically resected early gastric cancers: A pilot study
Ho Suk Kang, Mi Jung Kwon, Premi Haynes, Yan Liang, Yuqi Ren, Hyun Lim, Jae Seung Soh, Nan Young Kim, Hye Kyung Lee
Pathology - Research and Practice.2021; 222: 153434. CrossRef - Characteristics of metachronous gastric neoplasms after curative endoscopic submucosal dissection for early gastric neoplasms
Shan-Shan Xu, Ning-Li Chai, Xiao-Wei Tang, En-Qiang Linghu, Sha-Sha Wang, Bao Li
Chinese Medical Journal.2021; 134(21): 2603. CrossRef - Consenso mexicano sobre detección y tratamiento del cáncer gástrico incipiente
M.E. Icaza-Chávez, M.A. Tanimoto, F.M. Huerta-Iga, J.M. Remes-Troche, R. Carmona-Sánchez, A. Ángeles-Ángeles, F.J. Bosques-Padilla, J.M. Blancas-Valencia, G. Grajales-Figueroa, O.V. Hernández-Mondragón, A.I. Hernández-Guerrero, M.A. Herrera-Servín, F.D. H
Revista de Gastroenterología de México.2020; 85(1): 69. CrossRef - The Mexican consensus on the detection and treatment of early gastric cancer
M.E. Icaza-Chávez, M.A. Tanimoto, F.M. Huerta-Iga, J.M. Remes-Troche, R. Carmona-Sánchez, A. Ángeles-Ángeles, F.J. Bosques-Padilla, J.M. Blancas-Valencia, G. Grajales-Figueroa, O.V. Hernández-Mondragón, A.I. Hernández-Guerrero, M.Á. Herrera-Servín, F.D. H
Revista de Gastroenterología de México (English Edition).2020; 85(1): 69. CrossRef - Long‐term follow up of serum pepsinogens in patients with gastric cancer or dysplasia after Helicobacter pylori eradication
Gitark Noh, Nayoung Kim, Yonghoon Choi, Hye Seung Lee, Young Jae Hwang, Hee Jin Kim, Hyuk Yoon, Cheol Min Shin, Young Soo Park, Dong Ho Lee
Journal of Gastroenterology and Hepatology.2020; 35(9): 1540. CrossRef - Metachronous Gastric Cancer: Another Hurdle for Successful Endoscopic Treatment for Early Gastric Cancer?
Moon Won Lee, Gwang Ha Kim
Gut and Liver.2020; 14(2): 145. CrossRef - Clinical Outcomes of Metachronous Gastric Cancer after Endoscopic Resection for Early Gastric Cancer
Jue Lie Kim, Sang Gyun Kim, Jung Kim, Jae Yong Park, Hyo-Joon Yang, Hyun Ju Kim, Hyunsoo Chung
Gut and Liver.2020; 14(2): 190. CrossRef - Chemoprevention of gastric cancer development after Helicobacter pylori eradication therapy in an East Asian population: Meta-analysis
Mitsushige Sugimoto, Masaki Murata, Yoshio Yamaoka
World Journal of Gastroenterology.2020; 26(15): 1820. CrossRef - MULTIFOCAL EARLY GASTRIC CANCER IN A PATIENT WITH ATROPHIC GASTRITIS AND PERNICIOUS ANEMIA
Tommaso Zurleni, Michele Altomare, Giovanni Serio, Filippo Catalano
International Journal of Surgery Case Reports.2020;[Epub] CrossRef - Impact of the timing of Helicobacter pylori eradication on the risk of development of metachronous lesions after treatment of early gastric cancer: a population-based cohort study
Hyun Ju Kim, Yun Jin Kim, Seung In Seo, Woon Geon Shin, Chan Hyuk Park
Gastrointestinal Endoscopy.2020; 92(3): 613. CrossRef - Gastric cancer prevention strategies: A global perspective
Leonardo Henry Eusebi, Andrea Telese, Giovanni Marasco, Franco Bazzoli, Rocco Maurizio Zagari
Journal of Gastroenterology and Hepatology.2020; 35(9): 1495. CrossRef - Metachronous Gastric Cancer Occurring after Endoscopic Resection of Early Gastric Cancer
Gwang Ha Kim
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2020; 20(4): 295. CrossRef - Somatic alterations and mutational burden are potential predictive factors for metachronous development of early gastric cancer
Kazuhiro Sakuta, Yu Sasaki, Yasuhiko Abe, Hidenori Sato, Masakuni Shoji, Takao Yaoita, Makoto Yagi, Naoko Mizumoto, Yusuke Onozato, Takashi Kon, Ayumi Koseki, Sonoko Sato, Ryoko Murakami, Yuki Miyano, Yoshiyuki Ueno
Scientific Reports.2020;[Epub] CrossRef - Clinicopathologic Characteristics of Patients with Gastric Superficial Neoplasia and Risk Factors for Multiple Lesions after Endoscopic Submucosal Dissection in a Western Country
Gisela Brito-Gonçalves, Diogo Libânio, Pedro Marcos, Inês Pita, Rui Castro, Inês Sá, Mário Dinis-Ribeiro, Pedro Pimentel-Nunes
GE - Portuguese Journal of Gastroenterology.2020; 27(2): 76. CrossRef - Helicobacter pylori status and risks of metachronous recurrence after endoscopic resection of early gastric cancer: a systematic review and meta-analysis
Shiyu Xiao, Sizhu Li, Liya Zhou, Wenjun Jiang, Jinzhe Liu
Journal of Gastroenterology.2019; 54(3): 226. CrossRef - Helicobacter pylori Eradication for Metachronous Gastric Cancer: An Unsuitable Methodology Impeding Broader Clinical Usage
Alexios-Fotios A. Mentis, Efthimios Dardiotis
Frontiers in Oncology.2019;[Epub] CrossRef - Common Locations of Gastric Cancer: Review of Research from the Endoscopic Submucosal Dissection Era
Su Jin Kim, Cheol Woong Choi
Journal of Korean Medical Science.2019;[Epub] CrossRef - UEG Week 2019 Poster Presentations
United European Gastroenterology Journal.2019; 7(S8): 189. CrossRef - Helicobacter pylori Infection following Endoscopic Resection of Early Gastric Cancer
Lan Li, Chaohui Yu
BioMed Research International.2019; 2019: 1. CrossRef - Prophylaxis and early diagnosis of stomach cancer
I. G. Bakulin, S. S. Pirogov, N. V. Bakulina, E. A. Stadnik, N. N. Golubev
Dokazatel'naya gastroenterologiya.2018; 7(2): 44. CrossRef - Linked Color Imaging and Blue Laser Imaging for Upper Gastrointestinal Screening
Hiroyuki Osawa, Yoshimasa Miura, Takahito Takezawa, Yuji Ino, Tsevelnorov Khurelbaatar, Yuichi Sagara, Alan Kawarai Lefor, Hironori Yamamoto
Clinical Endoscopy.2018; 51(6): 513. CrossRef
- Impact of endoscopy intervals on metachronous gastric cancer after endoscopic submucosal dissection: Comparison between 1 year and half‐a‐year
- 9,505 View
- 283 Download
- 35 Web of Science
- 39 Crossref

- Can Endoscopic Ulcerations in Early Gastric Cancer Be Clearly Defined before Endoscopic Resection? A Survey among Endoscopists
- Sung Min Park, Byung-Wook Kim, Joon Sung Kim, Young Wook Kim, Gi Jun Kim, Seung Ji Ryu
- Clin Endosc 2017;50(5):473-478. Published online April 24, 2017
- DOI: https://doi.org/10.5946/ce.2016.143
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Early gastric cancer (EGC) with ulcerations can be treated via endoscopic resection (ER) when it is differentiated pathologically, limited to the mucosa, and <3 cm in diameter. The presence of ulceration is a key factor in deciding treatment strategies and is usually diagnosed during endoscopic examination. The aim of this study was to evaluate whether ulcerations in EGC can be clearly defined among endoscopists and which factors are related to the differences.
Methods
A survey questionnaire, composed of demographic features and endoscopic images of seven patients with EGC, was presented to the endoscopists via e-mail. The endoscopists were asked whether such patients have ulcerations in the lesions.
Results
The questionnaires were e-mailed to 197 endoscopists, and 103 doctors replied. The presence of an endoscopic ulceration was defined differently among the endoscopists, depending on the duration of endoscopic practice and the experience of endoscopic submucosal dissection (ESD). The differences were especially high in the lesions without mucosal breaks and converging folds, which were expected to be viewed as non-ulcerative.
Conclusions
Before ER, endoscopic ulcerations in EGC must be reviewed by experienced endoscopists to reduce overestimations, and adequate educational programs for trainees should be established. -
Citations
Citations to this article as recorded by- A standardized pathology report for gastric cancer: 2nd edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi,
Journal of Pathology and Translational Medicine.2023; 57(1): 1. CrossRef - A Standardized Pathology Report for Gastric Cancer: 2nd Edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi,
Journal of Gastric Cancer.2023; 23(1): 107. CrossRef - Applicability of endoscopic submucosal dissection for patients with early gastric cancer beyond the expanded indication for endoscopic submucosal dissection
Jeong Ho Song, Sejin Lee, Sung Hyun Park, Anastasios Kottikias, Aleisa Abdulmohsen, Nasser Alrashidi, Minah Cho, Yoo Min Kim, Hyoung-Il Kim, Woo Jin Hyung
Surgical Endoscopy.2022; 36(11): 8349. CrossRef - Construction and analysis of an ulcer risk prediction model after endoscopic submucosal dissection for early gastric cancer
San-Dong Gong, Huan Li, Yi-Bin Xie, Xiao-Hui Wang
World Journal of Gastrointestinal Oncology.2022; 14(9): 1823. CrossRef - Discrepancy between endoscopic and pathological ulcerative findings in clinical intramucosal early gastric cancer
Yohei Yabuuchi, Kohei Takizawa, Naomi Kakushima, Noboru Kawata, Masao Yoshida, Yoichi Yamamoto, Yoshihiro Kishida, Sayo Ito, Kenichiro Imai, Hirotoshi Ishiwatari, Kinichi Hotta, Hiroyuki Matsubayashi, Etsuro Bando, Masanori Terashima, Takashi Sugino, Hiro
Gastric Cancer.2021; 24(3): 691. CrossRef - Predictive Model of Nonneoplastic Pathology after Endoscopic Resection of Gastric Epithelial Neoplasia
Tae-Geun Gweon, Byung-Wook Kim, Joon Sung Kim, Sung Min Park, Jeong Seon Ji, Bo In Lee
Gut and Liver.2020; 14(2): 199. CrossRef - Endoscopic Factors that Can Predict Histological Ulcerations in Early Gastric Cancers
Jaesin Lee, Byung-Wook Kim, Cheal Wung Huh, Joon Sung Kim, Lee-So Maeng
Clinical Endoscopy.2020; 53(3): 328. CrossRef - What is the Most Precise Endoscopic Finding for Predicting the Clinicopathological Behaviors in Ulcerative Early Gastric Cancer?
Youngdae Kim
Clinical Endoscopy.2020; 53(3): 249. CrossRef - Risk Factors for Lymph Node Metastasis in Undifferentiated-Type Gastric Carcinoma
Myeong-Cherl Kook
Clinical Endoscopy.2019; 52(1): 15. CrossRef - Identification of Ulceration in Early Gastric Cancer before Resection is Not Easy: Need for a New Guideline for Endoscopic Submucosal Dissection Indication Based on Endoscopic Image
Hang Lak Lee
Clinical Endoscopy.2017; 50(5): 410. CrossRef
- A standardized pathology report for gastric cancer: 2nd edition
- 6,084 View
- 150 Download
- 11 Web of Science
- 10 Crossref

- Evaluation and Endoscopic Management of Esophageal Submucosal Tumor
- Weon Jin Ko, Ga Won Song, Joo Young Cho
- Clin Endosc 2017;50(3):250-253. Published online November 7, 2016
- DOI: https://doi.org/10.5946/ce.2016.109
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Submucosal tumors (SMTs) originate from tissues that constitute the submucosal layer and muscularis propria, and are covered by normal mucosa. Esophageal SMTs are rare, accounting for <1% of all esophageal tumors. However, the recent widespread use of endoscopy has led to a rapid increase in incidental detection of SMTs in Korea. Esophageal SMTs are benign in ≥90% of cases, but the possibility of malignancies such as gastrointestinal stromal tumor and malignant leiomyosarcoma still exists. Therefore, patients undergo resection in the presence of symptoms or the possibility of a malignant tumor. For resection of esophageal SMTs, surgical resection was the only option available in case of possible malignancy, but minimally invasive surgery by endoscopic resection is becoming more preferable to surgical resection with the development of endoscopic ultrasonography, endoscopic techniques, and other devices.
-
Citations
Citations to this article as recorded by- Chest pain in a patient with suicidal history
Chien-Ming Chiang, Hsueh-Chien Chiang, Jui-Wen Kang
Frontline Gastroenterology.2024; : flgastro-2023-102617. CrossRef - Cholangiocarcinoma With Rare Esophageal Metastasis
Mana Matsuoka, Katsumasa Kobayashi, Yukito Okura, Takahito Nozaka, Ayako Sato, Masato Yauchi, Taichi Matsumoto, Yohei Furumoto, Takao Horiuchi, Toru Asano
ACG Case Reports Journal.2022; 9(1): e00717. CrossRef - Natural History of Asymptomatic Esophageal Subepithelial Tumors of 30 mm or Less in Size
Seokin Kang, Do Hoon Kim, Yuri Kim, Dongsub Jeon, Hee Kyong Na, Jeong Hoon Lee, Ji Yong Ahn, Kee Wook Jung, Kee Don Choi, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung
Journal of Korean Medical Science.2022;[Epub] CrossRef - A Submucosal Tumor-like Lesion of the Cervical Esophagus Similar to the Tonsillar Structures of Waldeyer’s Ring: A Case Report
Shibo Song, Xiaolong Feng, Xudong Liu, Guiqi Wang, Liyan Xue
Medicina.2022; 58(12): 1804. CrossRef - Clinical study of submucosal tunneling endoscopic resection and endoscopic submucosal dissection in the treatment of submucosal tumor originating from the muscularis propria layer of the esophagus
Yue Zhang, Jing Wen, Shuxian Zhang, Xuyang Liang, Ling Ren, Lu Wang, Yunliang Sun, Shouying Li, Kun Wang, Shengxiang Lv, Xiao Qiao
Medicine.2022; 101(51): e32380. CrossRef - Role of endoscopic ultrasound in anticancer therapy: Current evidence and future perspectives
Andre Bratanic, Dorotea Bozic, Antonio Mestrovic, Dinko Martinovic, Marko Kumric, Tina Ticinovic Kurir, Josko Bozic
World Journal of Gastrointestinal Oncology.2021; 13(12): 1863. CrossRef - Left atrial appendage thrombus detected by transesophageal examination with linear endoscopic ultrasound
Kenji Ikezawa, Minoru Shigekawa, Kaoruko Sengoku, Teppei Yoshioka, Ryotaro Sakamori, Yasushi Sakata, Tetsuo Takehara
Clinical Case Reports.2019; 7(7): 1327. CrossRef - A Potentially Malignant Giant Esophageal Paraganglioma
Tammi Arbel Rubinstein, Gennady Kouniavsky, Bibi Kanengisser Pines, Efraim Idelevich, Li or Lazar, Amir Elami, Ilan Bar, Guy Pines
The Annals of Thoracic Surgery.2019; 108(6): e349. CrossRef - Strategy for esophageal non-epithelial tumors based on a retrospective analysis of a single facility
Tomoaki Aoki, Tetsu Nakamura, Taro Oshikiri, Hiroshi Hasegawa, Masashi Yamamoto, Yoshiko Matsuda, Shingo Kanaji, Kimihiro Yamashita, Takeru Matsuda, Yasuo Sumi, Satoshi Suzuki, Yoshihiro Kakeji
Esophagus.2018; 15(4): 286. CrossRef - Esophageal Squamous Cell Carcinoma Presenting as a Subepithelial Tumor
Soon Young Kim, Sang Kil Lee, Hyang Joo Ryu
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2017; 17(3): 144. CrossRef
- Chest pain in a patient with suicidal history
- 7,832 View
- 271 Download
- 11 Web of Science
- 10 Crossref

- Endoscopic Submucosal Dissection for Early Gastric Cancers with Uncommon Histology
- Gwang Ha Kim
- Clin Endosc 2016;49(5):434-437. Published online September 30, 2016
- DOI: https://doi.org/10.5946/ce.2016.127
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic submucosal dissection (ESD) enables en bloc curative resection of early gastric cancers (EGCs) with a negligible risk of lymph node metastasis (LNM). Although ESD for EGCs with absolute and expanded indications is safe, the results differ between EGCs with specialized and common histologies. EGC with papillary adenocarcinoma is a differentiated-type adenocarcinoma. At present, it is treated with ESD according to the same criteria as other differentiated-type adenocarcinomas. The LNM rate under the current indication criteria is high, and over half of the patients who undergo ESD as a primary treatment for EGC with papillary adenocarcinoma achieve an out-of-ESD result. Gastric carcinoma with lymphoid stroma in EGC has a low LNM rate and a favorable outcome, despite deep submucosal invasion. Patients with this gastric cancer subtype may be good candidates for ESD, even with deep submucosal invasion. Large-scale prospective multi-center studies with longer follow-up periods are needed to set proper ESD criteria for these tumors. Clinicians should be aware of these disease entities and ESD should be more carefully considered for EGCs with papillary adenocarcinoma and gastric carcinoma with lymphoid stroma.
-
Citations
Citations to this article as recorded by- Gastric carcinoma with lymphoid stroma derived from hamartomatous inverted polyp with osteoclast-like giant cells: a case report
Shoko Yamashita, Masaaki Nishi, Kozo Yoshikawa, Toshihiro Nakao, Takuya Tokunaga, Chie Takasu, Hideya Kashihara, Yuma Wada, Toshiaki Yoshimoto, Yosuke Iwakawa, Takeshi Oya, Koichi Tsuneyama, Mitsuo Shimada
International Cancer Conference Journal.2022; 11(3): 196. CrossRef - Endoscopic Submucosal Dissection of Papillary Gastric Adenocarcinoma; Systematic Review
Chang Seok Bang, Jae Jun Lee, Gwang Ho Baik
Journal of Clinical Medicine.2020; 9(5): 1465. CrossRef - Pitfalls in the Interpretation of Publications about Endoscopic Submucosal Dissection of Early Gastric Cancer with Undifferentiated-Type Histology
Chang Seok Bang, Gwang Ho Baik
Clinical Endoscopy.2019; 52(1): 30. CrossRef - A new Schiff base coordinated copper(II) compound induces apoptosis and inhibits tumor growth in gastric cancer
Yan Xia, Xingkai Liu, Luping Zhang, Jinzhu Zhang, Chaoying Li, Nan Zhang, Hong Xu, Yan Li
Cancer Cell International.2019;[Epub] CrossRef
- Gastric carcinoma with lymphoid stroma derived from hamartomatous inverted polyp with osteoclast-like giant cells: a case report
- 7,433 View
- 169 Download
- 7 Web of Science
- 4 Crossref

- Interpretation of Pathologic Margin after Endoscopic Resection of Gastrointestinal Stromal Tumor
- Sang Gyun Kim
- Clin Endosc 2016;49(3):229-231. Published online April 7, 2016
- DOI: https://doi.org/10.5946/ce.2016.035
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Interpretation of the pathologic margin of a specimen from a resected tumor is important because local recurrence can be predicted by the presence of tumor cells in the resection margin. Although a sufficient resection margin is recommended in the resection of gastrointestinal adenocarcinoma, it is not usually regarded strictly in cases of mesenchymal tumor, especially gastrointestinal stromal tumor (GIST), because the tumor is usually encapsulated or well demarcated, and not infiltrative. Therefore, margin positivity is not rare in the pathological evaluation of surgically or endoscopically resected GIST, and does not always indicate incomplete resection. Although a GIST may have a tumor-positive pathologic margin, complete resection may be achieved if no residual tumor is visible, and long-term survival can be predicted as in the cases with a negative pathologic margin.
-
Citations
Citations to this article as recorded by- Efficiency of an endoscopic resection strategy for management of submucosal tumors < 20 mm in the upper gastrointestinal tract
Fabrice Caillol, Elise Meunier, Christophe Zemmour, Jean-Philippe Ratone, Jerome Guiramand, Solene Hoibian, Yanis Dahel, Flora Poizat, Marc Giovannini
Endoscopy International Open.2022; 10(04): E347. CrossRef - Comparison of the treatment outcomes of endoscopic and surgical resection of GI stromal tumors in the stomach: a propensity score–matched case-control study
Ga Hee Kim, Kee Don Choi, Chung Sik Gong, In-Seob Lee, Young Soo Park, Minkyu Han, Hee Kyong Na, Ji Yong Ahn, Jeong Hoon Lee, Kee Wook Jung, Do Hoon Kim, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung
Gastrointestinal Endoscopy.2020; 91(3): 527. CrossRef - Is the Surgical Margin in Gastrointestinal Stromal Tumors Different
Piotr Rutkowski, Jacek Skoczylas, Piotr Wisniewski
Visceral Medicine.2018; 34(5): 347. CrossRef
- Efficiency of an endoscopic resection strategy for management of submucosal tumors < 20 mm in the upper gastrointestinal tract
- 7,468 View
- 82 Download
- 3 Web of Science
- 3 Crossref

- Clinical Application of Magnifying Endoscopy with Narrow-Band Imaging in the Stomach
- Kenshi Yao
- Clin Endosc 2015;48(6):481-490. Published online November 30, 2015
- DOI: https://doi.org/10.5946/ce.2015.48.6.481
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Magnifying endoscopy with narrow-band imaging (M-NBI) can visualize superficial microanatomies in the stomach. The normal morphology of the microanatomy visualized by M-NBI differs according to the part of the stomach. The gastric fundic glandular mucosa appears as a regular honeycomb-like subepithelial capillary network (SECN) pattern with a regular collecting venule pattern and regular oval crypt opening with circular marginal crypt epithelium (MCE) pattern. The gastric pyloric glandular mucosa displays a regular coil-shaped SECN pattern and regular polygonal or curved MCE pattern. For a diagnosis of early gastric cancer using M-NBI, the vessel plus surface classification system was developed. This system is clinically useful for the differential diagnosis of focal gastritis and small depressed cancer and for determining the horizontal extent of early gastric cancer for successful endoscopic resection. Advantages of M-NBI over conventional endoscopic imaging techniques with white light include accurate diagnosis and cost effectiveness. This technique is a breakthrough in the endoscopic diagnostic field.
-
Citations
Citations to this article as recorded by- Could near focus endoscopy, narrow-band imaging, and acetic acid improve the visualization of microscopic features of stomach mucosa?
Admir Kurtcehajic, Enver Zerem, Tomislav Bokun, Ervin Alibegovic, Suad Kunosic, Ahmed Hujdurovic, Amir Tursunovic, Kenana Ljuca
World Journal of Gastrointestinal Endoscopy.2024; 16(3): 157. CrossRef - The investigation of volatile organic compounds in diagnosing (early) esophageal squamous cell carcinoma and gastric adenocarcinoma
Hang Yang, Chengfang Xiang, Yi Mou, Xinyue Zhou, Wenwen Li, Yixiang Duan, Bing Hu
Journal of Cancer Research and Clinical Oncology.2023; 149(10): 7029. CrossRef - Approach for the Management of Indefinite-for-neoplasia Lesions Detected on Gastric Mucosal Biopsy
Dae Gon Ryu, Cheol Woong Choi
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2023; 23(1): 7. CrossRef - Magnifying Endoscopy with Narrow-Band Imaging for Duodenal Neuroendocrine Tumors
Gwang Ha Kim, Kiyoun Yi, Dong Chan Joo, Moon Won Lee, Hye Kyung Jeon, Bong Eun Lee
Journal of Clinical Medicine.2023; 12(9): 3106. CrossRef - Lightweight deep learning model incorporating an attention mechanism and feature fusion for automatic classification of gastric lesions in gastroscopic images
Lingxiao Wang, Yingyun Yang, Aiming Yang, Ting Li
Biomedical Optics Express.2023; 14(9): 4677. CrossRef - Advanced gastric cancer detected during regular follow-up after eradication of Helicobacter pylori
Masami Tanaka, Daisuke Kikuchi, Hiroyuki Odagiri, Atsuko Hosoi, Yugo Suzuki, Takayuki Okamura, Yorinari Ochiai, Junnosuke Hayasaka, Yutaka Mitsunaga, Kosuke Nomura, Satoshi Yamashita, Akira Matsui, Yutaka Takazawa, Shu Hoteya
Clinical Journal of Gastroenterology.2022; 15(2): 358. CrossRef - Usefulness of artificial intelligence in early gastric cancer
Alba Panarese
WArtificial Intelligence in Cancer.2022; 3(2): 17. CrossRef - Risk factors for early gastric cancer: focus on Helicobacter pylori gastritis
Hee Seok Moon
Journal of the Korean Medical Association.2022; 65(5): 259. CrossRef - Gastric Cancer
Dalton A. Norwood, Eleazar Montalvan-Sanchez, Ricardo L. Dominguez, Douglas R. Morgan
Gastroenterology Clinics of North America.2022; 51(3): 501. CrossRef - Demarcation Line Determination for Diagnosis of Gastric Cancer Disease Range Using Unsupervised Machine Learning in Magnifying Narrow-Band Imaging
Shunsuke Okumura, Misa Goudo, Satoru Hiwa, Takeshi Yasuda, Hiroaki Kitae, Yuriko Yasuda, Akira Tomie, Tatsushi Omatsu, Hiroshi Ichikawa, Nobuaki Yagi, Tomoyuki Hiroyasu
Diagnostics.2022; 12(10): 2491. CrossRef - Clinical Role of Magnifying Endoscopy with Narrow-band Imaging in the Diagnosis of Early Gastric Cancer
Soo In Choi
Journal of Digestive Cancer Research.2022; 10(2): 56. CrossRef - NBI utility in oncologic surgery: An organ by organ review
Francesca Boscolo Nata, Giancarlo Tirelli, Vincenzo Capriotti, Alberto Vito Marcuzzo, Erica Sacchet, Azzurra Nicole Šuran-Brunelli, Nicolò de Manzini
Surgical Oncology.2021; 36: 65. CrossRef - Magnifying Power: New Endoscopic Tools for the Diagnosis of Krukenberg Tumor
Hannah Ramrakhiani, Aarushi K. Thaker, Albert Pisani, Ankur Sangoi, George Triadafilopoulos
Digestive Diseases and Sciences.2021; 66(10): 3296. CrossRef - Identifying early gastric cancer under magnifying narrow-band images with deep learning: a multicenter study
Hao Hu, Lixin Gong, Di Dong, Liang Zhu, Min Wang, Jie He, Lei Shu, Yiling Cai, Shilun Cai, Wei Su, Yunshi Zhong, Cong Li, Yongbei Zhu, Mengjie Fang, Lianzhen Zhong, Xin Yang, Pinghong Zhou, Jie Tian
Gastrointestinal Endoscopy.2021; 93(6): 1333. CrossRef - Dynamic diagnosis of early gastric cancer with microvascular blood flow rate using magnifying endoscopy (with video): A pilot study
Hiroya Ueyama, Noboru Yatagai, Atsushi Ikeda, Yoichi Akazawa, Hiroyuki Komori, Tsutomu Takeda, Kohei Matsumoto, Kumiko Ueda, Kenshi Matsumoto, Daisuke Asaoka, Mariko Hojo, Takashi Yao, Akihito Nagahara
Journal of Gastroenterology and Hepatology.2021; 36(7): 1927. CrossRef - Diagnostic Ability of Magnifying Narrow-Band Imaging for the Extent of Early Gastric Cancer: A Systematic Review and Meta-Analysis
Yingying Hu, Xueqin Chen, Maher Hendi, Jianmin Si, Shujie Chen, Yanyong Deng, Bruno Annibale
Gastroenterology Research and Practice.2021; 2021: 1. CrossRef - Image-Enhanced Endoscopy and Its Corresponding Histopathology in the Stomach
Hisashi Doyama, Hiroyoshi Nakanishi, Kenshi Yao
Gut and Liver.2021; 15(3): 329. CrossRef - Systematic Review on Optical Diagnosis of Early Gastrointestinal Neoplasia
Andrej Wagner, Stephan Zandanell, Tobias Kiesslich, Daniel Neureiter, Eckhard Klieser, Josef Holzinger, Frieder Berr
Journal of Clinical Medicine.2021; 10(13): 2794. CrossRef - Sporadic fundic gland polyps with dysplasia or carcinoma: Clinical and endoscopic characteristics
Wataru Sano, Fumihiro Inoue, Daizen Hirata, Mineo Iwatate, Santa Hattori, Mikio Fujita, Yasushi Sano
World Journal of Gastrointestinal Oncology.2021; 13(7): 487. CrossRef - Sporadic fundic gland polyps with dysplasia or carcinoma: Clinical and endoscopic characteristics
Wataru Sano, Fumihiro Inoue, Daizen Hirata, Mineo Iwatate, Santa Hattori, Mikio Fujita, Yasushi Sano
World Journal of Gastrointestinal Oncology.2021; 13(7): 662. CrossRef - Controversies in EUS: Do we need miniprobes?
Hans Seifert, Pietro Fusaroli, PaoloGiorgio Arcidiacono, Barbara Braden, Felix Herth, Michael Hocke, Alberto Larghi, Bertrand Napoleon, Mihai Rimbas, BogdanSilvio Ungureanu, Adrian Sãftoiu, AnandV Sahai, ChristophF Dietrich
Endoscopic Ultrasound.2021; 10(4): 246. CrossRef - Application of Current Image-Enhanced Endoscopy in Gastric Diseases
Wansik Lee
Clinical Endoscopy.2021; 54(4): 477. CrossRef - Feasibility of standardized procedures of white light gastroscopy for clinical practice: A multicenter study in China
Qiang Wang, Sheng Yu Zhang, Xi Wu, Fang Yao, Wei Xun Zhou, Ning Li Chai, Shu Tian Zhang, Jian Yu Hao, Jing Wu, Ji Chang Zhang, Bao Hong Xu, Li Xia Hu, Ai Ming Yang
Journal of Digestive Diseases.2021; 22(11): 656. CrossRef - Convolutional neural network for the diagnosis of early gastric cancer based on magnifying narrow band imaging
Lan Li, Yishu Chen, Zhe Shen, Xuequn Zhang, Jianzhong Sang, Yong Ding, Xiaoyun Yang, Jun Li, Ming Chen, Chaohui Jin, Chunlei Chen, Chaohui Yu
Gastric Cancer.2020; 23(1): 126. CrossRef - Endoscopic acanthosis nigricans appearance: A novel specific marker for diagnosis of low‐grade intraepithelial neoplasia
Jinnian Cheng, Xianjun Xu, Qian Zhuang, Shengzheng Luo, Xiaoyuan Gong, Xiaowan Wu, Xinjian Wan, Hui Zhou
Journal of Gastroenterology and Hepatology.2020; 35(8): 1372. CrossRef - Clinicopathological features of early gastric cancers arising in Helicobacter pylori uninfected patients
Chiko Sato, Kingo Hirasawa, Yoko Tateishi, Yuichiro Ozeki, Atsushi Sawada, Ryosuke Ikeda, Takehide Fukuchi, Masafumi Nishio, Ryosuke Kobayashi, Makomo Makazu, Hiroaki Kaneko, Yoshiaki Inayama, Shin Maeda
World Journal of Gastroenterology.2020; 26(20): 2618. CrossRef - Narrowband imaging with near‐focus magnification for discriminating the gastric tumor margin before endoscopic resection: A prospective randomized multicenter trial
Jung‐Wook Kim, Yunho Jung, Jae‐Young Jang, Gwang Ha Kim, Byoung Wook Bang, Jun Chul Park, Hyuk Soon Choi, Jun‐Hyung Cho
Journal of Gastroenterology and Hepatology.2020; 35(11): 1930. CrossRef - Clinical applicability of gastroscopy with narrow-band imaging for the diagnosis of Helicobacter pylori gastritis, precancerous gastric lesion, and neoplasia
Jun-Hyung Cho, Seong Ran Jeon, So-Young Jin
World Journal of Clinical Cases.2020; 8(14): 2902. CrossRef - A new exploration of white globe appearance (WGA) in ulcerative lesions
Jinnian Cheng, Jie Xia, Qian Zhuang, Xianjun Xu, Xiaowan Wu, Xinjian Wan, Jing Wang, Hui Zhou
Zeitschrift für Gastroenterologie.2020; 58(08): 754. CrossRef - Tapering body stiffness shortens upper gastrointestinal examination via transoral insertion with ultrathin endoscope
Satoshi Ono, Shun Ito, Kyohei Maejima, Shosuke Hosaka, Kiyotaka Umeki, Shin-ichiro Sato
Endoscopy International Open.2020; 08(12): E1748. CrossRef - Diagnosis and treatment of the gastric adenoma and Helicobacter pylori-negative atrophic gastritis: clinical case
R.O. Kuvaev, O.B. Tkachenko, A.N. Sidorova, E.A. Krainova, S.V. Kashin, A.A. Kuvaeva
Dokazatel'naya gastroenterologiya.2020; 9(4): 83. CrossRef - Endoscopic microanatomy of the normal gastrointestinal mucosa with narrow band technology and magnification
Hugo Uchima, Kenshi Yao
Gastroenterología y Hepatología.2019; 42(2): 117. CrossRef - Endoscopic microanatomy of the normal gastrointestinal mucosa with narrow band technology and magnification
Hugo Uchima, Kenshi Yao
Gastroenterología y Hepatología (English Edition).2019; 42(2): 117. CrossRef - How to adjust endoscopic findings to histopathological findings of the stomach: a “histopathology-oriented” correspondence method helps to understand endoscopic findings
Yasuko Fujita, Mitsuo Kishimoto, Osamu Dohi, Kazuhiro Kamada, Atsushi Majima, Reiko Kimura-Tsuchiya, Nobuaki Yagi, Hideyuki Konishi, Yuji Naito, Yoshinori Harada, Hideo Tanaka, Eiichi Konishi, Tamotsu Sugai, Akio Yanagisawa
Gastric Cancer.2018; 21(3): 573. CrossRef - Diagnostic performance of routine esophagogastroduodenoscopy using magnifying endoscope with narrow‐band imaging for gastric cancer
Shoichi Yoshimizu, Yorimasa Yamamoto, Yusuke Horiuchi, Masami Omae, Toshiyuki Yoshio, Akiyoshi Ishiyama, Toshiaki Hirasawa, Tomohiro Tsuchida, Junko Fujisaki
Digestive Endoscopy.2018; 30(1): 71. CrossRef - Efficacy of narrow-band imaging for detecting intestinal metaplasia in adult patients with symptoms of dyspepsia
S. Sobrino-Cossío, J.M. Abdo Francis, F. Emura, E.S. Galvis-García, M.L. Márquez Rocha, G. Mateos-Pérez, C.B. González-Sánchez, N. Uedo
Revista de Gastroenterología de México (English Edition).2018; 83(3): 245. CrossRef - La eficacia de la imagen de banda estrecha para la detección de metaplasia intestinal en pacientes adultos con síntomas de dispepsia
S. Sobrino-Cossío, J.M. Abdo Francis, F. Emura, E.S. Galvis-García, M.L. Márquez Rocha, G. Mateos-Pérez, C.B. González-Sánchez, N. Uedo
Revista de Gastroenterología de México.2018; 83(3): 245. CrossRef - Role of SpyGlass-DStm in the preoperative assessment of pancreatic intraductal papillary mucinous neoplasm involving the main pancreatic duct
Takao Ohtsuka, Yoshitaka Gotoh, Yohei Nakashima, Yoshifumi Okayama, So Nakamura, Makiko Morita, Mohammed Y.F. Aly, Vittoria Vanessa D.M. Velasquez, Yasuhisa Mori, Yoshihiko Sadakari, Kohei Nakata, Yoshihiro Miyasaka, Kousei Ishigami, Nao Fujimori, Naoki M
Pancreatology.2018; 18(5): 566. CrossRef - A Theoretical Model: Elastic Analysis of the Evolution of the Crypt Opening Between the Fundic Gland and the Pyloric Gland
Fei Xiong, Xiao Gang Liu
Frontiers in Physiology.2018;[Epub] CrossRef - Advanced imaging and therapeutic endoscopy
Chaitanya Pant, Mojtaba S. Olyaee, Amit Rastogi
Techniques in Gastrointestinal Endoscopy.2017; 19(3): 151. CrossRef - An update on gastric cancer
Syed A. Ahmad, Brent T. Xia, Christina E. Bailey, Daniel E. Abbott, Beth A. Helmink, Meghan C. Daly, Ramya Thota, Cameron Schlegal, Leah K. Winer, S. Ameen Ahmad, Ali H. Al Humaidi, Alexander A Parikh
Current Problems in Surgery.2016; 53(10): 449. CrossRef
- Could near focus endoscopy, narrow-band imaging, and acetic acid improve the visualization of microscopic features of stomach mucosa?
- 18,265 View
- 792 Download
- 39 Web of Science
- 41 Crossref

- Usefulness of Ready-to-Use 0.4% Sodium Hyaluronate (Endo-Ease) in the Endoscopic Resection of Gastrointestinal Neoplasms
- Eun Ran Kim, Yun Gyoung Park, Byung-Hoon Min, Jun Haeng Lee, Poong-Lyul Rhee, Jae J. Kim, Jung Ho Park, Dong Il Park, Dong Kyung Chang
- Clin Endosc 2015;48(5):392-398. Published online September 30, 2015
- DOI: https://doi.org/10.5946/ce.2015.48.5.392
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Background/Aims Commercially available sodium hyaluronate solutions are usually too thick to inject through catheters and need dilution with normal saline (NS) before use, which increases the risk of contamination. We evaluated the usefulness of ready-to-use 0.4% sodium hyaluronate, Endo-Ease (EE; UNIMED Pharm. Inc., Seoul, Korea).
Methods We performed a prospective multicenter randomized study from May 2011 to September 2012. Patients requiring endoscopic resection (ER) for gastric or colorectal neoplasm at two referral hospitals were enrolled.
Results One hundred fifty-four patients (72 with a gastric neoplasm and 82 with a colorectal neoplasm) were included in intention-to-treat analysis. Thirty-seven gastric neoplasms and 43 colorectal neoplasms were enrolled in the EE group. The usefulness rate was significantly higher in the EE group than in the NS group (89.2% vs. 60.0% for gastric neoplasms and 95.3% vs. 67.7% for colorectal neoplasms,
p <0.001). In the EE group, the ease of mucosal resection was significantly higher than in the NS group (p <0.001). The injected volume was smaller in the EE group than in the NS group (p <0.05).Conclusions The use of EE reduced the need for additional injections and improved the ease of ER. A submucosal injection of EE is useful for the ER of both gastric and colorectal neoplasms.
-
Citations
Citations to this article as recorded by- Efficacy and safety of a submucosal injection solution of sodium alginate for endoscopic resection in a porcine model
Kyung Uk Jung, Yeon Jae Lee, Jae-Young Jang, Joo Young Cho
Scientific Reports.2024;[Epub] CrossRef - Injectable temperature-sensitive hydrogel facilitating endoscopic submucosal dissection
Ruifen Xu, Xiaoyu Yang, Tong Yi, Tao Tan, Zhongqi Li, Xuyang Feng, Jing Rao, Pinghong Zhou, Hao Hu, Yonghua Zhan
Frontiers in Bioengineering and Biotechnology.2024;[Epub] CrossRef - Hypertonic solution as an optimal submucosal injection solution for endoscopic resection of gastrointestinal mucosal lesions: Systematic review and network meta‐analysis
Li Gao, Jiawei Bai, Kai Liu, Lulu Wang, Shaohua Zhu, Xin Zhao, Ying Han, Zhiguo Liu
Digestive Endoscopy.2023;[Epub] CrossRef - Injectable Thermosensitive Chitosan Solution with β-Glycerophosphate as an Optimal Submucosal Fluid Cushion for Endoscopic Submucosal Dissection
Seung Jeong, Han Jo Jeon, Kyoung-Je Jang, Sangbae Park, Hyuk Soon Choi, Jong Hoon Chung
Polymers.2021; 13(11): 1696. CrossRef
- Efficacy and safety of a submucosal injection solution of sodium alginate for endoscopic resection in a porcine model
- 7,208 View
- 71 Download
- 4 Web of Science
- 4 Crossref

- Recent Advances in Endoscopic Papillectomy for Ampulla of Vater Tumors: Endoscopic Ultrasonography, Intraductal Ultrasonography, and Pancreatic Stent Placement
- Jimin Han, Dong Wook Lee, Ho Gak Kim
- Clin Endosc 2015;48(1):24-30. Published online January 31, 2015
- DOI: https://doi.org/10.5946/ce.2015.48.1.24
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Since it was first described nearly three decades ago, endoscopic papillectomy (EP) has been utilized as a less invasive, alternative therapy for adenoma of the major duodenal papilla. In this article, we review the recent advances in EP, especially those pertaining to endoscopic ultrasonography (EUS), intraductal ultrasonography (IDUS), and pancreatic stent placement for the prevention of postpapillectomy pancreatitis. Because EUS and IDUS have similar diagnostic accuracies, either modality can be used for the preprocedural evaluation of ampullary tumors. Nevertheless, further technical refinements are required for a more precise evaluation. Given the paucity of data on the usefulness of EUS and/or IDUS during follow-up after EP, a well-designed study is warranted. Furthermore, pancreatic stent placement appears to have a protective effect against postpapillectomy pancreatitis; however, a prospective, randomized, controlled study with a larger number of patients is needed to assess this finding. Moreover, since pancreatic stent placement after EP is not always successful, various novel techniques have been developed to ensure reliable stent placement. Despite the recent advances in EP, further technical refinements and studies are needed to confirm their efficacy.
-
Citations
Citations to this article as recorded by- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
Seung Joo Kang, Chung Hyun Tae, Chang Seok Bang, Cheol Min Shin, Young-Hoon Jeong, Miyoung Choi, Joo Ha Hwang, Yutaka Saito, Philip Wai Yan Chiu, Rungsun Rerknimitr, Christopher Khor, Vu Van Khien, Kee Don Choi, Ki-Nam Shim, Geun Am Song, Oh Young Lee
Clinical Endoscopy.2024; 57(2): 141. CrossRef - Effect of prophylactic endoscopic clipping for prevention of delayed bleeding after endoscopic papillectomy for ampullary neoplasm: a multicenter randomized trial
Se Woo Park, Tae Jun Song, Jin Seok Park, Jae Hyuk Jun, Tae Young Park, Dong Wook Oh, Sang Soo Lee, Myung-Hwan Kim
Endoscopy.2022; 54(08): 787. CrossRef - Efficacy analysis of hemostatic spray following endoscopic papillectomy: A multicenter comparative study
Kyong Joo Lee, Tae Hoon Lee, Jae Hee Cho, Jong Jin Hyun, Sung Ill Jang, Seok Jeong, Jin‐Seok Park, Jae Kook Yang, Don Haeng Lee, Dong Ki Lee, Sang Heum Park
Journal of Gastroenterology and Hepatology.2022; 37(11): 2138. CrossRef
- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
- 9,750 View
- 144 Download
- 3 Web of Science
- 3 Crossref

- Gastric Squamous Papilloma in a 52-Year-Old Female Patient
- Hyung Ha Jang, Hyung Wook Kim, Su Jin Kim, Choel Woong Choi, Su Bum Park, Byeong Jun Song, Dong Hoon Shin, Dae Hwan Kang
- Clin Endosc 2014;47(6):571-574. Published online November 30, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.6.571
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub A papilloma is a benign epithelial lesion characterized by finger-like projections of tissue lined by an overgrowth of squamous cells and a core of connective tissue. We report a case of squamous papilloma on the cardia in a 52-year-old asymptomatic female. Endoscopy showed a 1-cm sized is polyp with hyperemic change originating from the cardia adjacent to the esophagogastric junction, the biopsy of which suggested a diagnosis of squamous papilloma. Endoscopic mucosal resection was performed to obtain a definite diagnosis and the polyp was completely removed. The histological result was compatible with squamous papilloma, and its surrounding tissues showed foveolar epithelium, which suggested a stomach origin. This is the first report of endoscopic resection of a gastric squamous papilloma. Squamous papilloma should be considered in the differential diagnosis of a gastric polyp, especially one in the cardia. As the prognostic value of a squamous papilloma is not well known, we recommend endoscopic resection to treat a gastric squamous papilloma, when possible.
-
Citations
Citations to this article as recorded by- Overexpression of Neutrophil MMP-9 and HIF-1α May Contribute to the Finger-Like Projections Formation and Histo-Pathogenesis in Nasal Inverted Papilloma
Tao Li, Kai Sen Tan, Yan Yi Tu, Li Zhao, Jing Liu, Hsiao Hui Ong, De Yun Wang, Li Shi
Journal of Inflammation Research.2021; Volume 14: 2979. CrossRef
- Overexpression of Neutrophil MMP-9 and HIF-1α May Contribute to the Finger-Like Projections Formation and Histo-Pathogenesis in Nasal Inverted Papilloma
- 7,729 View
- 71 Download
- 1 Web of Science
- 1 Crossref

- Lymph Node Metastases in Esophageal Carcinoma: An Endoscopist's View
- Jin Woong Cho, Suck Chei Choi, Jae Young Jang, Sung Kwan Shin, Kee Don Choi, Jun Haeng Lee, Sang Gyun Kim, Jae Kyu Sung, Seong Woo Jeon, Il Ju Choi, Gwang Ha Kim, Sam Ryong Jee, Wan Sik Lee, Hwoon-Yong Jung, Korean ESD Study Group
- Clin Endosc 2014;47(6):523-529. Published online November 30, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.6.523
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub One of the most important prognostic factors in esophageal carcinoma is lymph node metastasis, and in particular, the number of affected lymph nodes, which influences long-term outcomes. The esophageal lymphatic system is connected longitudinally and transversally; thus, the pattern of lymph node metastases is very complex. Early esophageal cancer frequently exhibits skipped metastasis, and minimal surgery using sentinel node navigation cannot be performed. In Korea, most esophageal cancer cases are squamous cell carcinoma (SCC), although the incidence of adenocarcinoma has started to increase recently. Most previous reports have failed to differentiate between SCC and adenocarcinoma, despite the fact that the Union for International Cancer Control (7th edition) and American Joint Committee on Cancer staging systems both consider these separately because they differ in cause, biology, lymph node metastasis, and outcome. Endoscopic tumor resection is an effective and safe treatment for lesions with no associated lymph node metastasis. Esophageal mucosal cancer confined to the lamina propria is an absolute indication for endoscopic resection, and a lesion that has invaded the muscularis mucosae can be cured by local resection if invasion to the lymphatic system has not occurred.
-
Citations
Citations to this article as recorded by- The association between histological subtypes and lymph node metastasis and prognosis in early esophageal cancer: a population-based study
Jun-Peng Lin, Xiao-Feng Chen, Hang Zhou, Feng-Nian Zhuang, Hao He, Wei-Jie Chen, Feng Wang, Shuo-Yan Liu
European Journal of Cancer Prevention.2024; 33(2): 152. CrossRef - Application of the convolution neural network in determining the depth of invasion of gastrointestinal cancer: a systematic review and meta-analysis
Ruo Wu, Kaiwen Qin, Yuxin Fang, Yuyuan Xu, Haonan Zhang, Wenhua Li, Xiaobei Luo, Zelong Han, Side Liu, Qingyuan Li
Journal of Gastrointestinal Surgery.2024; 28(4): 538. CrossRef - Esophageal cancer
Daniel C. Eisner
JAAPA.2024; 37(4): 19. CrossRef - Gliomagenesis is orchestrated by the Oct3/4 regulatory network
Tatyana N. IGNATOVA, Hersh J. CHAITIN, Nickolay V. KUKEKOV, Oleg N. SUSLOV, Galina I. DULATOVA, Khalid A. HANAFY, Frank D. VRIONIS
Journal of Neurosurgical Sciences.2024;[Epub] CrossRef - Management of early oesophageal cancer: An overview
Gavin G Calpin, Matthew G Davey, Noel E Donlon
World Journal of Gastrointestinal Surgery.2024; 16(5): 1255. CrossRef - Surgical Approach to Esophagectomy Post CheckMate 577
Nikhil Panda, Lana Schumacher
Thoracic Surgery Clinics.2023; 33(2): 209. CrossRef - Mechanisms of esophageal cancer metastasis and treatment progress
Yusheng Wang, Wei Yang, Qianyun Wang, Yong Zhou
Frontiers in Immunology.2023;[Epub] CrossRef - Ten-year follow-up of endoscopic mucosal resection versus esophagectomy for esophageal intramucosal adenocarcinoma in the setting of Barrett’s esophagus: a Canadian experience
Alisha Fernandes, Chao Li, Daniel French, James Ellsmere
Surgical Endoscopy.2023; 37(11): 8735. CrossRef - Advanced Endoscopic Resection Techniques: Endoscopic Submucosal Dissection and Endoscopic Full-Thickness Resection
Phillip S. Ge, Hiroyuki Aihara
Digestive Diseases and Sciences.2022; 67(5): 1521. CrossRef - A Novel Ferroptosis-Related Gene Signature to Predict Prognosis of Esophageal Carcinoma
Jian Wang, Ziming Guo, Fei Sun, Tian Xu, Jianlin Wang, Jingping Yu, Dong-Hua Yang
Journal of Oncology.2022; 2022: 1. CrossRef - Recent advances in multidisciplinary therapy for adenocarcinoma of the esophagus and esophagogastric junction
Yi-Han Zheng, En-Hao Zhao
World Journal of Gastroenterology.2022; 28(31): 4299. CrossRef - The relationship between esophageal cancer mortality‐to‐incidence ratios of countries and ranking of world's health system
Ming‐Hui Chang, Shih‐Ming Huang, Wen‐Wei Sung, Tzu‐Wei Yang, Hsuan‐Yi Chen, Chang‐Cheng Su, Wei‐Liang Chen, Ming‐Chang Tsai, Chi‐Chih Wang
Advances in Digestive Medicine.2021; 8(4): 234. CrossRef - Staging esophageal cancer: low EUS accuracy in t2n0 patients
Germana de Nucci, Maria Chiara Petrone, Nicola Imperatore, Emanuele Asti, Gemma Rossi, Giampiero Manes, Maurizio Vecchi, Luca Pastorelli, Luigi Bonavina, Paolo Giorgio Arcidiacono
Endoscopy International Open.2021; 09(03): E313. CrossRef - Long non-coding RNA SPRY4-IT1 as a promising indicator for three field lymph-node dissection of thoracic esophageal carcinoma
Peng Qie, Qifan Yin, Xuejiao Xun, Yongbin Song, Shaohui Zhou, Huining Liu, Junpeng Feng, Ziqiang Tian
Journal of Cardiothoracic Surgery.2021;[Epub] CrossRef - A clinically interpretable convolutional neural network for the real-time prediction of early squamous cell cancer of the esophagus: comparing diagnostic performance with a panel of expert European and Asian endoscopists
Martin A. Everson, Luis Garcia-Peraza-Herrera, Hsiu-Po Wang, Ching-Tai Lee, Chen-Shuan Chung, Ping-Hsin Hsieh, Chien-Chuan Chen, Cheng-Hao Tseng, Ming-Hung Hsu, Tom Vercauteren, Sebastien Ourselin, Sergey Kashin, Raf Bisschops, Oliver Pech, Laurence Lovat
Gastrointestinal Endoscopy.2021; 94(2): 273. CrossRef - Pitfalls and Pearls in Esophageal Carcinoma
Sonia L. Betancourt-Cuellar, Diana P. Palacio, Marcelo F. Kuperman Benveniste, Yasmeen Mawlawi, Jeremy J. Erasmus
Seminars in Ultrasound, CT and MRI.2021; 42(6): 535. CrossRef - Effect of perioperative flurbiprofen axetil on long‐term survival of patients with esophageal carcinoma who underwent thoracoscopic esophagectomy: A retrospective study
Yanhu Xie, Di Wang, Chen Gao, Jicheng Hu, Min Zhang, Wei Gao, Shuhua Shu, Xiaoqing Chai
Journal of Surgical Oncology.2021; 124(4): 540. CrossRef - Long-term outcomes of an esophagus-preserving chemoradiotherapy strategy for patients with endoscopically unresectable stage I thoracic esophageal squamous cell carcinoma
Tatsuya Suwa, Yuichi Ishida, Yoshiharu Negoro, Fusako Kusumi, Yoshio Kadokawa, Rihito Aizawa, Toshifumi Nakajima, Yoshiaki Okamoto, Yoshishige Okuno, Kazunari Yamada, Masakazu Ogura, Masao Murakami, Takashi Mizowaki
Clinical and Translational Radiation Oncology.2021; 30: 88. CrossRef - Treating esophageal squamous cell carcinoma with ablation: the fear of what lies beneath
Elizabeth Anne Montgomery, Rehan Haidry
Gastrointestinal Endoscopy.2021; 94(4): 843. CrossRef - Lymph Node Involvement in Oesophageal Carcinoma: A Single-Centre Observational Study From Western India
Ajay K Boralkar , Abdul Rafe, Bhushan Bhalgat
Cureus.2021;[Epub] CrossRef - A nomogram for predicting lymph node metastasis in superficial esophageal squamous cell carcinoma
Weifeng Zhang, Han Chen, Guoxin Zhang, Guangfu Jin
The Journal of Biomedical Research.2021; 35(5): 361. CrossRef - Predicting lymph node metastases with endoscopic resection in cT2N0M0 oesophageal cancer: A systematic review and meta‐analysis
Ali Al‐Kaabi, Rachel S van der Post, Jonathan Huising, Camiel Rosman, Iris D Nagtegaal, Peter D Siersema
United European Gastroenterology Journal.2020; 8(1): 35. CrossRef - Intrapapillary capillary loop classification in magnification endoscopy: open dataset and baseline methodology
Luis C. García-Peraza-Herrera, Martin Everson, Laurence Lovat, Hsiu-Po Wang, Wen Lun Wang, Rehan Haidry, Danail Stoyanov, Sébastien Ourselin, Tom Vercauteren
International Journal of Computer Assisted Radiology and Surgery.2020; 15(4): 651. CrossRef - Clinical impact of FDG PET/CT in alimentary tract malignancies: an updated review
Esma A. Akin, Zain N. Qazi, Murat Osman, Robert K. Zeman
Abdominal Radiology.2020; 45(4): 1018. CrossRef - Comparison of general anesthesia and conscious sedation in procedure-related complications during esophageal endoscopic submucosal dissection
Seung Hyun Kim, Yong Seon Choi, Sang Kil Lee, Hanseul Oh, Seung Ho Choi
Surgical Endoscopy.2020; 34(8): 3560. CrossRef - Indications, contraindications and limitations of endoscopic therapy for Barrett’s esophagus and early esophageal adenocarcinoma
Carol Rouphael, Mythri Anil Kumar, Madhusudhan R. Sanaka, Prashanthi N. Thota
Therapeutic Advances in Gastroenterology.2020; 13: 175628482092420. CrossRef - Importance of investigating high-risk human papillomavirus in lymph node metastasis of esophageal adenocarcinoma
Preeti Sharma, Shweta Dutta Gautam, Shanmugarajah Rajendra
World Journal of Gastroenterology.2020; 26(21): 2729. CrossRef - Inaccurate pretreatment staging can impact survival in early stage esophageal adenocarcinoma
Anthony J. Scholer, Abhineet Uppal, Shu‐Ching Chang, Debopriya Ghosh, Mary Garland‐ Kledzik, Juan Santamaria‐Barria, Adam Khader, Ahmed Dehal, Trevan Fischer, Melanie Goldfarb
Journal of Surgical Oncology.2020; 122(5): 914. CrossRef - Development of a Novel Serum Exosomal MicroRNA Nomogram for the Preoperative Prediction of Lymph Node Metastasis in Esophageal Squamous Cell Carcinoma
Tong Liu, Lu-Tao Du, Yun-Shan Wang, Shan-Yu Gao, Juan Li, Pei-Long Li, Zhao-Wei Sun, Helen Binang, Chuan-Xin Wang
Frontiers in Oncology.2020;[Epub] CrossRef - A comprehensive methylation signature identifies lymph node metastasis in esophageal squamous cell carcinoma
Roshni Roy, Raju Kandimalla, Fuminori Sonohara, Masahiko Koike, Yasuhiro Kodera, Naoki Takahashi, Yasuhide Yamada, Ajay Goel
International Journal of Cancer.2019; 144(5): 1160. CrossRef - Artificial intelligence for the real‐time classification of intrapapillary capillary loop patterns in the endoscopic diagnosis of early oesophageal squamous cell carcinoma: A proof‐of‐concept study
M Everson, LCGP Herrera, W Li, I Muntion Luengo, O Ahmad, M Banks, C Magee, D Alzoubaidi, HM Hsu, D Graham, T Vercauteren, L Lovat, S Ourselin, S Kashin, Hsiu-Po Wang, Wen-Lun Wang, RJ Haidry
United European Gastroenterology Journal.2019; 7(2): 297. CrossRef - Early detection and therapeutics
Wladyslaw Januszewicz, Rebecca C. Fitzgerald
Molecular Oncology.2019; 13(3): 599. CrossRef - Endoscopic Submucosal Dissection for Esophageal Adenocarcinoma: A North American Perspective
Philippe Bouchard, Juan-Carlos Molina, Jonathan Cools-Lartigue, Jonathan Spicer, Carmen L. Mueller, Lorenzo E. Ferri
Journal of Gastrointestinal Surgery.2019; 23(6): 1087. CrossRef - Improved prognosis with induction chemotherapy in pathological complete responders after trimodality treatment for esophageal squamous cell carcinoma: Hypothesis generating for adjuvant treatment
Shao-Lun Lu, Feng-Ming Hsu, Chiao-Ling Tsai, Jang-Ming Lee, Pei-Ming Huang, Chih-Hung Hsu, Chia-Chi Lin, Yih-Leong Chang, Min-Shu Hsieh, Jason Chia-Hsien Cheng
European Journal of Surgical Oncology.2019; 45(8): 1498. CrossRef - Initial Evaluation of Computer-Assisted Radiologic Assessment for Renal Mass Edge Detection as an Indication of Tumor Roughness to Predict Renal Cancer Subtypes
Rahul Rajendran, Kevan Iffrig, Deepak K Pruthi, Allison Wheeler, Brian Neuman, Dharam Kaushik, Ahmed M Mansour, Karen Panetta, Sos Agaian, Michael A. Liss
Advances in Urology.2019; 2019: 1. CrossRef - Membrane Metalloendopeptidase (MME) Suppresses Metastasis of Esophageal Squamous Cell Carcinoma (ESCC) by Inhibiting FAK-RhoA Signaling Axis
Mengqing Li, Ling Wang, Yuting Zhan, Tingting Zeng, Xu Zhang, Xin-Yuan Guan, Yan Li
The American Journal of Pathology.2019; 189(7): 1462. CrossRef - Barrett oesophagus
Yonne Peters, Ali Al-Kaabi, Nicholas J. Shaheen, Amitabh Chak, Andrew Blum, Rhonda F. Souza, Massimiliano Di Pietro, Prasad G. Iyer, Oliver Pech, Rebecca C. Fitzgerald, Peter D. Siersema
Nature Reviews Disease Primers.2019;[Epub] CrossRef - Early Esophageal Cancer: A Gastroenterologist’s Disease
Joseph Spataro, Alvin M. Zfass, Mitchell Schubert, Tilak Shah
Digestive Diseases and Sciences.2019; 64(11): 3048. CrossRef - Endoscopic Techniques for Early Detection of Esophageal Cancer
Jae Myung Park
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2019; 19(3): 149. CrossRef - Endoscopic Treatment for Esophageal Cancer
Eun Jeong Gong, Do Hoon Kim
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2019; 19(3): 156. CrossRef - Endoskopische Therapieoptionen beim Adenokarzinom am ösophagogastralen Übergang
Seung-Hun Chon, Isabel Bartella, Martin Bürger
Der Onkologe.2019; 25(12): 1073. CrossRef - Treatment of early stage (T1) esophageal adenocarcinoma: Personalizing the best therapy choice
Lindsay Danielle Kumble, Elisabeth Silver, Aaron Oh, Julian A Abrams, Joshua R Sonett, Chin Hur
World Journal of Meta-Analysis.2019; 7(9): 406. CrossRef - Clinical diagnostic value of digestive endoscopic narrow-band imaging in early esophageal cancer
Zhenhua Su, Liang Wang, Sichen Wei, Xinliang Wei, Yu Kong, Weiwei Wang, Ruixue Guo, Xiaomeng Shi
Oncology Letters.2019;[Epub] CrossRef - Role of Perioperative Chemotherapy in Lymph Node-negative Esophageal Cancer After Resection
Yang Yang, Xia Zhou, Luoyong Tang, Xiaoling Xu, Xianghui Du, Guoqin Qiu
American Journal of Clinical Oncology.2019; 42(12): 924. CrossRef - Endoscopic Management of Early Adenocarcinoma and Squamous Cell Carcinoma of the Esophagus: Screening, Diagnosis, and Therapy
Massimiliano di Pietro, Marcia I. Canto, Rebecca C. Fitzgerald
Gastroenterology.2018; 154(2): 421. CrossRef - Elucidation of the Anatomical Mechanism of Nodal Skip Metastasis in Superficial Thoracic Esophageal Squamous Cell Carcinoma
Yuji Kumakura, Takehiko Yokobori, Tomonori Yoshida, Keigo Hara, Makoto Sakai, Makoto Sohda, Tatsuya Miyazaki, Hideaki Yokoo, Tadashi Handa, Tetsunari Oyama, Hiroshi Yorifuji, Hiroyuki Kuwano
Annals of Surgical Oncology.2018; 25(5): 1221. CrossRef - Endoscopic Treatment of Early-Stage Esophageal Cancer
Mariam Naveed, Nisa Kubiliun
Current Oncology Reports.2018;[Epub] CrossRef - Role of endoscopic therapy in early esophageal cancer
Sonika Malik, Gautam Sharma, Madhusudhan R Sanaka, Prashanthi N Thota
World Journal of Gastroenterology.2018; 24(35): 3965. CrossRef - Loss of PAR-3 protein expression is associated with invasion, lymph node metastasis, and poor survival in esophageal squamous cell carcinoma
Tomoko Kitaichi, Kohichiroh Yasui, Yasuyuki Gen, Osamu Dohi, Naoto Iwai, Akira Tomie, Nobuhisa Yamada, Kei Terasaki, Atsushi Umemura, Taichiro Nishikawa, Kanji Yamaguchi, Michihisa Moriguchi, Yoshio Sumida, Hironori Mitsuyoshi, Yuji Naito, Yoh Zen, Yoshit
Human Pathology.2017; 62: 134. CrossRef - Oesophageal adenocarcinoma has a higher risk of lymph node metastasis than squamous cell carcinoma: a propensity score-matched study
Han-Yu Deng, Zhi-Qiang Wang, Yun-Cang Wang, Gang Li, Jun Luo, Long-Qi Chen, Lun-Xu Liu, Qing-Hua Zhou, Yi-Dan Lin
European Journal of Cardio-Thoracic Surgery.2017; 52(5): 958. CrossRef - Prognostic significance of tumor length in patients receiving esophagectomy for esophageal cancer
Alexander C. Hollis, Lauren M. Quinn, James Hodson, Emily Evans, James Plowright, Ruksana Begum, Harriet Mitchell, Mike T. Hallissey, John L. Whiting, Ewen A. Griffiths
Journal of Surgical Oncology.2017; 116(8): 1114. CrossRef - New magnifying endoscopic classification for superficial esophageal squamous cell carcinoma
Su Jin Kim, Gwang Ha Kim, Moon Won Lee, Hye Kyung Jeon, Dong Hoon Baek, Bong Eun Lee, Geun Am Song
World Journal of Gastroenterology.2017; 23(24): 4416. CrossRef - Is endoscopic ultrasound examination necessary in the management of esophageal cancer?
Tomas DaVee, Jaffer A Ajani, Jeffrey H Lee
World Journal of Gastroenterology.2017; 23(5): 751. CrossRef - Endoscopic ultrasound staging for early esophageal cancer: Are we denying patients neoadjuvant chemo-radiation?
Carrie Luu, Marisa Amaral, Jason Klapman, Cynthia Harris, Khaldoun Almhanna, Sarah Hoffe, Jessica Frakes, Jose M Pimiento, Jacques P Fontaine
World Journal of Gastroenterology.2017; 23(46): 8193. CrossRef - Decreased expression of CD63 tetraspanin protein predicts elevated malignant potential in human esophageal cancer
Xiaojing Lai, Qing Gu, Xia Zhou, Wei Feng, Xiao Lin, Yan He, Jinming Cao, Pengfei Liu, Huojun Zhang, Xiao Zheng
Oncology Letters.2017; 13(6): 4245. CrossRef - Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis
Hariruk Yodying, Akihisa Matsuda, Masao Miyashita, Satoshi Matsumoto, Nobuyuki Sakurazawa, Marina Yamada, Eiji Uchida
Annals of Surgical Oncology.2016; 23(2): 646. CrossRef - Endoscopic Submucosal Dissection for Superficial Esophageal Neoplasm: A Growing Body of Evidence
Eun Jeong Gong, Hwoon-Yong Jung
Clinical Endoscopy.2016; 49(2): 101. CrossRef - FDG-PET/CT lymph node staging after neoadjuvant chemotherapy in patients with adenocarcinoma of the esophageal–gastric junction
Pavel Fencl, Otakar Belohlavek, Tomas Harustiak, Milada Zemanova
Abdominal Radiology.2016; 41(11): 2089. CrossRef - MiR-106b promotes migration and invasion through enhancing EMT via downregulation of Smad 7 in Kazakh’s esophageal squamous cell carcinoma
Fang Dai, Tao Liu, Shutao Zheng, Qing Liu, Chenchen Yang, Jian Zhou, Yumei Chen, Ilyar Sheyhidin, Xiaomei Lu
Tumor Biology.2016; 37(11): 14595. CrossRef - A Risk Prediction Model Based on Lymph-Node Metastasis in Poorly Differentiated–Type Intramucosal Gastric Cancer
Jeung Hui Pyo, Hyuk Lee, Byung-Hoon Min, Jun Haeng Lee, Min Gew Choi, Jun Ho Lee, Tae Sung Sohn, Jae Moon Bae, Kyoung-Mee Kim, Hyeon Seon Ahn, Sin-Ho Jung, Sung Kim, Jae J. Kim, Xin-Yuan Guan
PLOS ONE.2016; 11(5): e0156207. CrossRef - MicroRNA-92b represses invasion-metastasis cascade of esophageal squamous cell carcinoma
Gang Ma, Chao Jing, Lin Li, Furong Huang, Fang Ding, Baona Wang, Dongmei Lin, Aiping Luo, Zhihua Liu
Oncotarget.2016; 7(15): 20209. CrossRef - DNA polymerase iota (Pol ι) promotes invasion and metastasis of esophageal squamous cell carcinoma
Shitao Zou, Zeng-Fu Shang, Biao Liu, Shuyu Zhang, Jinchang Wu, Min Huang, Wei-Qun Ding, Jundong Zhou
Oncotarget.2016; 7(22): 32274. CrossRef - CTL- vs Treg lymphocyte-attracting chemokines, CCL4 and CCL20, are strong reciprocal predictive markers for survival of patients with oesophageal squamous cell carcinoma
J Y Liu, F Li, L P Wang, X F Chen, D Wang, L Cao, Y Ping, S Zhao, B Li, S H Thorne, B Zhang, P Kalinski, Y Zhang
British Journal of Cancer.2015; 113(5): 747. CrossRef - Metadherin is required for the proliferation, migration, and invasion of esophageal squamous cell carcinoma and its meta-analysis
Chenchen Yang, Shutao Zheng, Qing Liu, Tao Liu, Mang Lu, Fang Dai, Xiangpeng Gao, Ilyar Sheyhidin, Xiaomei Lu
Translational Research.2015; 166(6): 614. CrossRef - A Comparison of Postoperative Early Enteral Nutrition with Delayed Enteral Nutrition in Patients with Esophageal Cancer
Gongchao Wang, Hongbo Chen, Jun Liu, Yongchen Ma, Haiyong Jia
Nutrients.2015; 7(6): 4308. CrossRef
- The association between histological subtypes and lymph node metastasis and prognosis in early esophageal cancer: a population-based study
- 10,733 View
- 131 Download
- 69 Web of Science
- 65 Crossref

- Tissue Acquisition in Gastric Epithelial Tumor Prior to Endoscopic Resection
- Chan Gyoo Kim
- Clin Endosc 2013;46(5):436-440. Published online September 30, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.5.436
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Endoscopic forceps biopsy is essential before planning an endoscopic resection of upper gastrointestinal epithelial tumors. However, forceps biopsy is limited by its superficiality and frequency of sampling errors. Histologic discrepancies between endoscopic forceps biopsies and resected specimens are frequent. Factors associated with such histologic discrepancies are tumor size, macroscopic type, surface color, and the type of medical facility. Precise targeting of biopsies is recommended to achieve an accurate diagnosis, curative endoscopic resection, and a satisfactory oncologic outcome. Multiple deep forceps biopsies can induce mucosal ulceration in early gastric cancer. Endoscopic resection for early gastric cancer with ulcerative findings is associated with piecemeal resection, incomplete resection, and a risk for procedure-related complications such as bleeding and perforation. Such active ulcers caused by forceps biopsy and following submucosal fibrosis might also be mistaken as an indication for more aggressive procedures, such as gastrectomy with D2 lymph node dissection. Proton pump inhibitors might be prescribed to facilitate the healing of biopsy-induced ulcers if an active ulcer is predicted after deep biopsy. It is unknown which time interval from biopsy to endoscopic resection is appropriate for a safe procedure and a good oncologic outcome. Further investigations are needed to conclude the appropriate time interval.
-
Citations
Citations to this article as recorded by- Success rate of current human-derived gastric cancer organoids establishment and influencing factors: A systematic review and meta-analysis
Kai-Lin Jiang, Xiang-Xiang Wang, Xue-Jiao Liu, Li-Kun Guo, Yong-Qi Chen, Qing-Ling Jia, Ke-Ming Yang, Jiang-Hong Ling
World Journal of Gastrointestinal Oncology.2024; 16(4): 1626. CrossRef - Clinical value and influencing factors of establishing stomach cancer organoids by endoscopic biopsy
Jie Li, Yan Chen, Yingyi Zhang, Xiaobo Peng, Meihong Wu, Ling Chen, Xianbao Zhan
Journal of Cancer Research and Clinical Oncology.2023; 149(7): 3803. CrossRef - Chinese integrated guideline on the management of gastric precancerous conditions and lesions
Ping Wang, Peng Li, Yingxuan Chen, Li Li, Yuanyuan Lu, Weixun Zhou, Liqun Bian, Beihua Zhang, Xiaolan Yin, Junxiang Li, Jie Chen, Shutian Zhang, Yongquan Shi, Xudong Tang
Chinese Medicine.2022;[Epub] CrossRef - Clinical applicability of gastroscopy with narrow-band imaging for the diagnosis of Helicobacter pylori gastritis, precancerous gastric lesion, and neoplasia
Jun-Hyung Cho, Seong Ran Jeon, So-Young Jin
World Journal of Clinical Cases.2020; 8(14): 2902. CrossRef - Gastric Cancer Caused by Adenoma: Predictive Factors Associated with Lesions Other Than the Expanded Indications
Seong Hwan Park, Kee Don Choi, Kyoungwon Jung, Yangsoon Park, Sunpyo Lee, Eun Jeong Gong, Hee Kyong Na, Ji Yong Ahn, Kee Wook Jung, Jeong Hoon Lee, Do Hoon Kim, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung
Gut and Liver.2018; 12(3): 246. CrossRef - Risk factors associated with histological upgrade of gastric low‐grade dysplasia on pretreatment biopsy
Lang Yang, Peng Jin, Xin Wang, Tong Zhang, Yu Qi He, Xiao Jun Zhao, Na Li, Guang Zhi Yang, Jian Qiu Sheng
Journal of Digestive Diseases.2018; 19(10): 596. CrossRef - Endoscopic predictors for undifferentiated histology in differentiated gastric neoplasms prior to endoscopic resection
Ji Min Choi, Sang Gyun Kim, Hyo-Joon Yang, Joo Hyun Lim, Jeongmin Choi, Jong Pil Im, Joo Sung Kim, Woo Ho Kim, Hyun Chae Jung
Surgical Endoscopy.2016; 30(1): 89. CrossRef - Guidance on the effective use of upper gastrointestinal histopathology
Maurice B Loughrey, Brian T Johnston
Frontline Gastroenterology.2014; 5(2): 88. CrossRef - Endoscopic features suggesting gastric cancer in biopsy-proven gastric adenoma with high-grade neoplasia
Jung Ho Kim
World Journal of Gastroenterology.2014; 20(34): 12233. CrossRef
- Success rate of current human-derived gastric cancer organoids establishment and influencing factors: A systematic review and meta-analysis
- 5,530 View
- 36 Download
- 9 Crossref

- The Clinical Significance and Management of Noncurative Endoscopic Resection in Early Gastric Cancer
- Jun Heo, Seong Woo Jeon
- Clin Endosc 2013;46(3):235-238. Published online May 31, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.3.235
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Nowadays, endoscopic mucosal resection or endoscopic submucosal dissection has shown effectiveness equivalent to that of gastrectomy and has emerged as a popular technique for curative treatment of gastric cancer. However, noncurative resection or resection beyond the indication may lead to lymphatic and extended organ metastasis resulting in loss of the opportunity for full recovery. Therefore, it is an important issue to decide the range of curative resection in the endoscopic resection field. Furthermore, management of noncurative endoscopic resection in early gastric cancer is also important. The most favorable treatment after noncurative resection would be surgery. However, other noninvasive treatments such as argon plasma coagulation, additional endoscopic resection and close observation for recurrence are thought to be the optional treatments after the noncurative resection. In the future, prospective research studies and observations are expected to verify the effectiveness of noninvasive treatments.
-
Citations
Citations to this article as recorded by- Improved pentamethine cyanine nanosensors for optoacoustic imaging of pancreatic cancer
Matthew D. Laramie, Benjamin L. Fouts, William M. MacCuaig, Emmanuel Buabeng, Meredith A. Jones, Priyabrata Mukherjee, Bahareh Behkam, Lacey R. McNally, Maged Henary
Scientific Reports.2021;[Epub] CrossRef - Additional laparoscopic gastrectomy after noncurative endoscopic submucosal dissection for early gastric cancer: A single-center experience
Yan-Tao Tian, Fu-Hai Ma, Gui-Qi Wang, Yue-Ming Zhang, Li-Zhou Dou, Yi-Bin Xie, Yu-Xin Zhong, Ying-Tai Chen, Quan Xu, Dong-Bing Zhao
World Journal of Gastroenterology.2019; 25(29): 3996. CrossRef - Risk stratification and management of non-curative resection after endoscopic submucosal dissection for early gastric cancer
Jae Pil Han, Su Jin Hong, Hee Kyung Kim, Yun Nah Lee, Tae Hee Lee, Bong Min Ko, Joo Young Cho
Surgical Endoscopy.2016; 30(1): 184. CrossRef - Additional Gastrectomy after Non-Curative Endoscopic Submucosal Dissection for Early Gastric Cancer
Yeon Soo Chang
The Journal of Minimally Invasive Surgery.2016; 19(1): 3. CrossRef - Highlights of the 48th Seminar of Korean Society of Gastrointestinal Endoscopy
Kwang An Kwon, Il Ju Choi, Eun Young Kim, Seok Ho Dong, Ki Baik Hahm
Clinical Endoscopy.2013; 46(3): 203. CrossRef
- Improved pentamethine cyanine nanosensors for optoacoustic imaging of pancreatic cancer
- 5,429 View
- 55 Download
- 5 Crossref


 KSGE
KSGE


 First
First Prev
Prev



