Search
- Page Path
- HOME > Search
Review
- Clinical meaning of sarcopenia in patients undergoing endoscopic treatment
- Hiroyuki Hisada, Yosuke Tsuji, Hikaru Kuribara, Ryohei Miyata, Kaori Oshio, Satoru Mizutani, Hideki Nakagawa, Rina Cho, Nobuyuki Sakuma, Yuko Miura, Hiroya Mizutani, Daisuke Ohki, Seiichi Yakabi, Yu Takahashi, Yoshiki Sakaguchi, Naomi Kakushima, Nobutake Yamamichi, Mitsuhiro Fujishiro
- Received July 26, 2023 Accepted August 18, 2023 Published online March 22, 2024
- DOI: https://doi.org/10.5946/ce.2023.193 [Epub ahead of print]
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - With increasing global life expectancy, the significance of geriatric assessment parameters has increased. Sarcopenia is a crucial assessment parameter and is defined as the age-related loss of muscle mass and strength. Sarcopenia is widely acknowledged as a risk factor for postoperative complications in diverse advanced malignancies and has a detrimental effect on the long-term prognosis. While most studies have primarily concentrated on the correlation between sarcopenia and advanced cancer, more recent investigations have focused on the relationship between sarcopenia and early-stage cancer. Endoscopic submucosal dissection (ESD), which is less invasive than surgical intervention, is extensively employed in the management of early-stage cancer, although it is associated with complications such as bleeding and perforation. In recent years, several reports have revealed the adverse consequences of sarcopenia in patients with early-stage cancer undergoing ESD. This literature review briefly summarizes the recent studies on the association between sarcopenia and ESD.
- 2,059 View
- 43 Download

Systematic Review and Meta-analysis
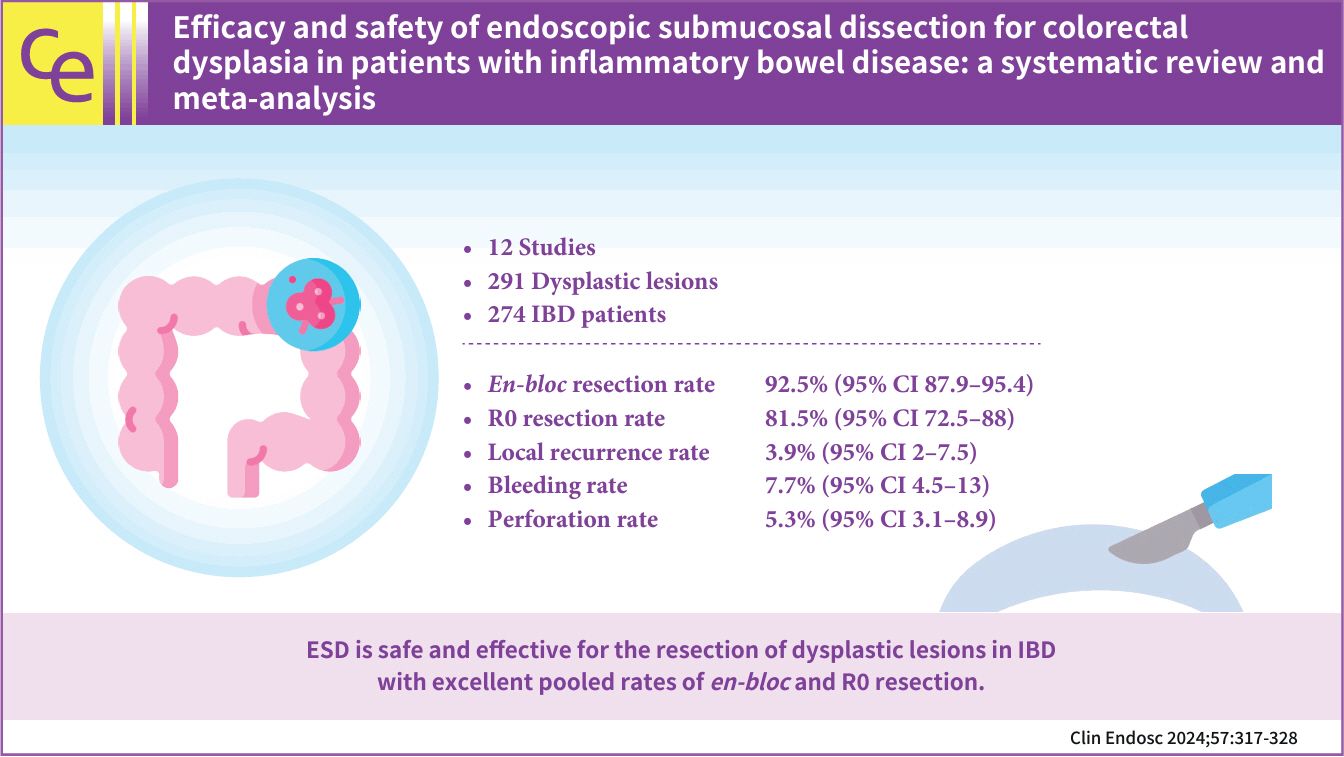
- Efficacy and safety of endoscopic submucosal dissection for colorectal dysplasia in patients with inflammatory bowel disease: a systematic review and meta-analysis
- Talia F. Malik, Vaishnavi Sabesan, Babu P. Mohan, Asad Ur Rahman, Mohamed O. Othman, Peter V. Draganov, Gursimran S. Kochhar
- Clin Endosc 2024;57(3):317-328. Published online February 29, 2024
- DOI: https://doi.org/10.5946/ce.2023.205
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub 
- Background
/Aims: In this meta-analysis, we studied the safety and efficacy of endoscopic submucosal dissection (ESD) for colorectal dysplasia in patients with inflammatory bowel disease (IBD).
Methods
Multiple databases were searched, and studies were retrieved based on pre-specified criteria until October 2022. The outcomes assessed were resection rates, procedural complications, local recurrence, metachronous tumors, and the need for surgery after ESD in IBD. Standard meta-analysis methods were followed using the random-effects model, and I2% was used to assess heterogeneity.
Results
Twelve studies comprising 291 dysplastic lesions in 274 patients were included with a median follow-up of 25 months. The pooled en-bloc resection, R0 resection, and curative resection rates were 92.5% (95% confidence interval [CI], 87.9%–95.4%; I2=0%), 81.5% (95% CI, 72.5%–88%; I2=43%), and 48.9% (95% CI, 32.1%–65.9%; I2=87%), respectively. The local recurrence rate was 3.9% (95% CI, 2%–7.5%; I2=0%). The pooled rates of bleeding and perforation were 7.7% (95% CI, 4.5%–13%; I2=10%) and 5.3% (95% CI, 3.1%–8.9%; I2=0%), respectively. The rates of metachronous recurrence and additional surgery following ESD were 10% (95% CI, 5.2%–18.2%; I2=55%) and 13% (95% CI, 8.5%–19.3%; I2=54%), respectively.
Conclusions
ESD is safe and effective for the resection of dysplastic lesions in IBD with an excellent pooled rate of en-bloc and R0 resection.
- 2,542 View
- 62 Download

Original Article
-
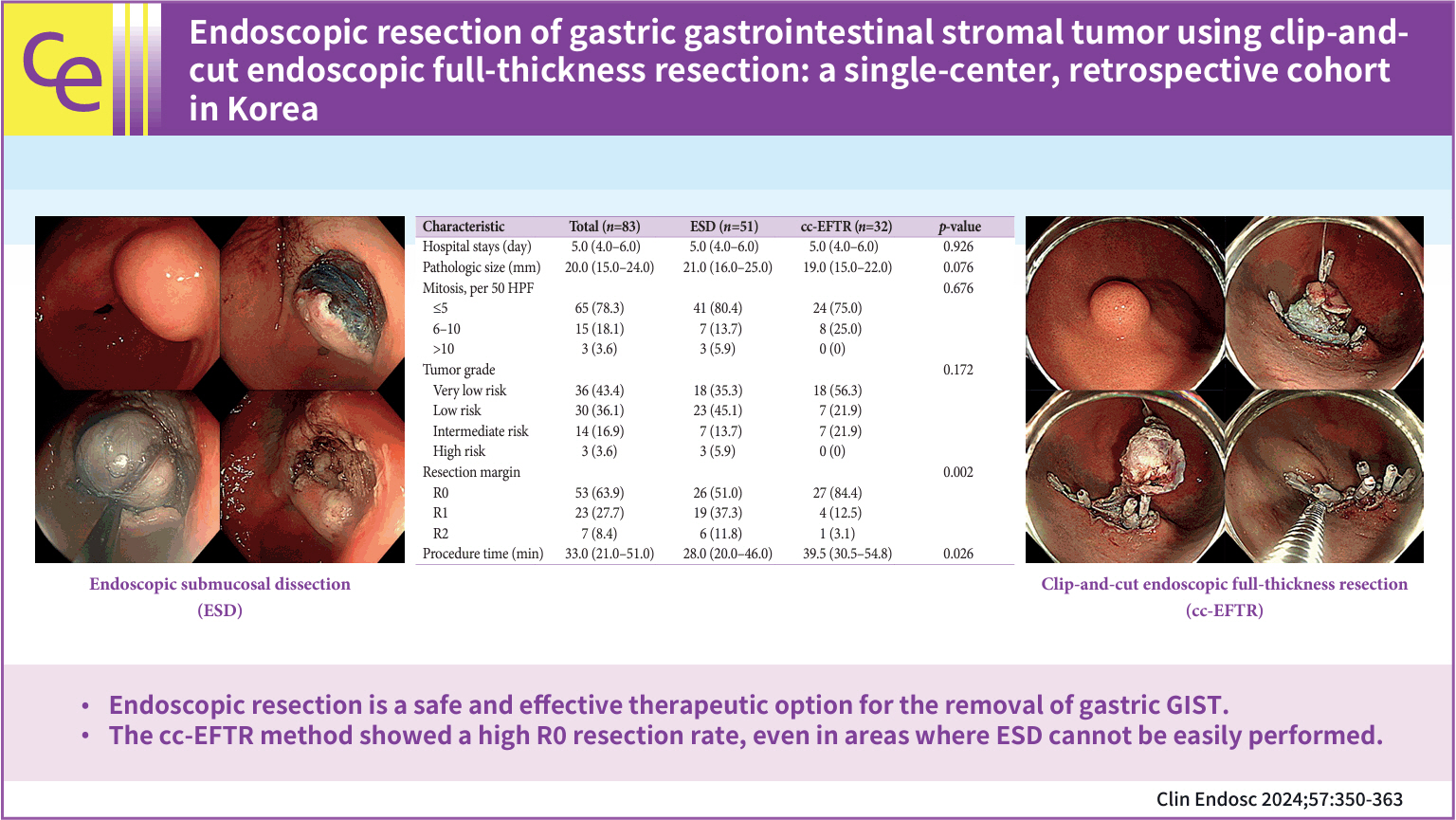
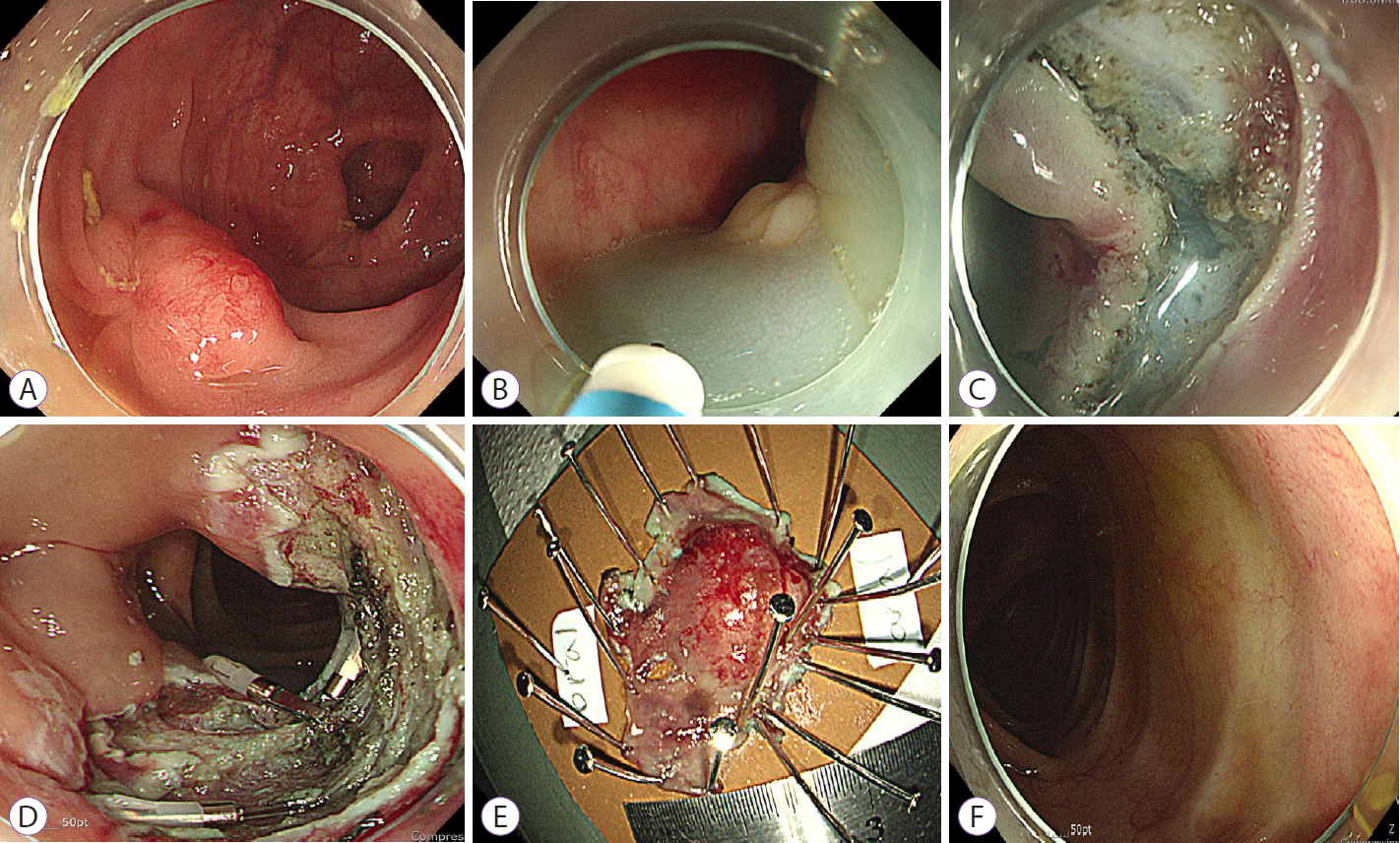
Endoscopic resection of gastric gastrointestinal stromal tumor using clip-and-cut endoscopic full-thickness resection: a single-center, retrospective cohort in Korea

- Yuri Kim, Ji Yong Ahn, Hwoon-Yong Jung, Seokin Kang, Ho June Song, Kee Don Choi, Do Hoon Kim, Jeong Hoon Lee, Hee Kyong Na, Young Soo Park
- Clin Endosc 2024;57(3):350-363. Published online February 15, 2024
- DOI: https://doi.org/10.5946/ce.2023.144
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub 
- Background
/Aims: To overcome the technical limitations of classic endoscopic resection for gastric gastrointestinal stromal tumors (GISTs), various methods have been developed. In this study, we examined the role and feasibility of clip-and-cut procedures (clip-and-cut endoscopic full-thickness resection [cc-EFTR]) for gastric GISTs.
Methods
Medical records of 83 patients diagnosed with GISTs after endoscopic resection between 2005 and 2021 were retrospectively reviewed. Moreover, clinical characteristics and outcomes were analyzed.
Results
Endoscopic submucosal dissection (ESD) and cc-EFTR were performed in 51 and 32 patients, respectively. The GISTs were detected in the upper third of the stomach for ESD (52.9%) and cc-EFTR (90.6%). Within the cc-EFTR group, a majority of GISTs were located in the deep muscularis propria or serosal layer, accounting for 96.9%, as opposed to those in the ESD group (45.1%). The R0 resection rates were 51.0% and 84.4% in the ESD and cc-EFTR groups, respectively. Seven (8.4%) patients required surgical treatment (six patients underwent ESD and one underwent cc-EFTR,) due to residual tumor (n=5) and post-procedure adverse events (n=2). Patients undergoing R0 or R1 resection did not experience recurrence during a median 14-month follow-up period, except for one patient in the ESD group.
Conclusions
cc-EFTR displayed a high R0 resection rate; therefore, it is a safe and effective therapeutic option for small gastric GISTs. -
Citations
Citations to this article as recorded by- Endoscopic resection penetrating the muscularis propria for gastric gastrointestinal stromal tumors: advances and challenges
Jin Woong Cho
Clinical Endoscopy.2024; 57(3): 329. CrossRef
- Endoscopic resection penetrating the muscularis propria for gastric gastrointestinal stromal tumors: advances and challenges
- 2,511 View
- 67 Download
- 1 Web of Science
- 1 Crossref

Systematic Review and Meta-analysis
- Comparison of scissor-type knife to non-scissor-type knife for endoscopic submucosal dissection: a systematic review and meta-analysis
- Harishankar Gopakumar, Ishaan Vohra, Srinivas Reddy Puli, Neil R Sharma
- Clin Endosc 2024;57(1):36-47. Published online January 5, 2024
- DOI: https://doi.org/10.5946/ce.2023.122

-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: Scissor-type endoscopic submucosal dissection (ST-ESD) knives can reduce the adverse events associated with ESDs. This study aimed to compare ST-ESD and non-scissor-type (NST)-ESD knives.
Methods
We identified ten studies that compared the performance characteristics and safety profiles of ST-ESD and NST-ESD knives. Fixed- and random-effects models were used to calculate the pooled proportions. Heterogeneity was assessed using the I2 test.
Results
On comparing ST-ESD knives to NST-ESD knives, the weighted odds of en bloc resection was 1.61 (95% confidence interval [CI], 0.90–2.90; p=0.14), R0 resection was 1.10 (95% CI, 0.71–1.71; p=0.73), delayed bleeding was 0.40 (95% CI, 0.17–0.90; p=0.03), perforation was 0.35 (95% CI, 0.18–0.70; p<0.01) and ESD self-completion by non-experts was 1.89 (95% CI, 1.20–2.95; p<0.01). There was no heterogeneity, with an I2 score of 0% (95% CI, 0%–54.40%).
Conclusions
The findings of reduced odds of perforation, a trend toward reduced delayed bleeding, and an improvement in the rates of en bloc and R0 resection with ST-ESD knives compared to NST-ESD knives support the use of ST-ESD knives when non-experts perform ESDs or as an adjunct tool for challenging ESD procedures.
- 2,267 View
- 141 Download

Review
- Management of complications related to colorectal endoscopic submucosal dissection
- Tae-Geun Gweon, Dong-Hoon Yang
- Clin Endosc 2023;56(4):423-432. Published online July 27, 2023
- DOI: https://doi.org/10.5946/ce.2023.104

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Compared to endoscopic mucosal resection (EMR), colonoscopic endoscopic submucosal dissection (C-ESD) has the advantages of higher en bloc resection rates and lower recurrence rates of colorectal neoplasms. Therefore, C-ESD is considered an effective treatment method for laterally spread tumors and early colorectal cancer. However, C-ESD is technically more difficult and requires a longer procedure time than EMR. In addition to therapeutic efficacy and procedural difficulty, safety concerns should always be considered when performing C-ESD in clinical practice. Bleeding and perforation are the main adverse events associated with C-ESD and can occur during C-ESD or after the completion of the procedure. Most bleeding associated with C-ESD can be managed endoscopically, even if it occurs during or after the procedure. More recently, most perforations identified during C-ESD can also be managed endoscopically, unless the mural defect is too large to be sutured with endoscopic devices or the patient is hemodynamically unstable. Delayed perforations are quite rare, but they require surgical treatment more frequently than endoscopically identified intraprocedural perforations or radiologically identified immediate postprocedural perforations. Post-ESD coagulation syndrome is a relatively underestimated adverse event, which can mimic localized peritonitis from perforation. Here, we classify and characterize the complications associated with C-ESD and recommend management options for them.
-
Citations
Citations to this article as recorded by- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
Seung Joo Kang, Chung Hyun Tae, Chang Seok Bang, Cheol Min Shin, Young-Hoon Jeong, Miyoung Choi, Joo Ha Hwang, Yutaka Saito, Philip Wai Yan Chiu, Rungsun Rerknimitr, Christopher Khor, Vu Van Khien, Kee Don Choi, Ki-Nam Shim, Geun Am Song, Oh Young Lee
Clinical Endoscopy.2024; 57(2): 141. CrossRef - Is there a best choice of equipment for colorectal endoscopic submucosal dissection?
Francesco Cocomazzi, Sonia Carparelli, Nunzia Labarile, Antonio Capogreco, Marco Gentile, Roberta Maselli, Jahnvi Dhar, Jayanta Samanta, Alessandro Repici, Cesare Hassan, Francesco Perri, Antonio Facciorusso
Expert Review of Medical Devices.2024; : 1. CrossRef
- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
- 1,728 View
- 130 Download
- 4 Web of Science
- 2 Crossref

Original Articles
-
Significance of rescue hybrid endoscopic submucosal dissection in difficult colorectal cases

- Hayato Yamaguchi, Masakatsu Fukuzawa, Takashi Kawai, Takahiro Muramatsu, Taisuke Matsumoto, Kumiko Uchida, Yohei Koyama, Akira Madarame, Takashi Morise, Shin Kono, Sakiko Naito, Naoyoshi Nagata, Mitsushige Sugimoto, Takao Itoi
- Clin Endosc 2023;56(6):778-789. Published online July 26, 2023
- DOI: https://doi.org/10.5946/ce.2022.268

-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub 
- Background
/Aims: Hybrid endoscopic submucosal dissection (ESD), in which an incision is made around a lesion and snaring is performed after submucosal dissection, has some advantages in colorectal surgery, including shorter procedure time and preventing perforation. However, its value for rescue resection in difficult colorectal ESD cases remains unclear. This study evaluated the utility of rescue hybrid ESD (RH-ESD).
Methods
We divided 364 colorectal ESD procedures into the conventional ESD group (C-ESD, n=260), scheduled hybrid ESD group (SH-ESD, n=69), and RH-ESD group (n=35) and compared their clinical outcomes.
Results
Resection time was significantly shorter in the following order: RH-ESD (149 [90–197] minutes) >C-ESD (90 [60–140] minutes) >SH-ESD (52 [29–80] minutes). The en bloc resection rate increased significantly in the following order: RH-ESD (48.6%), SH-ESD (78.3%), and C-ESD (97.7%). An analysis of factors related to piecemeal resection of RH-ESD revealed that the submucosal dissection rate was significantly lower in the piecemeal resection group (25% [20%–30%]) than in the en bloc resection group (40% [20%–60%]).
Conclusions
RH-ESD was ineffective in terms of curative resection because of the low en bloc resection rate, but was useful for avoiding surgery. -
Citations
Citations to this article as recorded by- Planned Hybrid Endoscopic Submucosal Dissection as Alternative for Colorectal Neoplasms: A Propensity Score-Matched Study
Yu-xin Zhang, Xun Liu, Fang Gu, Shi-gang Ding
Digestive Diseases and Sciences.2024; 69(3): 949. CrossRef - Understanding hybrid endoscopic submucosal dissection subtleties
João Paulo de Souza Pontual, Alexandre Moraes Bestetti, Diogo Turiani Hourneaux de Moura
Clinical Endoscopy.2023; 56(6): 738. CrossRef
- Planned Hybrid Endoscopic Submucosal Dissection as Alternative for Colorectal Neoplasms: A Propensity Score-Matched Study
- 2,224 View
- 122 Download
- 2 Web of Science
- 2 Crossref

-
Usefulness of the S-O clip for duodenal endoscopic submucosal dissection: a propensity score-matched study

- Ippei Tanaka, Dai Hirasawa, Hiroaki Saito, Junichi Akahira, Tomoki Matsuda
- Clin Endosc 2023;56(6):769-777. Published online May 24, 2023
- DOI: https://doi.org/10.5946/ce.2022.195

-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub 
- Background
/Aims: Endoscopic submucosal dissection (ESD) for superficial non-ampullary duodenal tumors (SNADETs) is associated with a high rate of en bloc resection. However, the technique for ESD remains challenging. Recent studies have demonstrated the effectiveness of S-O clips in colonic and gastric ESD. We evaluated the efficacy and safety of duodenal ESD using an S-O clip for SNADETs.
Methods
Consecutive patients who underwent ESD for SNADETs between January 2011 and December 2021 were retrospectively enrolled. Propensity score matching analysis was used to compare patients who underwent duodenal ESD with the S-O clip (S-O group) and those who underwent conventional ESD (control group). Intraoperative perforation rate was the primary outcome, while procedure time and R0 resection rate were the secondary outcomes.
Results
After propensity score matching, 16 pairs were created: 43 and 17 in the S-O and control groups, respectively. The intraoperative perforation rate in the S-O group was significantly lower than that in the control group (p=0.033). A significant difference was observed in the procedure time between the S-O and control groups (39±9 vs. 82±30 minutes, respectively; p=0.003).
Conclusions
The S-O clip reduced the intraoperative perforation rate and procedure time, which may be useful and effective in duodenal ESD.
- 2,400 View
- 105 Download

- Management of esophageal neoplasms by endoscopic submucosal dissection: experience over 100 consecutive procedures
- Josué Aliaga Ramos, Yoshinori Morita, Takashi Toyonaga, Danilo Carvalho, Moises Salgado Pedrosa, Vitor N. Arantes
- Clin Endosc 2023;56(5):613-622. Published online May 17, 2023
- DOI: https://doi.org/10.5946/ce.2022.245

-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: Endoscopic submucosal dissection (ESD) is currently considered the first-line treatment for the eradication of superficial neoplasms of the esophagus in Eastern countries. However, in the West, particularly in Latin America, the experience with esophageal ESD is still limited because of the high technical complexity required for its execution. This study aimed to present the results of the clinical application of ESD to manage superficial esophageal neoplasms in a Latin American center in over 100 consecutive cases.
Methods
This retrospective study included consecutive patients who underwent endoscopic ESD for superficial esophageal neoplasms between 2009 and 2022. The following clinical outcomes were assessed: en bloc, complete, and curative resection rates, local recurrence, adverse events, and procedure-related mortality.
Results
Esophageal ESD was performed mainly for squamous cell carcinoma (66.6%), high-grade intraepithelial neoplasia (17.1%), and adenocarcinoma (11.4%). En bloc and complete resection rates were 96.2% and 81.0%, respectively. The curative resection rate was 64.8%. Adverse events occurred in six cases (5.7%). Endoscopic follow-up was performed for an average period of 29.7 months.
Conclusions
ESD performed by trained operators is feasible, safe, and clinically effective for managing superficial neoplastic lesions of the esophagus in Latin America.
- 1,739 View
- 82 Download

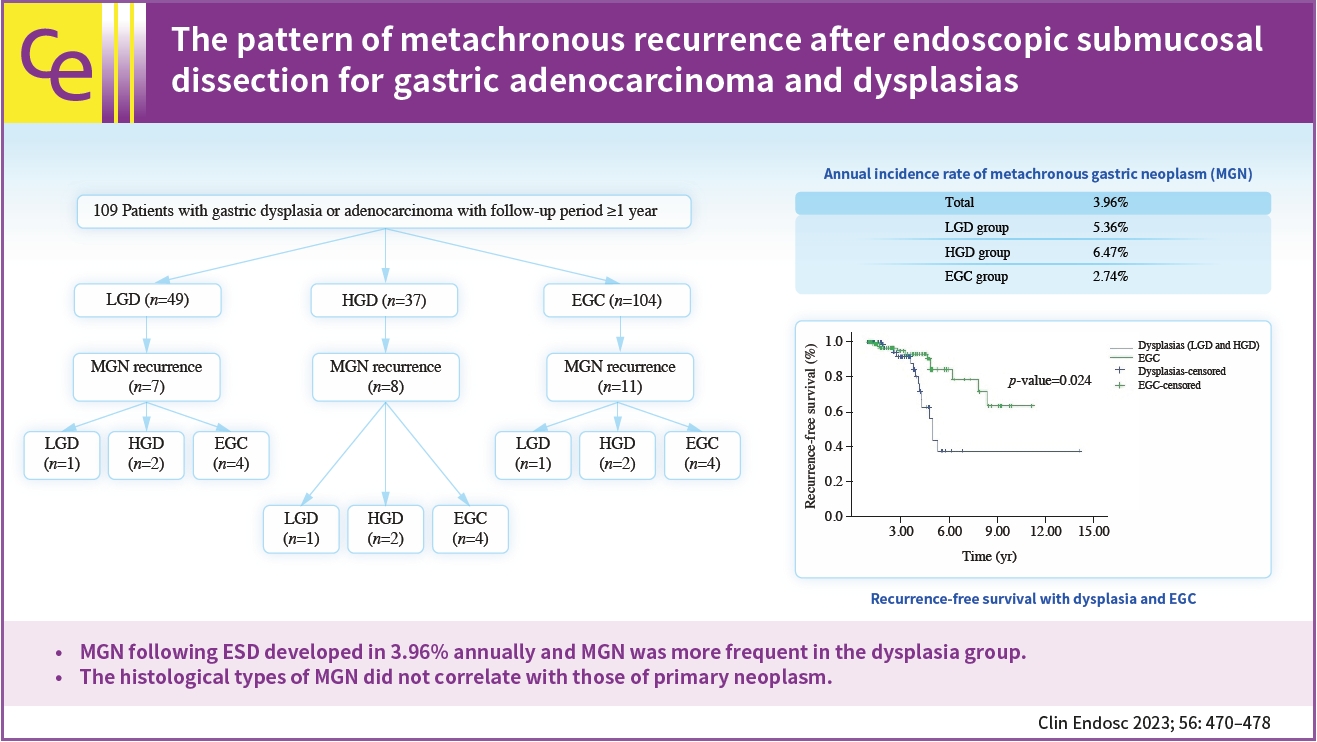
- The pattern of metachronous recurrence after endoscopic submucosal dissection for gastric adenocarcinoma and dysplasias
- Sunah Suk, Yeon Joo Seo, Dae Young Cheung, Han Hee Lee, Jin Il Kim, Soo-Heon Park
- Clin Endosc 2023;56(4):470-478. Published online April 18, 2023
- DOI: https://doi.org/10.5946/ce.2022.259
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: Metachronous recurrence incidences and risk factors following endoscopic submucosal dissection (ESD) for gastric adenocarcinoma and dysplasias were investigated.
Methods
Retrospective review of electronic medical records of patients who underwent gastric ESD at The Catholic University of Korea, Yeouido St. Mary’s Hospital.
Results
A total of 190 subjects were enrolled for analysis during the study period. The mean age was 64.4 years-old and the male sex occupied 73.7%. The mean observation period following ESD was 3.45 years. The annual incidence rate of metachronous gastric neoplasms (MGN) was about 3.96%. The annual incidence rate was 5.36% for the low-grade dysplasia group, 6.47% for the high-grade dysplasia group, and 2.74% for the EGC group. MGN was more frequent in the dysplasia group than in the EGC group (p<0.05). For those with MGN development, the mean time interval from ESD to MGN was 4.1 (±1.8) years. By using the Kaplan–Meier model, the estimated mean MGN free survival time was 9.97 years (95% confidence interval, 8.53–11.40) The histological types of MGN were not related to the primary histology types.
Conclusions
MGN following ESD developed in 3.96% annually and MGN was more frequent in the dysplasia group. The histological types of MGN did not correlate with those of primary neoplasm. -
Citations
Citations to this article as recorded by- Research Progress in ESD Treatment of Early Gastric Cancer
亭 贺
Advances in Clinical Medicine.2024; 14(02): 4201. CrossRef
- Research Progress in ESD Treatment of Early Gastric Cancer
- 1,366 View
- 75 Download
- 1 Crossref

- Endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma: long-term follow-up in a Western center
- Andreas Probst, Alanna Ebigbo, Stefan Eser, Carola Fleischmann, Tina Schaller, Bruno Märkl, Stefan Schiele, Bernd Geissler, Gernot Müller, Helmut Messmann
- Clin Endosc 2023;56(1):55-64. Published online January 13, 2023
- DOI: https://doi.org/10.5946/ce.2022.093
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic submucosal dissection (ESD) has been established as a treatment modality for superficial esophageal squamous cell carcinoma (ESCC). Long-term follow-up data are lacking in Western countries. The aim of this study was to analyze long-term survival in a Western center.
Methods
Patients undergoing ESD for ESCC were included. The analysis was performed retrospectively using a prospectively collected database.
Results
R0 resection rate was 96.7% (59/61 lesions in 58 patients). Twenty-seven patients (46.6%) fulfilled the curative resection criteria (M1/M2) (group A), 11 patients (19.0%) had M3 lesions without lymphovascular invasion (LVI) (group B), and 20 patients (34.5%) had lesions with submucosal invasion or LVI (group C). Additional treatment was recommended after non-curative resection. It was not performed in 20/31 patients (64.5%), mainly because of comorbidities (75%). Twenty-nine out of 58 (50.0%) patients died during a mean follow-up of 3.7 years. Death was related to ESCC in 17.2% (5/29) of patients. The disease-specific survival rate after curative resection was 100%. Overall survival rates after 5 years were 61.5%, 63.6% and 28.1% for groups A, B, and C, respectively. The overall survival was significantly worse after non-curative resection (p=0.038).
Conclusions
Non-curative resection is frequent after ESD for ESCC in Western patients. The long-term prognosis is limited and mainly determined by comorbidity. Early diagnosis and pre-interventional assessments need to be improved. -
Citations
Citations to this article as recorded by- Endoscopic submucosal dissection for early esophageal squamous cell carcinoma: long-term results from a Western cohort
Ilse N. Beaufort, Charlotte N. Frederiks, Anouk Overwater, Lodewijk A.A. Brosens, Arjun D. Koch, Roos E. Pouw, Jacques J.G.H.M. Bergman, Bas L.A.M. Weusten
Endoscopy.2024; 56(05): 325. CrossRef - Endoscopic Submucosal Dissection for Esophageal Cancer: Current and Future
Yuki Okubo, Ryu Ishihara
Life.2023; 13(4): 892. CrossRef
- Endoscopic submucosal dissection for early esophageal squamous cell carcinoma: long-term results from a Western cohort
- 2,266 View
- 136 Download
- 2 Web of Science
- 2 Crossref

- Endoscopic submucosal dissection in colorectal neoplasia performed with a waterjet system-assisted knife: higher en-bloc resection rate than conventional technique
- Paolo Cecinato, Matteo Lucarini, Francesco Azzolini, Mariachiara Campanale, Fabio Bassi, Annalisa Cippitelli, Romano Sassatelli
- Clin Endosc 2022;55(6):775-783. Published online October 6, 2022
- DOI: https://doi.org/10.5946/ce.2022.099

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Colorectal endoscopic submucosal dissection (ESD) is burdened by its associated high risk of adverse events and long procedure time. Recently, a waterjet-assisted knife was introduced to simplify and speed up the procedure. The aim of this study was to evaluate the efficacy and safety of waterjet-assisted ESD (WESD) compared to that of the conventional ESD (CESD) technique.
Methods
The charts of 254 consecutive patients who underwent colorectal ESD between January 2014 and February 2021 for colorectal neoplasms were analyzed. The primary outcome was the en-bloc resection rate. Secondary outcomes were complete and curative resection rates, the need to switch to a hybrid ESD, procedure speed, the adverse event rates, and the recurrence rates.
Results
Approximately 174 neoplasias were considered, of which, 123 were removed by WESD and 51 by CESD. The en-bloc resection rate was higher in the WESD group (94.3% vs. 84.3%). Complete resection rates and curative resection rates were similar. The need to switch to a hybrid ESD was greater during CESD (39.2% vs. 13.8%). Procedure speed and adverse event rates were similar. During follow-up, one recurrence occurred after a WESD.
Conclusions
WESD allows a high rate of en-bloc resections and less frequently requires a rescue switch to the hybrid ESD compared to CESD. -
Citations
Citations to this article as recorded by- Is there a best choice of equipment for colorectal endoscopic submucosal dissection?
Francesco Cocomazzi, Sonia Carparelli, Nunzia Labarile, Antonio Capogreco, Marco Gentile, Roberta Maselli, Jahnvi Dhar, Jayanta Samanta, Alessandro Repici, Cesare Hassan, Francesco Perri, Antonio Facciorusso
Expert Review of Medical Devices.2024; : 1. CrossRef - Colorectal Endoscopic Submucosal Dissection: Performance of a Novel Hybrid-Technology Knife in an Animal Trial
Jérémie Jacques, Horst Neuhaus, Markus D. Enderle, Ulrich Biber, Walter Linzenbold, Martin Schenk, Kareem Khalaf, Alessandro Repici
Diagnostics.2023; 13(21): 3347. CrossRef
- Is there a best choice of equipment for colorectal endoscopic submucosal dissection?
- 2,025 View
- 127 Download
- 3 Web of Science
- 2 Crossref

-
Intralesional steroid infusion using a spray tube to prevent stenosis after endoscopic submucosal dissection of esophageal cancer

- Atsushi Goto, Takeshi Okamoto, Ryo Ogawa, Kouichi Hamabe, Shinichi Hashimoto, Jun Nishikawa, Taro Takami
- Clin Endosc 2022;55(4):520-524. Published online July 28, 2022
- DOI: https://doi.org/10.5946/ce.2021.262
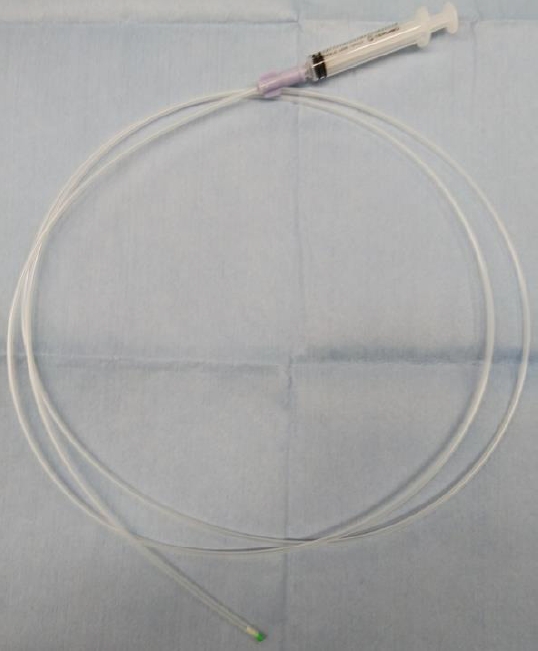
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Intralesional steroid injections have been administered as prophylaxis for stenosis after esophageal endoscopic submucosal dissection. However, this method carries a risk of potential complications such as perforation because a fine needle is used to directly puncture the postoperative ulcer. We devised a new method of steroid intralesional infusion using a spray tube and evaluated its efficacy and safety.
Methods
Intralesional steroid infusion using a spray tube was performed on 27 patients who underwent endoscopic submucosal dissection for superficial esophageal cancer with three-quarters or more of the lumen circumference resected. The presence or absence of stenosis, complications, and the number of endoscopic balloon dilations (EBDs) performed were evaluated after treatment.
Results
Although stenosis was not observed in 22 of the 27 patients, five patients had stenosis and dysphagia requiring EBD. The stenosis in these five patients was relieved after four EBDs. No complications related to intralesional steroid infusion using the spray tube were observed.
Conclusions
Intralesional steroid infusion using a spray tube is a simple and safe technique that is adequately effective in preventing stenosis Clinical trial number (UMIN000037567).
- 2,736 View
- 133 Download
- 1 Web of Science

Review
- Endoscopic treatment for rectal neuroendocrine tumor: which method is better?
- Seung Min Hong, Dong Hoon Baek
- Clin Endosc 2022;55(4):496-506. Published online July 11, 2022
- DOI: https://doi.org/10.5946/ce.2022.115

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Recently, research on rectal neuroendocrine tumors (NETs) has increased during the last few decades. Rectal NETs measuring <10 mm without atypical features and confined to the submucosal layer have only 1% risk of metastasis, and the long-term survival probability of patients without metastasis at the time of diagnosis is approximately 100%. Therefore, the current guidelines suggest endoscopic resection of rectal NETs of <10 mm is regarded as a safe therapeutic option. However, there are currently no clear recommendations for technique selection for endoscopic resection. The choice of treatment modality for rectal NETs should be based on the lesion size, endoscopic characteristics, grade of differentiation, depth of vertical involvement, lymphovascular invasion, and risk of metastasis. Moreover, the complete resection rate, complications, and experience at the center should be considered. Modified endoscopic mucosal resection is the most suitable resection method for rectal NETs of <10 mm, because it is an effective and safe technique that is relatively simple and less time-consuming compared with endoscopic submucosal dissection. Endoscopic submucosal dissection should be considered when the tumor size is >10 mm, suctioning is not possible due to fibrosis in the lesion, or when the snaring for modified endoscopic mucosal resection does not work well.
-
Citations
Citations to this article as recorded by- Treatment strategy and post‐treatment management of colorectal neuroendocrine tumor
Masau Sekiguchi, Takahisa Matsuda, Yutaka Saito
DEN Open.2024;[Epub] CrossRef - Comparison of endoscopic resection therapies for rectal neuroendocrine tumors
Meijiao Lu, Hongxia Cui, Mingjie Qian, Yating Shen, Jianhong Zhu
Minimally Invasive Therapy & Allied Technologies.2024; : 1. CrossRef - A Review of Colonoscopy in Intestinal Diseases
Seung Min Hong, Dong Hoon Baek
Diagnostics.2023; 13(7): 1262. CrossRef - Treatment of localized well-differentiated rectal neuroendocrine tumors: A focused review
Shigenobu Emoto, Hiroaki Nozawa, Kazuhito Sasaki, Koji Murono, Hiroyuki Matsuzaki, Yuichiro Yokoyama, Shinya Abe, Yuzo Nagai, Yuichiro Yoshioka, Takahide Shinagawa, Hirofumi Sonoda, Soichiro Ishihara
Formosan Journal of Surgery.2023; 56(3): 73. CrossRef - Clinical application of endoscopic ultrasonography in the management of rectal neuroendocrine tumors
Soo-Young Na, Seong Jung Kim, Hyoun Woo Kang
International Journal of Gastrointestinal Intervention.2023; 12(3): 105. CrossRef - Endoscopic submucosal dissection coupled with �modified clip coupled with elastic ring� traction removing rectal neuroendocrine tumor
Jing Zhou, Li-Sheng Wang, De-Feng Li, Rui-Yue Shi
Revista Española de Enfermedades Digestivas.2023;[Epub] CrossRef
- Treatment strategy and post‐treatment management of colorectal neuroendocrine tumor
- 3,994 View
- 285 Download
- 6 Web of Science
- 6 Crossref

Original Articles
-
Efficacy of the pocket-creation method with a traction device in endoscopic submucosal dissection for residual or recurrent colorectal lesions

- Daisuke Ide, Tomohiko Richard Ohya, Mitsuaki Ishioka, Yuri Enomoto, Eisuke Nakao, Yuki Mitsuyoshi, Junki Tokura, Keigo Suzuki, Seiichi Yakabi, Chihiro Yasue, Akiko Chino, Masahiro Igarashi, Akio Nakashima, Masayuki Saruta, Shoichi Saito, Junko Fujisaki
- Clin Endosc 2022;55(5):655-664. Published online May 31, 2022
- DOI: https://doi.org/10.5946/ce.2022.009
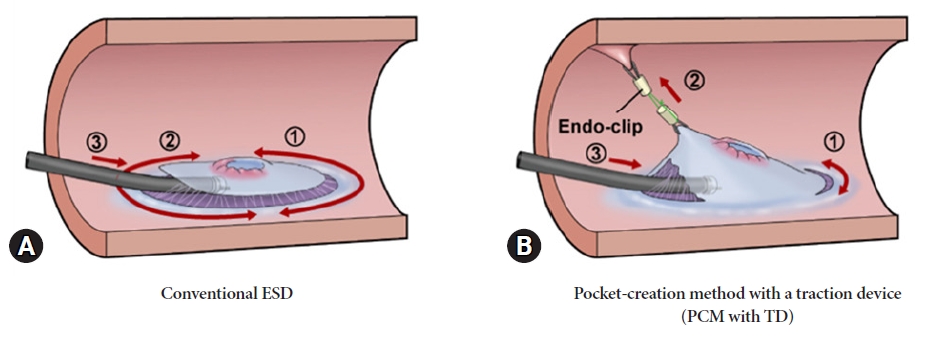
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic submucosal dissection (ESD) for residual or recurrent colorectal lesions after incomplete resection is challenging because of severe fibrosis. This study aimed to compare the efficacy of the pocket-creation method (PCM) with a traction device (TD) with that of conventional ESD for residual or recurrent colorectal lesions.
Methods
We retrospectively studied 72 patients with residual or recurrent colorectal lesions resected using ESD. Overall, 31 and 41 lesions were resected using PCM with TD and conventional ESD methods, respectively. We compared patient background and treatment outcomes between the PCM with TD and conventional ESD groups, respectively. The primary endpoints were en bloc resection and R0 resection rates. The secondary endpoints were the dissection speed and incidence of adverse events.
Results
En bloc resection was feasible in all cases with PCM with TD, but failed in 22% of cases of conventional ESD. The R0 resection rates for PCM with TD and conventional ESD were 97% and 66%, respectively. Dissection was significantly faster in the PCM with TD group (13.0 vs. 7.9 mm2/min). Perforation and postoperative bleeding were observed in one patient in each group.
Conclusions
PCM with TD is an effective method for treating residual or recurrent colorectal lesions after incomplete resection. -
Citations
Citations to this article as recorded by- Novel adjustable traction “noose knot” method for colorectal endoscopic submucosal dissection
Junki Tokura, Daisuke Ide, Keigo Suzuki, Chihiro Yasue, Akiko Chino, Masahiro Igarashi, Shoichi Saito
Endoscopy.2024; 56(S 01): E55. CrossRef - Efficacy and safety of salvage endoscopy in the treatment of residual or recurrent colorectal neoplasia after endoscopic resection: a systematic review and meta-analysis
Juan Du, Ting Zhang, Lei Wang, Hao Zhang, Wenquan Yi
Surgical Endoscopy.2024; 38(6): 3027. CrossRef - Is there a best choice of equipment for colorectal endoscopic submucosal dissection?
Francesco Cocomazzi, Sonia Carparelli, Nunzia Labarile, Antonio Capogreco, Marco Gentile, Roberta Maselli, Jahnvi Dhar, Jayanta Samanta, Alessandro Repici, Cesare Hassan, Francesco Perri, Antonio Facciorusso
Expert Review of Medical Devices.2024; : 1. CrossRef - Difficult colorectal polypectomy: Technical tips and recent advances
Sukit Pattarajierapan, Hiroyuki Takamaru, Supakij Khomvilai
World Journal of Gastroenterology.2023; 29(17): 2600. CrossRef - Endoscopic full-thickness resection versus endoscopic submucosal dissection for challenging colorectal lesions: a randomized trial
Gianluca Andrisani, Cesare Hassan, Margherita Pizzicannella, Francesco Pugliese, Massimiliano Mutignani, Chiara Campanale, Giorgio Valerii, Carmelo Barbera, Giulio Antonelli, Francesco Maria Di Matteo
Gastrointestinal Endoscopy.2023; 98(6): 987. CrossRef - Combination of endoscopic submucosal dissection techniques, a practical solution for difficult cases
Dong-Hoon Yang
Clinical Endoscopy.2022; 55(5): 626. CrossRef
- Novel adjustable traction “noose knot” method for colorectal endoscopic submucosal dissection
- 4,545 View
- 304 Download
- 7 Web of Science
- 6 Crossref

- Comparison between a novel core knife and the conventional IT knife 2 for endoscopic submucosal dissection of gastric mucosal lesions
- Myeongsoon Park, Jin Wook Lee, Dong Woo Shin, Jungseok Kim, Yoo Jin Lee, Ju Yup Lee, Kwang Bum Cho
- Clin Endosc 2022;55(6):767-774. Published online May 25, 2022
- DOI: https://doi.org/10.5946/ce.2022.002

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Few studies have compared the performances of endoscopic knives. This study aimed to compare the therapeutic outcomes of a novel core knife and the conventional IT knife 2 for endoscopic submucosal dissection (ESD) of gastric mucosal lesions.
Methods
This prospective, non-inferiority trial included patients diagnosed with gastric adenoma or early-stage adenocarcinoma at Keimyung University Dongsan Hospital between June and November 2020. The patients were randomly assigned to either the core knife or the IT knife 2 group. The operators and assistants scored the knives’ grip convenience and cutting abilities.
Results
A total of 39 patients were enrolled (core knife group, 20 patients; IT knife 2 group, 19 patients). There were no significant between-group differences in operator-assessed grip convenience (9.600 vs. 9.526, p=0.753), cutting ability (9.600 vs. 9.105, p=0.158), or assistant-assessed grip convenience (9.500 vs. 9.368, p=0.574).
Conclusions
The core knife achieved therapeutic outcomes that were comparable to those of the IT knife 2 for ESD of gastric mucosal lesions.
- 2,203 View
- 114 Download

-
Feasibility and safety of endoscopic submucosal dissection for lesions in proximity to a colonic diverticulum

- Nobuaki Ikezawa, Takashi Toyonaga, Shinwa Tanaka, Tetsuya Yoshizaki, Toshitatsu Takao, Hirofumi Abe, Hiroya Sakaguchi, Kazunori Tsuda, Satoshi Urakami, Tatsuya Nakai, Taku Harada, Kou Miura, Takahisa Yamasaki, Stuart Kostalas, Yoshinori Morita, Yuzo Kodama
- Clin Endosc 2022;55(3):417-425. Published online May 12, 2022
- DOI: https://doi.org/10.5946/ce.2021.245
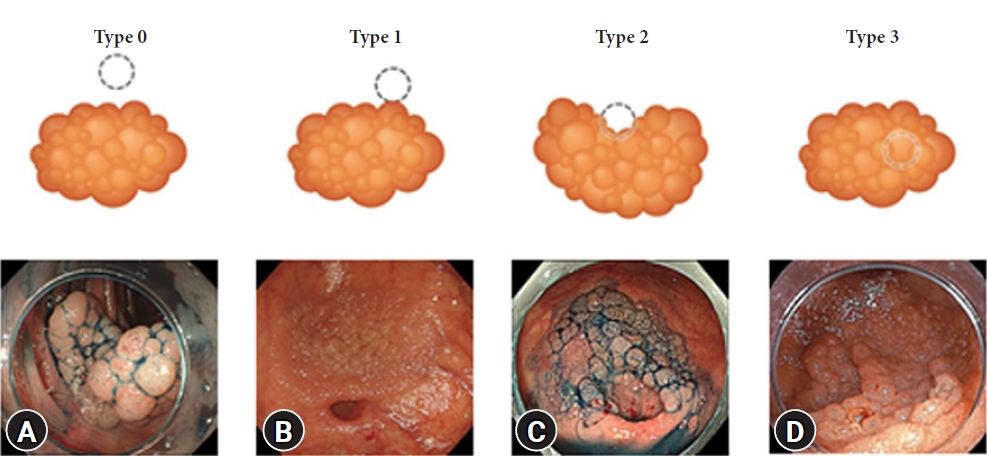
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic submucosal dissection (ESD) for diverticulum-associated colorectal lesions is generally contraindicated because of the high risk of perforation. Several studies on patients with such lesions treated with ESD have been reported recently. However, the feasibility and safety of ESD for lesions in proximity to a colonic diverticulum (D-ESD) have not been fully clarified. The aim of this study was to evaluate the feasibility and safety of D-ESD.
Methods
D-ESD was defined as ESD for lesions within approximately 3 mm of a diverticulum. Twenty-six consecutive patients who underwent D-ESD were included. Two strategic approaches were used depending on whether submucosal dissection of the diverticulum-related part was required (strategy B) or not (strategy A). Treatment outcomes and adverse events associated with each strategy were analyzed.
Results
The en bloc resection rate was 96.2%. The rates of R0 and curative resection in strategies A and B were 80.8%, 73.1%, 84.6%, and 70.6%, respectively. Two cases of intraoperative perforation and one case of delayed perforation occurred. The delayed perforation case required emergency surgery, but the other cases were managed conservatively.
Conclusions
D-ESD may be a feasible treatment option. However, it should be performed in a high-volume center by expert hands because it requires highly skilled endoscopic techniques. -
Citations
Citations to this article as recorded by- Endoscopic submucosal dissection for diverticulum using combination of countertraction and circumferential-inversion method
Hiroshi Takayama, Yoshinori Morita, Toshitatsu Takao, Douglas Motomura, Madoka Takao, Takashi Toyonaga, Yuzo Kodama
Endoscopy.2024; 56(S 01): E91. CrossRef - Traction-assisted endoscopic submucosal dissection for resection of ileocecal valve neoplasia: a French retrospective multicenter case series
Clara Yzet, Timothée Wallenhorst, Jérémie Jacques, Mariana Figueiredo Ferreira, Jérôme Rivory, Florian Rostain, Louis-Jean Masgnaux, Jean Grimaldi, Romain Legros, Pierre Lafeuille, Jérémie Albouys, Fabien Subtil, Marion Schaefer, Mathieu Pioche
Endoscopy.2024;[Epub] CrossRef - The role of cap-assisted endoscopy and its future implications
Sol Kim, Bo-In Lee
Clinical Endoscopy.2024; 57(3): 293. CrossRef - Successful planned piecemeal endoscopic resection using gel immersion and an over-the-scope clip for a lesion extensively extended into the colonic diverticulum
Tomoaki Tashima, Takahiro Muramatsu, Tomonori Kawasaki, Tsubasa Ishikawa, Shomei Ryozawa
VideoGIE.2023; 8(4): 167. CrossRef - Future therapeutic implications of new molecular mechanism of colorectal cancer
Sen Lu, Cheng-You Jia, Jian-She Yang
World Journal of Gastroenterology.2023; 29(16): 2359. CrossRef - Iatrogenic colorectal perforation caused by a clip
Hirotaka Oura, Yasuki Hatayama, Erika Nomura, Harutoshi Sugiyama, Daisuke Murakami, Makoto Arai, Takayoshi Nishino
Endoscopy.2023; 55(S 01): E1091. CrossRef
- Endoscopic submucosal dissection for diverticulum using combination of countertraction and circumferential-inversion method
- 3,732 View
- 171 Download
- 4 Web of Science
- 6 Crossref

- Outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms in patients with liver cirrhosis
- Young Kwon Choi, Jin Hee Noh, Do Hoon Kim, Hee Kyong Na, Ji Yong Ahn, Jeong Hoon Lee, Kee Wook Jung, Kee Don Choi, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung
- Clin Endosc 2022;55(3):381-389. Published online April 20, 2022
- DOI: https://doi.org/10.5946/ce.2021.242

-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: The treatment of superficial esophageal neoplasms (SENs) in cirrhotic patients is challenging and rarely investigated. We evaluated the outcomes of endoscopic submucosal dissection (ESD) to determine the efficacy and safety of treating SENs in patients with liver cirrhosis.
Methods
The baseline characteristics and treatment outcomes of patients who underwent ESD for SENs between November 2005 and December 2017 were retrospectively reviewed.
Results
ESD was performed in 437 patients with 481 SENs, including 15 cirrhotic patients with 17 SENs. En bloc resection (88.2% vs. 97.0%) and curative resection (64.7% vs. 78.9%) rates were not different between the cirrhosis and non-cirrhosis groups (p=0.105 and p=0.224, respectively). Bleeding was more common in cirrhotic patients (p=0.054), and all cases were successfully controlled endoscopically. The median procedure and hospitalization duration did not differ between the groups. Overall survival was lower in cirrhotic patients (p=0.003), while disease-specific survival did not differ between the groups (p=0.85).
Conclusions
ESD could be a safe and effective treatment option for SENs in patients with cirrhosis. Detailed preprocedural assessments are needed, including determination of liver function, esophageal varix status, and remaining life expectancy, to identify patients who will obtain the greatest benefit. -
Citations
Citations to this article as recorded by- Clinical outcomes of endoscopic submucosal dissection for esophageal squamous cell carcinoma with esophageal varices: Multicenter retrospective study
Yosuke Toya, Waku Hatta, Tomohiro Shimada, Tamotsu Matsuhashi, Takeharu Shiroki, Yu Sasaki, Tetsuya Tatsuta, Jun Nakamura, Norihiro Hanabata, Yohei Horikawa, Ko Nagino, Tomoyuki Koike, Atsushi Masamune, Yoshihiro Harada, Tetsuya Ohira, Katsunori Iijima, Y
Digestive Endoscopy.2024; 36(3): 314. CrossRef - Risk associated with endoscopic treatment of early upper gastrointestinal cancer in patients with liver cirrhosis and management strategies
Yu-Yong Tan, Yu-Min Qing, Jian Gong, De-Liang Liu
World Chinese Journal of Digestology.2024; 32(2): 102. CrossRef - Endoscopic submucosal dissection for early cancers or precancerous lesions of the upper GI tract in cirrhotic patients with esophagogastric varices: 10-year experience from a large tertiary center in China
Shuai Zhang, Ying-Di Liu, Ning-Li Chai, Yi Yao, Fei Gao, Bo Liu, Zhan-Di He, Lu Bai, Xin Huang, Chao Gao, En-Qiang Linghu, Lian-Yong Li
Gastrointestinal Endoscopy.2023; 97(6): 1031. CrossRef - Endoscopic Submucosal Dissection for Treatment of Early-Stage Cancer or Precancerous Lesion in the Upper Gastrointestinal Tract in Patients with Liver Cirrhosis
Yuyong Tan, Yumin Qing, Deliang Liu, Jian Gong
Journal of Clinical Medicine.2023; 12(20): 6509. CrossRef - Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasia in close proximity to esophageal varices: a multicenter international experience
Shruti Mony, Bing Hu, Abel Joseph, Hiroyuki Aihara, Lorenzo Ferri, Amit Bhatt, Amit Mehta, Peng-Sheng Ting, Alex Chen, Andrew Kalra, Jad Farha, Manabu Onimaru, Long He, Qi Luo, Andrew Y. Wang, Haruhiro Inoue, Saowanee Ngamruengphong
Endoscopy.2023;[Epub] CrossRef
- Clinical outcomes of endoscopic submucosal dissection for esophageal squamous cell carcinoma with esophageal varices: Multicenter retrospective study
- 2,703 View
- 129 Download
- 4 Web of Science
- 5 Crossref

- Efficacy and Safety of Endoscopic Submucosal Dissection for Superficial Gastric Neoplasms: A Latin American Cohort Study
- Fernando Palacios-Salas, Harold Benites-Goñi, Luis Marin-Calderón, Paulo Bardalez-Cruz, Jorge Vásquez-Quiroga, Edgar Alva-Alva, Bryan Medina-Morales, Jairo Asencios-Cusihuallpa
- Clin Endosc 2022;55(2):248-255. Published online November 12, 2021
- DOI: https://doi.org/10.5946/ce.2021.192

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic submucosal dissection (ESD) is the preferred technique for treating early gastric cancer (EGC). However, very few studies have been conducted in South America. This study aimed to assess the efficacy and safety of ESD for EGC.
Methods
We analyzed data from a prospective cohort from 2013 to 2020. A total of 152 superficial gastric neoplasms that fulfilled the absolute or expanded criteria for ESD were included. Outcomes were en bloc, R0, and curative resection rates, incidence of adverse events, and length of procedure.
Results
The age of the enrolled patients was 68.4±11.3 years. The number of included patients based on the absolute and expanded indications was 150 and 2, respectively. En bloc, R0, and curative resections were achieved in 98.0%, 96.1%, and 89.5% of the cases, respectively. Bleeding and perforation were reported in 5.9% and 6.6% of the cases, respectively. Histopathological examination revealed lowgrade dysplasia, high-grade dysplasia, well-differentiated adenocarcinoma, and poorly differentiated adenocarcinoma in 13, 20, 117, and 2 cases, respectively.
Conclusions
Our study shows that ESD performed by properly trained endoscopists in reference centers is safe and effective, with comparable therapeutic outcomes to those reported in the Eastern series. -
Citations
Citations to this article as recorded by- Short-Term and Long-Term Outcomes of Liver Cirrhosis in Gastric Neoplasm Patients Undergoing Endoscopic Submucosal Dissection
Xu-Rui Liu, Lian-Shuo Li, Fei Liu, Zi-Wei Li, Xiao-Yu Liu, Wei Zhang, Dong Peng
Journal of Laparoendoscopic & Advanced Surgical Techniques.2023; 33(7): 640. CrossRef - Endoscopic submucosal dissection for early gastric cancer: It is time to consider the quality of its outcomes
Gwang Ha Kim
World Journal of Gastroenterology.2023; 29(43): 5800. CrossRef - Endoscopic diagnosis of early gastric cancer
Dong Chan Joo, Gwang Ha Kim
Journal of the Korean Medical Association.2022; 65(5): 267. CrossRef - Therapeutic approach to non-curative resection after endoscopic treatment in early gastric cancer
Eun Jeong Gong, Chang Seok Bang
Journal of the Korean Medical Association.2022; 65(5): 284. CrossRef
- Short-Term and Long-Term Outcomes of Liver Cirrhosis in Gastric Neoplasm Patients Undergoing Endoscopic Submucosal Dissection
- 3,965 View
- 218 Download
- 5 Web of Science
- 4 Crossref

- Clinical Impact of Different Reconstruction Methods on Remnant Gastric Cancer at the Anastomotic Site after Distal Gastrectomy
- Kei Matsumoto, Shinwa Tanaka, Takashi Toyonaga, Nobuaki Ikezawa, Mari Nishio, Masanao Uraoka, Tomoatsu Yoshihara, Hiroya Sakaguchi, Hirofumi Abe, Tetsuya Yoshizaki, Madoka Takao, Toshitatsu Takao, Yoshinori Morita, Hiroshi Yokozaki, Yuzo Kodama
- Clin Endosc 2022;55(1):86-94. Published online August 13, 2021
- DOI: https://doi.org/10.5946/ce.2021.084
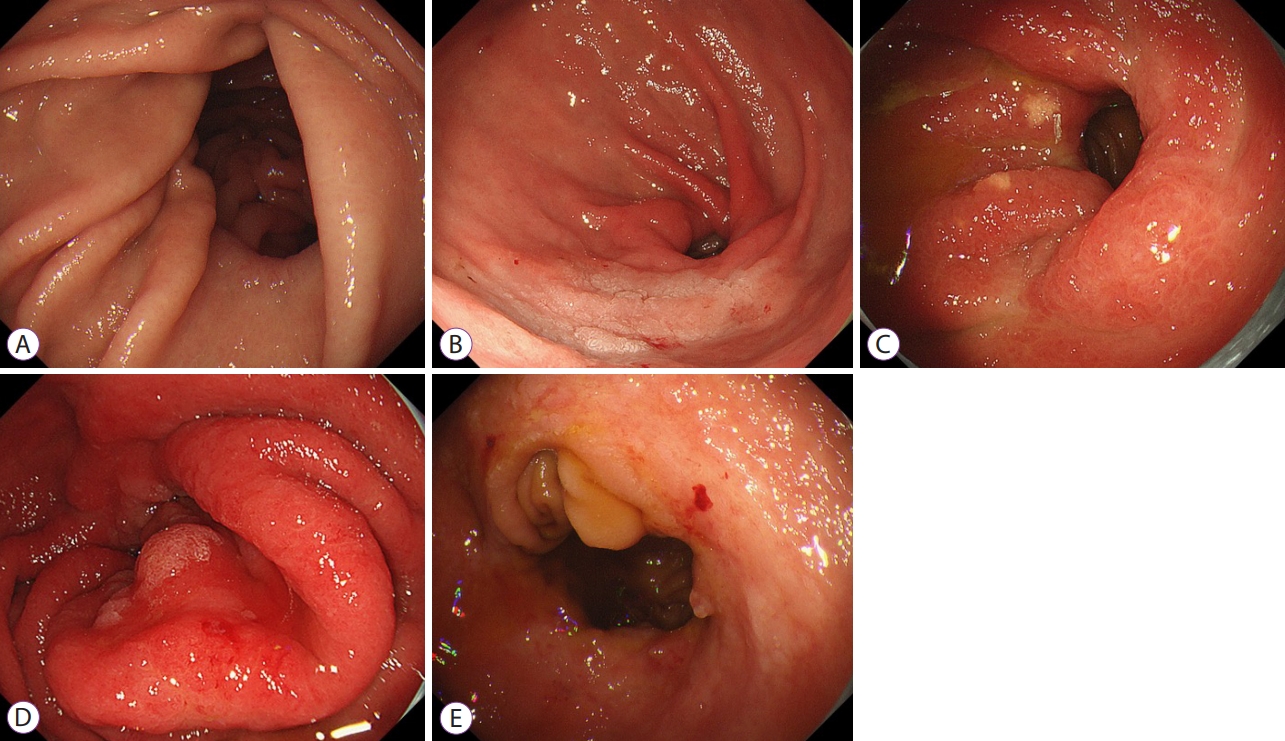
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: The anastomotic site after distal gastrectomy is the area most affected by duodenogastric reflux. Different reconstruction methods may affect the lesion characteristics and treatment outcomes of remnant gastric cancers at the anastomotic site. We retrospectively investigated the clinicopathologic and endoscopic submucosal dissection outcomes of remnant gastric cancers at the anastomotic site.
Methods
We recruited 34 consecutive patients who underwent endoscopic submucosal dissection for remnant gastric cancer at the anastomotic site after distal gastrectomy. Clinicopathology and treatment outcomes were compared between the Billroth II and non-Billroth II groups.
Results
The tumor size in the Billroth II group was significantly larger than that in the non-Billroth II group (22 vs. 19 mm; p=0.048). More severe gastritis was detected endoscopically in the Billroth II group (2 vs. 1.33; p=0.0075). Moreover, operation time was longer (238 vs. 121 min; p=0.004) and the frequency of bleeding episodes was higher (7.5 vs. 3.1; p=0.014) in the Billroth II group.
Conclusions
Compared to remnant gastric cancers in non-Billroth II patients, those in the Billroth II group had larger lesions with a background of severe remnant gastritis. Endoscopic submucosal dissection for remnant gastric cancers in Billroth II patients involved longer operative times and more frequent bleeding episodes than that in patients without Billroth II.
- 4,131 View
- 144 Download

- Clinical Outcomes and Adverse Events of Gastric Endoscopic Submucosal Dissection of the Mid to Upper Stomach under General Anesthesia and Monitored Anesthetic Care
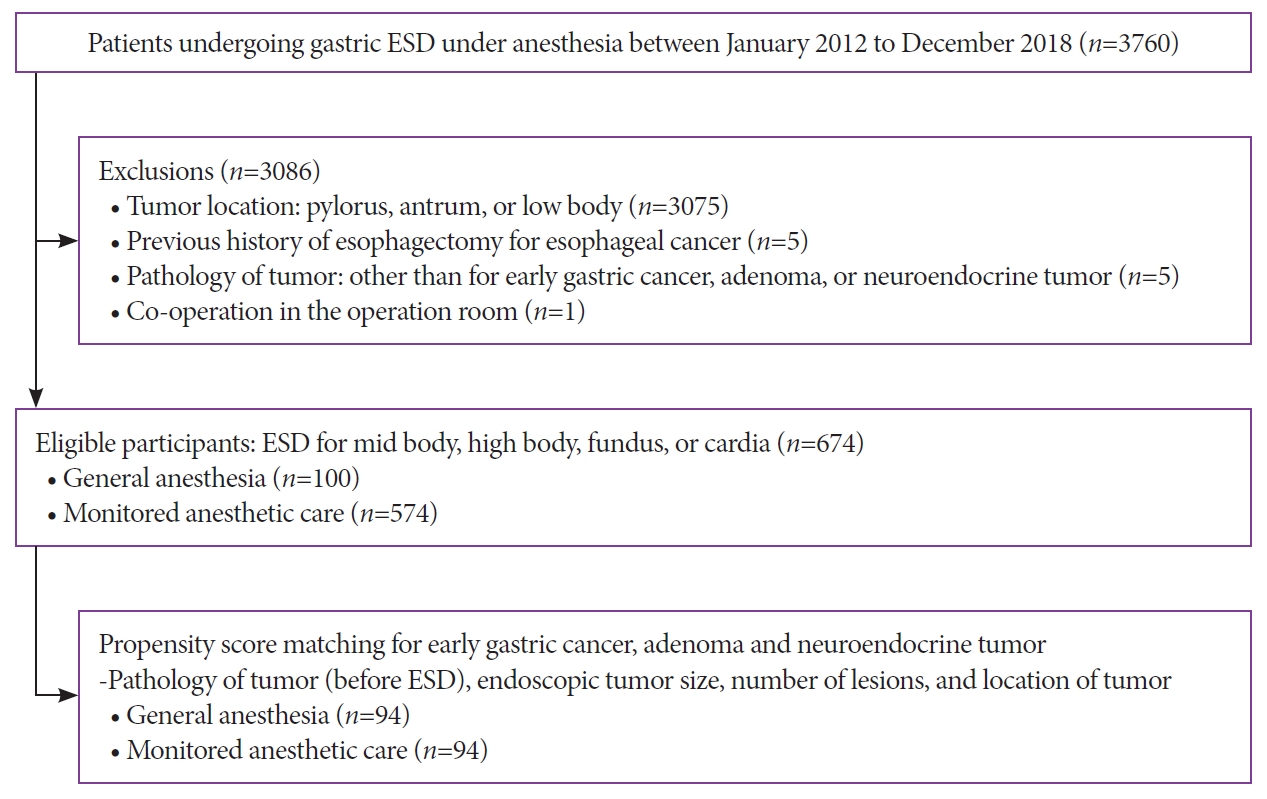
- Jong-In Chang, Tae Jun Kim, Na Young Hwang, Insuk Sohn, Yang Won Min, Hyuk Lee, Byung-Hoon Min, Jun Haeng Lee, Poong-Lyul Rhee, Jae J Kim
- Clin Endosc 2022;55(1):77-85. Published online July 5, 2021
- DOI: https://doi.org/10.5946/ce.2021.002
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic submucosal dissection (ESD) of gastric tumors in the mid-to-upper stomach is a technically challenging procedure. This study compared the therapeutic outcomes and adverse events of ESD of tumors in the mid-to-upper stomach performed under general anesthesia (GA) or monitored anesthesia care (MAC).
Methods
Between 2012 and 2018, 674 patients underwent ESD for gastric tumors in the midbody, high body, fundus, or cardia (100 patients received GA; 574 received MAC). The outcomes of the propensity score (PS)-matched (1:1) patients receiving either GA or MAC were analyzed.
Results
The PS matching identified 94 patients who received GA and 94 patients who received MAC. Both groups showed high rates of en bloc resection (GA, 95.7%; MAC, 97.9%; p=0.68) and complete resection (GA, 81.9%; MAC, 84.0%; p=0.14). There were no significant differences between the rates of adverse events (GA, 16.0%; MAC, 8.5%; p=0.18) in the anesthetic groups. Logistic regression analysis indicated that the method of anesthesia did not affect the rates of complete resection or adverse events.
Conclusions
ESD of tumors in the mid-to-upper stomach at our high-volume center had good outcomes, regardless of the method of anesthesia. Our results demonstrate no differences between the efficacies and safety of ESD performed under MAC and GA. -
Citations
Citations to this article as recorded by- General Anesthesia and Endoscopic Upper Gastrointestinal Tumor Resection
Seung Hyun Kim
Journal of Digestive Cancer Research.2023; 11(3): 125. CrossRef - Is General Anesthesia Needed in Endoscopic Submucosal Dissection for Lesions Located in the Mid to Upper Stomach?
Prasit Mahawongkajit, Jirawat Swangsri
Clinical Endoscopy.2022; 55(1): 43. CrossRef - Therapeutic approach to non-curative resection after endoscopic treatment in early gastric cancer
Eun Jeong Gong, Chang Seok Bang
Journal of the Korean Medical Association.2022; 65(5): 284. CrossRef - Paneth Cell Carcinoma of the Stomach
Jun Wan Kim, Gwang Ha Kim, Kyung Bin Kim
The Korean Journal of Gastroenterology.2022; 80(1): 34. CrossRef - Comparing Different Anesthesia Methods on Anesthetic Effect and Postoperative Pain in Patients with Early Gastric Cancer during Endoscopic Submucosal Dissection
Jie Zhang, Yanlei Chen, Zhiwu Liu, Zhihao Pan, Jinghua Pan
Journal of Oncology.2022; 2022: 1. CrossRef
- General Anesthesia and Endoscopic Upper Gastrointestinal Tumor Resection
- 3,709 View
- 173 Download
- 5 Web of Science
- 5 Crossref

Case Report
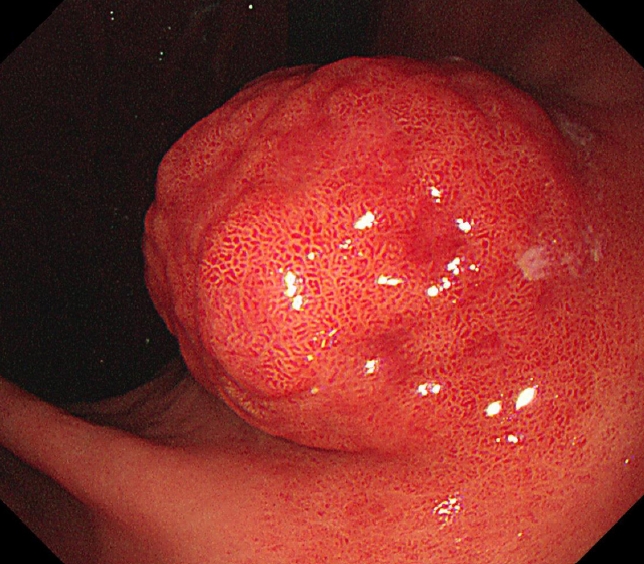
- Gastric Angiolipoma Resected with Endoscopic Submucosal Dissection
- Sang Myung Yeo, Jae Kwang Lee, Hyun Soo Kim, Chang Geun Park, Jae Kwon Jung, Dae Jin Kim, Yun Jin Chung, Han Jun Ryu
- Clin Endosc 2021;54(3):432-435. Published online March 15, 2021
- DOI: https://doi.org/10.5946/ce.2020.146
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Angiolipoma is a benign fatty neoplasm that has components of proliferating blood vessels. These types of lesions commonly occur in the subcutaneous tissue of the limbs and trunk. Angiolipoma in the gastrointestinal tract is extremely rare, and the final diagnosis generally depends on histological examination of the excised biopsy. In most previously reported cases, the lesions were diagnosed and treated with surgical management. In this study, we report a case of gastric angiolipoma of approximately 4 cm in size that was diagnosed and treated with endoscopic submucosal dissection.
-
Citations
Citations to this article as recorded by- A rare cause of upper gastrointestinal bleeding in an elderly female: Gastric Angiolipoma
Xiufan Ni, Sujun Gao, Lei Chen, Li Zhang, Jian Yin, Zhen Zhu
Revista Española de Enfermedades Digestivas.2023;[Epub] CrossRef - A Huge Gastric Angiolipoma Presenting with Acute Upper Gastrointestinal
Hemorrhage: A Case Report
Joo Hyeok Choi, Sung Bin Park, Jong Beum Lee, Tae-Jin Lee, Hyun Jeong Park, Eun Sun Lee
Current Medical Imaging Formerly Current Medical Imaging Reviews.2023;[Epub] CrossRef
- A rare cause of upper gastrointestinal bleeding in an elderly female: Gastric Angiolipoma
- 3,733 View
- 80 Download
- 2 Web of Science
- 2 Crossref

Original Article
- Endoscopic Submucosal Dissection versus Surgery for Undifferentiated-Type Early Gastric Cancer: A Systematic Review and Meta-Analysis
- Cheal-Wung Huh, Dae Won Ma, Byung-Wook Kim, Joon Sung Kim, Seung Jae Lee
- Clin Endosc 2021;54(2):202-210. Published online February 17, 2021
- DOI: https://doi.org/10.5946/ce.2020.121
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The use of endoscopic submucosal dissection (ESD) for treating undifferentiated-type early gastric cancer is controversial. The objective of this study was to perform a meta-analysis to compare the long-term outcomes of ESD and surgery for undifferentiated-type early gastric cancer.
Methods
The PubMed, Cochrane Library, and EMBASE databases were used to search for relevant studies comparing ESD and surgery for undifferentiated-type early gastric cancer. The methodological quality of the included publications was evaluated using the Risk of Bias Assessment tool for Nonrandomized Studies. The rates of overall survival, recurrence, adverse event, and complete resection were determined. Odds ratios (ORs) and 95% confidence intervals (CIs) were also evaluated.
Results
This meta-analysis enrolled five studies with 429 and 1,236 participants undergoing ESD and surgery, respectively. No significant difference was found in the overall survival rate between the ESD and surgery groups (OR, 2.29; 95% CI, 0.98–5.36; p=0.06). However, ESD was associated with a higher recurrence rate and a lower complete resection rate. The adverse event rate was similar between the two groups.
Conclusions
ESD with meticulous surveillance esophagogastroduodenoscopy may be as effective and safe as surgery in patients with undifferentiated-type early gastric cancer. Further large-scale, randomized, controlled studies from additional regions are required to confirm these findings. -
Citations
Citations to this article as recorded by- Curative criteria for endoscopic treatment of gastric cancer
João A. Cunha Neves, Pedro G. Delgado-Guillena, Patrícia Queirós, Diogo Libânio, Enrique Rodríguez de Santiago
Best Practice & Research Clinical Gastroenterology.2024; 68: 101884. CrossRef - Chinese national clinical practice guidelines on the prevention, diagnosis, and treatment of early gastric cancer
Peng Li, Ziyu Li, Enqiang Linghu, Jiafu Ji
Chinese Medical Journal.2024; 137(8): 887. CrossRef - Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach
Tae-Han Kim, In-Ho Kim, Seung Joo Kang, Miyoung Choi, Baek-Hui Kim, Bang Wool Eom, Bum Jun Kim, Byung-Hoon Min, Chang In Choi, Cheol Min Shin, Chung Hyun Tae, Chung sik Gong, Dong Jin Kim, Arthur Eung-Hyuck Cho, Eun Jeong Gong, Geum Jong Song, Hyeon-Su Im
Journal of Gastric Cancer.2023; 23(1): 3. CrossRef - Endoscopic Resection of Early Gastric Cancer and Pre-Malignant Gastric Lesions
Ana Clara Vasconcelos, Mário Dinis-Ribeiro, Diogo Libânio
Cancers.2023; 15(12): 3084. CrossRef - Performance of endoscopic submucosal dissection for undifferentiated early gastric cancer: a multicenter retrospective cohort
Apostolis Papaefthymiou, Michel Kahaleh, Arnaud Lemmers, Sandro Sferrazza, Maximilien Barret, Katsumi Yamamoto, Pierre Deprez, José C. Marín-Gabriel, George Tribonias, Hong Ouyang, Federico Barbaro, Oleksandr Kiosov, Stefan Seewald, Gaurav Patil, Shaimaa
Endoscopy International Open.2023; 11(07): E673. CrossRef - A meta-analysis of the impact on gastrectomy versus endoscopic submucosal dissection for early stomach cancer
Rajesh K. Singh
International Journal of Clinical Medical Research.2023; 1(3): 37. CrossRef - A meta-analysis of the impact on gastrectomy versus endoscopic submucosal dissection for early stomach cancer
Rajesh K. Singh
International Journal of Clinical Medical Research.2023;[Epub] CrossRef - Long-term outcomes of endoscopic submucosal dissection and surgery for undifferentiated intramucosal gastric cancer regardless of size
Gil Ho Lee, Eunyoung Lee, Bumhee Park, Jin Roh, Sun Gyo Lim, Sung Jae Shin, Kee Myung Lee, Choong-Kyun Noh
World Journal of Gastroenterology.2022; 28(8): 840. CrossRef - Comparison of long-term outcomes of endoscopic submucosal dissection and surgery for undifferentiated-type early gastric cancer meeting the expanded criteria: a systematic review and meta-analysis
Hyo-Joon Yang, Jie-Hyun Kim, Na Won Kim, Il Ju Choi
Surgical Endoscopy.2022; 36(6): 3686. CrossRef - Endoscopic submucosal dissection versus surgery for patients with undifferentiated early gastric cancer
Harold Benites-Goñi, Fernando Palacios-Salas, Andrea Carlin-Ronquillo, Carlos Díaz-Arocutipa, Alejandro Piscoya, Adrián Hernández
Revista Española de Enfermedades Digestivas.2022;[Epub] CrossRef - The future of endoscopic resection for early gastric cancer
Raquel Ortigão, Diogo Libânio, Mário Dinis‐Ribeiro
Journal of Surgical Oncology.2022; 125(7): 1110. CrossRef - Endoscopic treatment for early gastric cancer
Ji Yong Ahn
Journal of the Korean Medical Association.2022; 65(5): 276. CrossRef - Therapeutic approach to non-curative resection after endoscopic treatment in early gastric cancer
Eun Jeong Gong, Chang Seok Bang
Journal of the Korean Medical Association.2022; 65(5): 284. CrossRef - Long-Term Outcomes of Endoscopic Submucosal Dissection of Undifferentiated-Type Early Gastric Cancer
Chang Seok Bang
Clinical Endoscopy.2021; 54(2): 143. CrossRef
- Curative criteria for endoscopic treatment of gastric cancer
- 4,844 View
- 199 Download
- 13 Web of Science
- 14 Crossref

Case Reports
- Successful Endoscopic Resection of Gastric Mucosa-Associated Lymphoid Tissue Lymphoma Unresponsive to Helicobacter pylori Eradication Therapy
- Jeongmin Choi
- Clin Endosc 2022;55(1):136-140. Published online November 16, 2020
- DOI: https://doi.org/10.5946/ce.2020.232

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Eradication of Helicobacter pylori is the first-line treatment for gastric mucosa-associated lymphoid tissue (MALT) lymphomas; however, lesions may persist in 20% of patients after initial treatment, thereby necessitating the use of an additional therapeutic approach. Other treatment options include radiation therapy, chemotherapy, endoscopic resection, rituximab therapy, or watchful waiting. We present a case of localized gastric MALT lymphoma that did not respond to H. pylori eradication therapy. The patient waited for 12 months but the tumor showed no signs of regression endoscopically. Histologic examination revealed residual MALT lymphoma. The tumor was then successfully treated using endoscopic submucosal dissection and the patient remained disease-free for 4 years. To our knowledge, this is the first case in which a gastric MALT lymphoma was treated with endoscopic submucosal dissection. In conclusion, endoscopic resection may be recommended as second-line therapy for properly selected patients with gastric MALT lymphoma as it is effective and minimally invasive.
-
Citations
Citations to this article as recorded by- A Common Symptom With an Uncommon Diagnosis: A Case of Primary Esophageal Diffuse Large B-cell Lymphoma
Shruthi Narasimha, Rasiq Zackria, Jonathan Hughes, Vijay Jayaraman
Cureus.2024;[Epub] CrossRef - A Case of Esophageal MALT Lymphoma Mimicking a Subepithelial Tumor
Ha Eun Lee, Gwang Ha Kim, Min Ji Kim, Kyung Bin Kim, Dong Chan Joo, Hye Kyung Jeon, Moon Won Lee, Bong Eun Lee
The Korean Journal of Gastroenterology.2024; 83(4): 157. CrossRef - Clinical Management of Patients with Gastric MALT Lymphoma: A Gastroenterologist’s Point of View
Tamara Matysiak-Budnik, Kateryna Priadko, Céline Bossard, Nicolas Chapelle, Agnès Ruskoné-Fourmestraux
Cancers.2023; 15(15): 3811. CrossRef - Endoscopic Submucosal Dissection for Treatment of Localized Gastric Mucosa-associated Lymphoid Tissue Lymphoma: A Case Series
Jun-young Seo, Kee Don Choi, In Hye Song, Young Soo Park, Hee Kyong Na, Ji Yong Ahn, Jeong Hoon Lee, Kee Wook Jung, Do Hoon Kim, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2023; 23(3): 188. CrossRef - Bacteria-Mediated Oncogenesis and the Underlying Molecular Intricacies: What We Know So Far
Shashanka K. Prasad, Smitha Bhat, Dharini Shashank, Akshatha C. R., Sindhu R., Pornchai Rachtanapun, Devananda Devegowda, Prasanna K. Santhekadur, Sarana Rose Sommano
Frontiers in Oncology.2022;[Epub] CrossRef - Primary Esophageal Lymphoma: Clinical Experience in Diagnosis and Treatment
Junchi Qu, Yanyan Zhuang, Dandan Zheng, Fengting Huang, Shineng Zhang
Cureus.2021;[Epub] CrossRef
- A Common Symptom With an Uncommon Diagnosis: A Case of Primary Esophageal Diffuse Large B-cell Lymphoma
- 4,307 View
- 207 Download
- 5 Web of Science
- 6 Crossref

- Perforation of a Gastric Tear during Esophageal Endoscopic Submucosal Dissection under General Anesthesia
- Tomoaki Yamasaki, Yuhei Sakata, Takehisa Suekane, Hiroko Nebiki
- Clin Endosc 2021;54(6):916-919. Published online November 12, 2020
- DOI: https://doi.org/10.5946/ce.2020.220
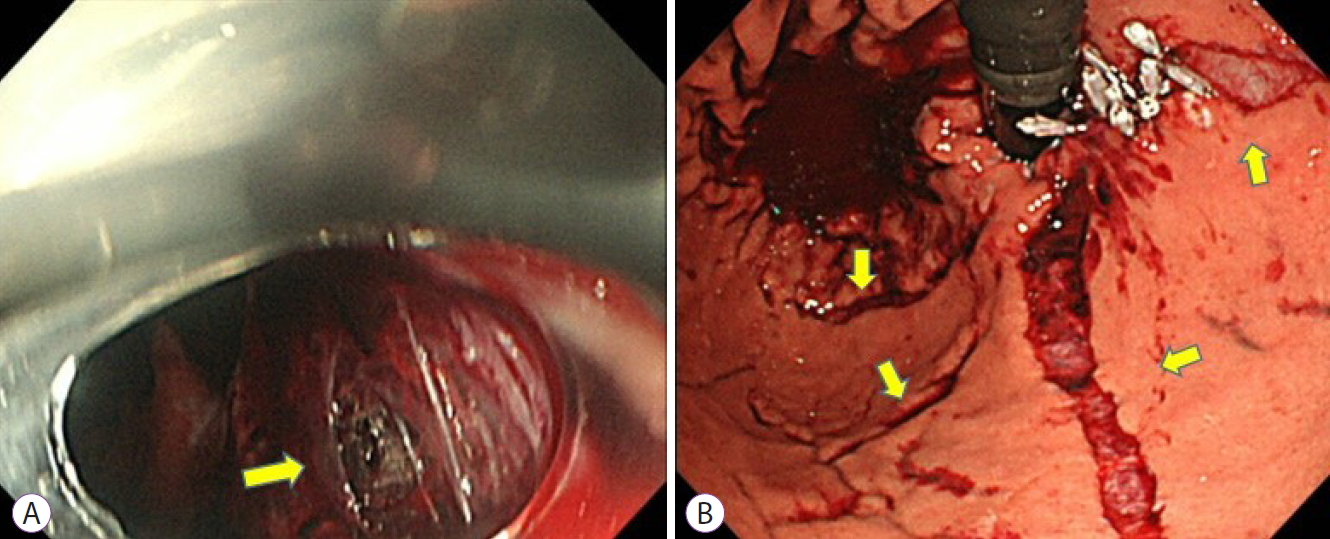
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Mallory-Weiss tears (MWT) are occasionally encountered during endoscopic procedures. Esophageal endoscopic submucosal dissection (ESD) is widely performed under general anesthesia to avoid unexpected body movements. We present the case of a 68-year-old woman with squamous cell carcinoma. Although ESD was performed under general anesthesia, a gastric perforation at the MWT caused by gastric inflation was observed after the procedure. The perforation was closed endoscopically, and she was discharged without any sequelae. Although general anesthesia is useful for esophageal ESD, it should be noted that it can cause MWT, and in rare cases, gastric perforation, due to gastric inflation during the procedure.
-
Citations
Citations to this article as recorded by- Gastric Perforation Encountered during Duodenal Stent Insertion
Sung Woo Ko, Hoonsub So, Sung Jo Bang
The Korean Journal of Gastroenterology.2022; 80(5): 221. CrossRef
- Gastric Perforation Encountered during Duodenal Stent Insertion
- 3,798 View
- 77 Download
- 1 Web of Science
- 1 Crossref

- Successful Endoscopic Resection of Residual Colonic Mucosa-Associated Lymphoid Tissue Lymphoma after Polypectomy
- Jeongmin Choi
- Clin Endosc 2021;54(5):759-762. Published online November 6, 2020
- DOI: https://doi.org/10.5946/ce.2020.233
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Mucosa-associated lymphoid tissue (MALT) lymphomas are typically found in the stomach, while colonic MALT lymphoma is rarely found. Considering its rarity, definitive treatment of colonic MALT lymphoma has not been established. Different from that in the stomach, Helicobacter pylori infection might play a minor role while determining the treatment of colonic MALT lymphoma. If colonic MALT lymphoma is localized, treatment options are surgical resection, radiation, endoscopic resection, or combination therapy. Here, we report a case of residual colonic MALT lymphoma after endoscopic mucosal resection, which was a 1.5-cm-sized tumor confined to the superficial wall of the rectum. The lesion was successfully treated using the endoscopic submucosal dissection technique. The patient remained disease-free for 4 years. This case provides rationale for endoscopic submucosal dissection treatment as a salvage therapy for residual tumors in properly selected patients with colonic MALT lymphoma.
-
Citations
Citations to this article as recorded by- A rare cause of hematochezia: colonic extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALToma): A case report and literature review
Chien-Hung Lu, Wei-Yu Kao, Chun-Chao Chang, Yu-An Kan
Medicine.2023; 102(21): e33869. CrossRef - Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma in the Gastrointestinal Tract in the Modern Era
Eri Ishikawa, Masanao Nakamura, Akira Satou, Kazuyuki Shimada, Shotaro Nakamura
Cancers.2022; 14(2): 446. CrossRef - Successful Endoscopic Resection of Primary Rectal Mucosa-Associated Lymphoid Tissue Lymphoma by Endoscopic Submucosal Dissection: A Case Report
Jian Han, Zhe Zhu, Chao Zhang, Hua-ping Xie
Frontiers in Medicine.2021;[Epub] CrossRef
- A rare cause of hematochezia: colonic extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALToma): A case report and literature review
- 3,972 View
- 100 Download
- 3 Web of Science
- 3 Crossref

Original Article
-
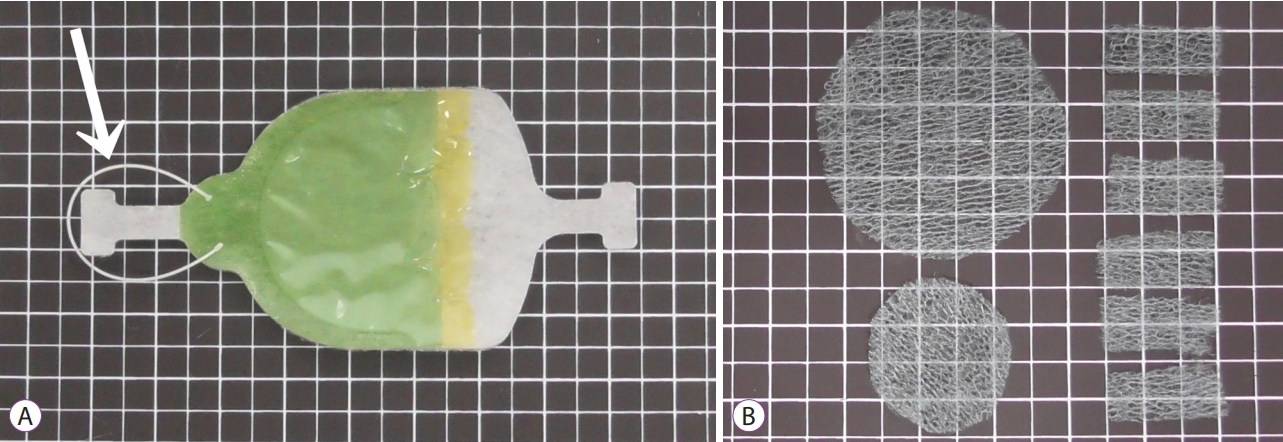
Efficacy of the Envelope Method in Applying Polyglycolic Acid Sheets to Post-Endoscopic Submucosal Dissection Ulcers in Living Pigs

- Hiroya Sakaguchi, Toshitatsu Takao, Yoshitaka Takegawa, Yuki Koga, Kazunori Yamanaka, Masataka Sagata, Shinwa Tanaka, Yoshinori Morita, Takashi Toyonaga, Yuzo Kodama
- Clin Endosc 2021;54(1):64-72. Published online July 16, 2020
- DOI: https://doi.org/10.5946/ce.2020.014
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Application of polyglycolic acid (PGA) sheets using fibrin glue in post-endoscopic submucosal dissection (ESD) ulcers to prevent bleeding has been reported to be difficult with the conventional delivery method because of gravity. This study assessed the usefulness of the envelope-based delivery system with and against gravity in living pigs.
Methods
PGA sheets were applied on post-ESD ulcers with and against gravity six times each using the conventional and envelope methods, respectively. The PGA sheet delivery time and the endoscopic and histological findings of the treated ulcer floors were compared.
Results
With gravity, the median PGA sheet application time was 1.00 (0.68–1.30) min/cm2 and 0.32 (0.18–0.52) min/cm2 with the conventional and envelope techniques (p=0.002), respectively, and against gravity, it was 1.20 (1.13–1.63) min/cm2 and 0.50 (0.39–0.58) min/cm2 (p=0.002), respectively. Against gravity, the endoscopic and histological findings revealed that the conventional group had insufficient fixation of the PGA sheets, but the envelope groups had sufficient fixation. The results with gravity were similar between the groups.
Conclusions
The envelope method makes it possible to deliver PGA sheets to the stomach quickly and cover ulcers appropriately both with and against gravity in living pigs. -
Citations
Citations to this article as recorded by- Endoscopic sealing hemostasis with polyglycolic acid sheet and fibrin glue as a novel endoscopic hemostatic technique: a report of three cases
Kai Korekawa, Atsushi Kunimitsu
Clinical Journal of Gastroenterology.2024;[Epub] CrossRef - Clinical Impact of Different Reconstruction Methods on Remnant Gastric Cancer at the Anastomotic Site after Distal Gastrectomy
Kei Matsumoto, Shinwa Tanaka, Takashi Toyonaga, Nobuaki Ikezawa, Mari Nishio, Masanao Uraoka, Tomoatsu Yoshihara, Hiroya Sakaguchi, Hirofumi Abe, Tetsuya Yoshizaki, Madoka Takao, Toshitatsu Takao, Yoshinori Morita, Hiroshi Yokozaki, Yuzo Kodama
Clinical Endoscopy.2022; 55(1): 86. CrossRef - The importance of pH adjustment for preventing fibrin glue dissolution in the stomach: an in vitro study
Yoshitaka Takegawa, Toshitatsu Takao, Hiroya Sakaguchi, Tatsuya Nakai, Kazuhiro Takeo, Yoshinori Morita, Takashi Toyonaga, Yuzo Kodama
Scientific Reports.2022;[Epub] CrossRef - A Novel Self-Assembled Gel for Gastric Endoscopic Submucosal Dissection-Induced Ulcer: A Preclinical Study in a Porcine Model
Meng Li, Haifeng Jin, Changpei Shi, Bin Lyu, Xiao Ying, Yuan Shi
Frontiers in Pharmacology.2021;[Epub] CrossRef
- Endoscopic sealing hemostasis with polyglycolic acid sheet and fibrin glue as a novel endoscopic hemostatic technique: a report of three cases
- 4,908 View
- 118 Download
- 5 Web of Science
- 4 Crossref

Review
- Photodynamic Therapy for Esophageal Cancer
- Takahiro Inoue, Ryu Ishihara
- Clin Endosc 2021;54(4):494-498. Published online May 19, 2020
- DOI: https://doi.org/10.5946/ce.2020.073
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Photodynamic therapy, a curative local treatment for esophageal squamous cell carcinoma, involves a photosensitizing drug (photosensitizer) with affinity for tumors and a photodynamic reaction triggered by laser light. Previously, photodynamic therapy was used to treat superficial esophageal squamous cell carcinoma judged to be difficult to undergo endoscopic resection. Recently, photodynamic therapy has mainly been performed for local failure after chemoradiotherapy. Although surgery is the most promising treatment for local failure after chemoradiotherapy, its morbidity and mortality rates are high. Endoscopic resection is feasible for local failure after chemoradiotherapy but requires advanced skills, and its indication is limited to within the submucosal layer by depth. Photodynamic therapy is less invasive than surgery and has a wider indication than endoscopic resection. Porfimer sodium (a first-generation photosensitizer) causes a high frequency of side effects related to photosensitivity and requires the long-term sunshade period. Talaporfin (a second-generation photosensitizer) requires a much shorter sun-shade period than porfimer sodium. Photodynamic therapy will profoundly change treatment strategies for local failure after chemoradiotherapy.
-
Citations
Citations to this article as recorded by- Aluminium phthalocyanine-mediated photodynamic therapy induces ATM-related DNA damage response and apoptosis in human oesophageal cancer cells
Onyisi Christiana Didamson, Rahul Chandran, Heidi Abrahamse
Frontiers in Oncology.2024;[Epub] CrossRef - An Ailment with Which I Will Contend: A Narrative Review of 5000 Years of Esophagogastric Cancers and Their Treatments, with Special Emphasis on Recent Advances in Immunotherapeutics
C. Beau Hilton, Steven Lander, Michael K. Gibson
Cancers.2024; 16(3): 618. CrossRef - Western outcomes of circumferential endoscopic submucosal dissection for early esophageal squamous cell carcinoma
Enrique Rodríguez de Santiago, Laurelle van Tilburg, Pierre H. Deprez, Mathieu Pioche, Roos E. Pouw, Michael J. Bourke, Stefan Seewald, Bas L.A.M. Weusten, Jeremie Jacques, Sara Leblanc, Pedro Barreiro, Arnaud Lemmers, Adolfo Parra-Blanco, Ricardo Küttner
Gastrointestinal Endoscopy.2024; 99(4): 511. CrossRef - Interventional gastroenterology in oncology
Vaibhav Wadhwa, Nicole Patel, Dheera Grover, Faisal S. Ali, Nirav Thosani
CA: A Cancer Journal for Clinicians.2023; 73(3): 286. CrossRef - Utilizing 4D Printing to Design Smart Gastroretentive, Esophageal, and Intravesical Drug Delivery Systems
Dina B. Mahmoud, Michaela Schulz‐Siegmund
Advanced Healthcare Materials.2023;[Epub] CrossRef - Recent Advances in Green Metallic Nanoparticles for Enhanced Drug Delivery in Photodynamic Therapy: A Therapeutic Approach
Alexander Chota, Blassan P. George, Heidi Abrahamse
International Journal of Molecular Sciences.2023; 24(5): 4808. CrossRef - Amino Acid Derivatives of Chlorin-e6—A Review
Maria da Graça H. Vicente, Kevin M. Smith
Molecules.2023; 28(8): 3479. CrossRef - Stimuli-responsive heparin-drug conjugates co-assembled into stable nanomedicines for cancer therapy
Zaixiang Fang, Ling Lin, Zhiqian Li, Lei Gu, Dayi Pan, Yunkun Li, Jie Chen, Haitao Ding, Xiaohe Tian, Qiyong Gong, Kui Luo
Acta Biomaterialia.2023;[Epub] CrossRef - Idarubicin and IR780 co-loaded PEG-b-PTMC nanoparticle for non-Hodgkin’s lymphoma therapy by photothermal/photodynamic strategy
Shanshan Weng, Luqi Pan, Dawei Jiang, Wenxia Xie, Zhiyuan Zhang, Changcan Shi, Bin Liang, Shenghao Wu
Materials & Design.2023; 230: 112008. CrossRef - Protein Photodamaging Activity and Photocytotoxic Effect of an Axial-Connecting Phosphorus(V)porphyrin Trimer
Kazutaka Hirakawa, Naoki Kishimoto, Yoshinobu Nishimura, Yuko Ibuki, Masaaki Fuki, Shigetoshi Okazaki
Chemical Research in Toxicology.2023;[Epub] CrossRef - Nanomedicine in Clinical Photodynamic Therapy for the Treatment of Brain Tumors
Hyung Shik Kim, Dong Yun Lee
Biomedicines.2022; 10(1): 96. CrossRef - Poly(styrene-co-maleic acid) Micelle of Photosensitizers for Targeted Photodynamic Therapy, Exhibits Prolonged Singlet Oxygen Generating Capacity and Superior Intracellular Uptake
Gahininath Yadavrao Bharate, Haibo Qin, Jun Fang
Journal of Personalized Medicine.2022; 12(3): 493. CrossRef - Deep-Learning for the Diagnosis of Esophageal Cancers and Precursor Lesions in Endoscopic Images: A Model Establishment and Nationwide Multicenter Performance Verification Study
Eun Jeong Gong, Chang Seok Bang, Kyoungwon Jung, Su Jin Kim, Jong Wook Kim, Seung In Seo, Uhmyung Lee, You Bin Maeng, Ye Ji Lee, Jae Ick Lee, Gwang Ho Baik, Jae Jun Lee
Journal of Personalized Medicine.2022; 12(7): 1052. CrossRef - Palliation of Malignant Dysphagia: Dilation, Stents, Cryoablation or PDT: The GI Perspective
Daniel J. Ellis, Nisa M. Kubiliun, Anna Tavakkoli
Foregut: The Journal of the American Foregut Society.2022; 2(2): 186. CrossRef - Palliation of Malignant Dysphagia: Dilation, Stents, Cryoablation or Photodynamic Therapy—A Surgical Perspective
Uzma Rahman, Olugbenga T. Okusanya
Foregut: The Journal of the American Foregut Society.2022; 2(2): 180. CrossRef - Endoscopic Treatment for Disease Persistence/Recurrence after Definitive Chemoradiotherapy for Esophageal Cancer
Prabin Sharma, Rani Modayil, Stavros N. Stavropoulos
Foregut: The Journal of the American Foregut Society.2022; 2(2): 132. CrossRef - Targeted chemo-photodynamic therapy toward esophageal cancer by GSH-sensitive theranostic nanoplatform
Guodong Ren, ZiCheng Wang, Yafei Tian, Jinyao Li, Yingyu Ma, Liang Zhou, Chengwu Zhang, Lixia Guo, Haipeng Diao, Lihong Li, Li Lu, Sufang Ma, Zhifang Wu, Lili Yan, Wen Liu
Biomedicine & Pharmacotherapy.2022; 153: 113506. CrossRef - Therapeutic effects of in-vivo radiodynamic therapy (RDT) for lung cancer treatment: a combination of 15MV photons and 5-aminolevulinic acid (5-ALA)
Dae-Myoung Yang, Dusica Cvetkovic, Lili Chen, C-M Charlie Ma
Biomedical Physics & Engineering Express.2022; 8(6): 065031. CrossRef - Laser induced thermotherapy of multiple actinic keratosis
Tatiana Evgenievna Sukhova, Yulia Vladimirovna Molochkova, Anna Igorevna Pronina
Russian Journal of Skin and Venereal Diseases.2022; 25(3): 181. CrossRef - Novel sulfonamide porphyrin TBPoS-2OH used in photodynamic therapy for malignant melanoma
Zhaohai Pan, Jiaojiao Fan, Qi Xie, Xin Zhang, Wen Zhang, Qing Ren, Minjing Li, Qiusheng Zheng, Jun Lu, Defang Li
Biomedicine & Pharmacotherapy.2021; 133: 111042. CrossRef - Photodynamic Therapy—An Up-to-Date Review
Adelina-Gabriela Niculescu, Alexandru Mihai Grumezescu
Applied Sciences.2021; 11(8): 3626. CrossRef - Targeted Photodynamic Diagnosis and Therapy for Esophageal Cancer: Potential Role of Functionalized Nanomedicine
Onyisi Christiana Didamson, Heidi Abrahamse
Pharmaceutics.2021; 13(11): 1943. CrossRef
- Aluminium phthalocyanine-mediated photodynamic therapy induces ATM-related DNA damage response and apoptosis in human oesophageal cancer cells
- 7,128 View
- 252 Download
- 15 Web of Science
- 22 Crossref

Original Articles
- Efficacy and Safety of Endoscopic Treatment for Gastrointestinal Stromal Tumors in the Upper Gastrointestinal Tract
- Cicilia Marcella, Shakeel Sarwar, Hui Ye, Rui Hua Shi
- Clin Endosc 2020;53(4):458-465. Published online March 17, 2020
- DOI: https://doi.org/10.5946/ce.2019.121
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic treatment (ET) has been applied for decades to treat subepithelial tumors, including gastrointestinal stromal tumors (GISTs). However, the efficacy of ET remains debatable. In this study, we evaluated the efficacy and safety of ET for GISTs in the upper gastrointestinal tract.
Methods
This retrospective single-center study included 97 patients who underwent ET. All patients were enrolled from July 2014 to July 2018. Parameters such as demographics, size, resection margin, complications, pathological features, procedure time, total cost, and follow-up were investigated and analyzed.
Results
Our study achieved 100% en bloc resection and 77.4% (72/93) R0 resection. The most common location was the fundus with a mean tumor size of 2.1±1.43 cm. The mean age, procedure time, hospital stay, and cost were 59.7±11.29 years, 64.7±35.23 minutes, 6.8 days, and 5,337 dollars, respectively. According to National Institutes of Health classification, 63 (64.9%), 26 (26.8%), 5 (5.2%), and 3 (3.1%) patients belonged to the very low, low, intermediate, and high risk classification, respectively. Immunohistochemistry results showed a 100% positive rate of CD34, DOG-1, CD117, and Ki67. A mean follow-up of 21.3±13.0 months showed no recurrence or metastasis.
Conclusions
ET is effective and safe for curative removal of GISTs in the upper gastrointestinal tract, and it can be a treatment of choice for patients with no metastasis. -
Citations
Citations to this article as recorded by- Comparison of endoscopic full-thickness resection and cap-assisted endoscopic full-thickness resection in the treatment of small (≤1.5 cm) gastric GI stromal tumors
Jinping Yang, Muhan Ni, Jingwei Jiang, Ximei Ren, Tingting Zhu, Shouli Cao, Shahzeb Hassan, Ying Lv, Xiaoqi Zhang, Yongyue Wei, Lei Wang, Guifang Xu
Gastrointestinal Endoscopy.2022; 95(4): 660. CrossRef - The necessarity of treatment for small gastric subepithelial tumors (1–2 cm) originating from muscularis propria: an analysis of 972 tumors
Jinlong Hu, Xinzhu Sun, Nan Ge, Sheng Wang, Jintao Guo, Xiang Liu, Guoxin Wang, Siyu Sun
BMC Gastroenterology.2022;[Epub] CrossRef - Natural History of Asymptomatic Esophageal Subepithelial Tumors of 30 mm or Less in Size
Seokin Kang, Do Hoon Kim, Yuri Kim, Dongsub Jeon, Hee Kyong Na, Jeong Hoon Lee, Ji Yong Ahn, Kee Wook Jung, Kee Don Choi, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung
Journal of Korean Medical Science.2022;[Epub] CrossRef - Massive Digestive Hemorrhagia Revealing a Gastro-Intestinal Stromal Tumor of the Jejunum
Yasmine Cherouaqi, Fatima zahra Belabbes, Hanane Delsa, Anass Nadi, Fedoua Rouibaa
Cureus.2021;[Epub] CrossRef - Endoscopic Treatment for Gastrointestinal Stromal Tumors in the Upper Gastrointestinal Tract
In Kyung Yoo, Joo Young Cho
Clinical Endoscopy.2020; 53(4): 383. CrossRef - Recent advances in the management of gastrointestinal stromal tumor
Monjur Ahmed
World Journal of Clinical Cases.2020; 8(15): 3142. CrossRef
- Comparison of endoscopic full-thickness resection and cap-assisted endoscopic full-thickness resection in the treatment of small (≤1.5 cm) gastric GI stromal tumors
- 4,658 View
- 150 Download
- 6 Web of Science
- 6 Crossref

-
Efficacy and Safety of Complete Endoscopic Resection of Colorectal Neoplasia Using a Stepwise Endoscopic Protocol with SOUTEN, a Novel Multifunctional Snare

- Shinji Yoshii, Marina Kubo, Mio Matsumoto, Takefumi Kikuchi, Yasunari Takakuwa
- Clin Endosc 2020;53(2):206-212. Published online February 27, 2020
- DOI: https://doi.org/10.5946/ce.2019.117
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: A multifunctional snare SOUTEN has a sharp tip at the top of the snare loop that enables incision of the mucosa, dissection of the submucosal layer, and snaring of lesion. This study assessed the efficacy and safety of complete endoscopic resection of colorectal neoplasia using SOUTEN.
Methods
We analyzed the rates of gross en bloc resection and complete resections of 108 consecutive tumors from 69 patients resected by precutting endoscopic mucosal resection (precutting), hybrid endoscopic submucosal dissection (hybrid), or conventional endoscopic submucosal dissection (conventional) using SOUTEN.
Results
Out of the 108 tumors, 50 were resected by precutting, 27 were resected by hybrid after attempting precutting, and the remaining 31 were resected by conventional after attempting precutting and hybrid resections. The median tumor sizes were 14.5 mm for precutting, 16.4 mm for hybrid, and 21.1 mm for conventional. The success rate of gross en bloc resection and histological complete resection were 100% and 94.0% for precutting, 96.4% and 96.4% for hybrid, and 100% and 100% for conventional method, respectively. No procedure-related complication occurred.
Conclusions
By using SOUTEN, precutting and hybrid were successfully performed on 10–30 mm tumors with a shorter procedure time than conventional without major complications. -
Citations
Citations to this article as recorded by- Hybrid Endoscopic Resection With Endo-knife and Snare for Colorectal Lesions: A Systematic Review and Meta-analysis
Shinji Yoshii, Takefumi Kikuchi, Yuki Hayashi, Masahiro Nojima, Hiro-o Yamano, Hiroshi Nakase
Techniques and Innovations in Gastrointestinal Endoscopy.2023; 25(2): 135. CrossRef - Standard Endoscopic Mucosal Resection vs Precutting Endoscopic Mucosal Resection Using Novel Disk-Tip Snare for Colorectal Lesions
Naohisa Yoshida, Ken Inoue, Hikaru Hashimoto, Reo Kobayashi, Yuri Tomita, Satoshi Sugino, Ryohei Hirose, Osamu Dohi, Yukiko Morinaga, Yutaka Inada, Takaaki Murakami, Yoshito Itoh
Digestive Diseases and Sciences.2023; 68(5): 2030. CrossRef - Outpatient hybrid endoscopic submucosal dissection with SOUTEN for early gastric cancer, followed by endoscopic suturing of the mucosal defect: A case report
Renma Ito, Kazuhiro Miwa, Yutaka Matano
World Journal of Gastrointestinal Surgery.2023; 15(8): 1831. CrossRef - Understanding hybrid endoscopic submucosal dissection subtleties
João Paulo de Souza Pontual, Alexandre Moraes Bestetti, Diogo Turiani Hourneaux de Moura
Clinical Endoscopy.2023; 56(6): 738. CrossRef - A knife plus a snare, but how will it fare?
Kavel Visrodia, Amrita Sethi
Gastrointestinal Endoscopy.2021; 93(3): 679. CrossRef - Freestyle endoscopic submucosal dissection using a multifunctional snare
Jun Arimoto, Hideyuki Chiba, Naoya Okada, Hiroki Kuwabara, Michiko Nakaoka
VideoGIE.2021; 6(11): 501. CrossRef
- Hybrid Endoscopic Resection With Endo-knife and Snare for Colorectal Lesions: A Systematic Review and Meta-analysis
- 4,832 View
- 110 Download
- 5 Web of Science
- 6 Crossref

Case Report
- Endoscopic Submucosal Dissection of a Colonic Calcifying Fibrous Tumor
- Jaeyoung Kim, Seongyul Ryu, Yeon-Ji Kim
- Clin Endosc 2020;53(4):487-490. Published online January 21, 2020
- DOI: https://doi.org/10.5946/ce.2019.138

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - A 49-year-old woman was referred to our hospital for further treatment due to the suspicion of a submucosal tumor in a routine screening colonoscopy. On colonoscopy, a 1-cm sized subepithelial mass with normal overlying mucosa in the hepatic flexure was found. Endoscopic ultrasonography (EUS) showed a homogenous hypoechoic lesion arising from the second and third layer. We were unable to make a final diagnosis because the lesion showed a small tumor with atypical macroscopic morphology including EUS findings. Therefore, endoscopic submucosal dissection was performed for the diagnostic treatment of the tumor. Submucosal dissection was performed just above the muscle layer, and the tumor was removed completely and reliably without any acute complications such as perforation. Based on histopathological findings, we diagnosed a benign, calcifying fibrous tumor (CFT). The present case is the first report of successful endoscopic diagnosis and treatment of colonic CFT mimicking a submucosal tumor.
-
Citations
Citations to this article as recorded by- Feasibility of endoscopic resection and impact of endoscopic ultrasound-based surveillance on colorectal subepithelial tumors
Eun Young Park, Dong Hoon Baek, Seung Min Hong, Bong Eun Lee, Moon Won Lee, Gwang Ha Kim, Geun Am Song
Surgical Endoscopy.2023; 37(9): 6867. CrossRef - Submucosal Necrotic Nodule of the Colon: An Enigmatic Entity Potentially Related to Anisakis Infection
Raul S. Gonzalez, Laura G. Pastrián, Sergey Pyatibrat, Hernan Dario Quiceno Arias, Yolanda Rodriguez Gil, Adam L. Booth, Itziar de la Peña Navarro, Maddi Garmendia-Irizar, Jennifer R. Lapointe, Mousa Mobarki, Luiz Miguel Nova-Camacho, Gina Parini, Estefan
Archives of Pathology & Laboratory Medicine.2023; 147(11): 1315. CrossRef
- Feasibility of endoscopic resection and impact of endoscopic ultrasound-based surveillance on colorectal subepithelial tumors
- 4,125 View
- 95 Download
- 2 Web of Science
- 2 Crossref


 KSGE
KSGE


 First
First Prev
Prev



