Search
- Page Path
- HOME > Search
- Korean guidelines for postpolypectomy colonoscopic surveillance: 2022 revised edition
- Su Young Kim, Min Seob Kwak, Soon Man Yoon, Yunho Jung, Jong Wook Kim, Sun-Jin Boo, Eun Hye Oh, Seong Ran Jeon, Seung-Joo Nam, Seon-Young Park, Soo-Kyung Park, Jaeyoung Chun, Dong Hoon Baek, Mi-Young Choi, Suyeon Park, Jeong-Sik Byeon, Hyung Kil Kim, Joo Young Cho, Moon Sung Lee, Oh Young Lee, Korean Society of Gastrointestinal Endoscopy, Korean Society of Gastroenterology, Korean Association for the Study of Intestinal Diseases
- Clin Endosc 2022;55(6):703-725. Published online October 13, 2022
- DOI: https://doi.org/10.5946/ce.2022.136
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
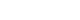
ePub - Colonoscopic polypectomy is effective in decreasing the incidence and mortality of colorectal cancer (CRC). Premalignant polyps discovered during colonoscopy are associated with the risk of metachronous advanced neoplasia. Postpolypectomy surveillance is the most important method for the management of advanced metachronous neoplasia. A more efficient and evidence-based guideline for postpolypectomy surveillance is required because of limited medical resources and concerns regarding colonoscopy complications. In these consensus guidelines, an analytic approach was used to address all reliable evidence to interpret the predictors of CRC or advanced neoplasia during surveillance colonoscopy. The key recommendations state that the high-risk findings for metachronous CRC following polypectomy are as follows: (1) adenoma ≥10 mm in size; (2) 3 to 5 (or more) adenomas; (3) tubulovillous or villous adenoma; (4) adenoma containing high-grade dysplasia; (5) traditional serrated adenoma; (6) sessile serrated lesion (SSL) containing any grade of dysplasia; (7) serrated polyp of at least 10 mm in size; and (8) 3 to 5 (or more) SSLs. More studies are needed to fully comprehend the patients most likely to benefit from surveillance colonoscopy and the ideal surveillance interval to prevent metachronous CRC.
-
Citations
Citations to this article as recorded by- Association between Atherosclerosis and High-Risk Colorectal Adenomas based on Cardio-Ankle Vascular Index and Ankle-Brachial Index
Jung Ho Lee, Hyunseok Cho, Sang Hoon Lee, Sung Joon Lee, Chang Don Kang, Dae Hee Choi, Jin Myung Park, Seung-Joo Nam, Tae Suk Kim, Ji Hyun Kim, Sung Chul Park
The Korean Journal of Gastroenterology.2024; 83(4): 143. CrossRef - A survey of current practices in post-polypectomy surveillance in Korea
Jeongseok Kim, Tae-Geun Gweon, Min Seob Kwak, Su Young Kim, Seong Jung Kim, Hyun Gun Kim, Eun Ran Kim, Sung Noh Hong, Eun Sun Kim, Chang Mo Moon, Dae Seong Myung, Dong Hoon Baek, Shin Ju Oh, Hyun Jung Lee, Ji Young Lee, Yunho Jung, Jaeyoung Chun, Dong-Hoo
Intestinal Research.2024; 22(2): 186. CrossRef - Korean Guidelines for Postpolypectomy Colonoscopic Surveillance: 2022 Revision
Su Young Kim
The Korean Journal of Medicine.2023; 98(3): 102. CrossRef - Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials
Mizuki Nagai, Sho Suzuki, Yohei Minato, Fumiaki Ishibashi, Kentaro Mochida, Ken Ohata, Tetsuo Morishita
Clinical Endoscopy.2023; 56(5): 553. CrossRef - Understanding colorectal polyps to prevent colorectal cancer
Dong-Hoon Yang
Journal of the Korean Medical Association.2023; 66(11): 626. CrossRef - Classification and endoscopic diagnosis of colorectal polyps
Ji Hyun Kim, Sung Chul Park
Journal of the Korean Medical Association.2023; 66(11): 633. CrossRef - Endoscopic treatment of colorectal polyps and early colorectal cancer
Yunho Jung
Journal of the Korean Medical Association.2023; 66(11): 642. CrossRef - Strategy for post-polypectomy colonoscopy surveillance: focus on the revised Korean guidelines
Yong Soo Kwon, Su Young Kim
Journal of the Korean Medical Association.2023; 66(11): 652. CrossRef
- Association between Atherosclerosis and High-Risk Colorectal Adenomas based on Cardio-Ankle Vascular Index and Ankle-Brachial Index
- 5,241 View
- 515 Download
- 8 Web of Science
- 8 Crossref

- 2021 Korean Society of Gastrointestinal Endoscopy Clinical Practice Guidelines for Endoscopic Sedation
- Hong Jun Park, Byung-Wook Kim, Jun Kyu Lee, Yehyun Park, Jin Myung Park, Jun Yong Bae, Seung Young Seo, Jae Min Lee, Jee Hyun Lee, Hyung Ku Chon, Jun-Won Chung, Hyun Ho Choi, Myung Ha Kim, Dong Ah Park, Jae Hung Jung, Joo Young Cho, Endoscopic Sedation Committee of Korean Society of Gastrointestinal Endoscopy
- Clin Endosc 2022;55(2):167-182. Published online February 22, 2022
- DOI: https://doi.org/10.5946/ce.2021.282
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Sedation can resolve anxiety and fear in patients undergoing endoscopy. The use of sedatives has increased in Korea. Appropriate sedation is a state in which the patient feels subjectively comfortable while maintaining the airway reflex for stable spontaneous breathing. The patient should maintain a state of consciousness to the extent that he or she can cooperate with the needs of the medical staff. Despite its benefits, endoscopic sedation has been associated with cardiopulmonary complications. Cardiopulmonary complications are usually temporary. Most patients recover without sequelae. However, they may progress to serious complications, such as cardiovascular collapse. Therefore, it is essential to screen high-risk patients before sedation and reduce complications by meticulous monitoring. Additionally, physicians should be familiar with the management of emergencies. The first Korean clinical practice guideline for endoscopic sedation was developed based on previous worldwide guidelines for endoscopic sedation using an adaptation process. The guideline consists of nine recommendations based on a critical review of currently available data and expert consensus when the guideline was drafted. These guidelines should provide clinicians, nurses, medical school students, and policy makers with information on how to perform endoscopic sedation with minimal risk.
-
Citations
Citations to this article as recorded by- Using Clinical-based Discharge Criteria to Discharge Patients After Endoscopy Procedures Under Drug-induced Intravenous Sedation in the Outpatient Care Unit: An Observational Study
Liangyu Fang, Lina Chen, Bingbing Wu, Yinchuan Xu, Laijuan Chen
Journal of PeriAnesthesia Nursing.2024;[Epub] CrossRef - Experience of organizing outpatient anesthetic care at Endoscopy centre of Multidisciplinary city clinic
O. V. Makarov, S. A. Osipov, E. P. Rodionov, A. A. Malyshev, I. Yu. Korzheva, L. M. Avramenko, Z. Z. Loseva, I. V. Balykov, L. A. Baichorova, E. I. Alikhanova, A. V. Vlasenko, E. A. Evdokimov, V. I. Makovey, V. V. Erofeev
Medical alphabet.2023; (6): 50. CrossRef - Anesthesia care provider sedation versus conscious sedation for endoscopic ultrasound–guided tissue acquisition: a retrospective cohort study
Sneha Shaha, Yinglin Gao, Jiahao Peng, Kendrick Che, John J. Kim, Wasseem Skef
Clinical Endoscopy.2023; 56(5): 658. CrossRef - Current status of the gastric cancer screening program in Korea
Young-Il Kim, Il Ju Choi
Journal of the Korean Medical Association.2022; 65(5): 250. CrossRef - In pursuit of the right plan for airway management in gastrointestinal endoscopic procedures…the battle half won?
Upender Gowd, SukhminderJit Singh Bajwa, Madhuri Kurdi, Gaurav Sindwani
Indian Journal of Anaesthesia.2022; 66(10): 683. CrossRef - Drugs used for sedation in gastrointestinal endoscopy
Jun Kyu Lee
Journal of the Korean Medical Association.2022; 65(11): 735. CrossRef
- Using Clinical-based Discharge Criteria to Discharge Patients After Endoscopy Procedures Under Drug-induced Intravenous Sedation in the Outpatient Care Unit: An Observational Study
- 5,715 View
- 568 Download
- 4 Web of Science
- 6 Crossref

- Clinical Practice Guidelines for the Endoscopic Management of Peripancreatic Fluid Collections
- Chi Hyuk Oh, Jun Kyu Lee, Tae Jun Song, Jin-Seok Park, Jae Min Lee, Jun Hyuk Son, Dong Kee Jang, Miyoung Choi, Jeong-Sik Byeon, In Seok Lee, Soo Teik Lee, Ho Soon Choi, Ho Gak Kim, Hoon Jai Chun, Chan Guk Park, Joo Young Cho
- Clin Endosc 2021;54(4):505-521. Published online July 27, 2021
- DOI: https://doi.org/10.5946/ce.2021.185
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic ultrasonography-guided intervention has gradually become a standard treatment for peripancreatic fluid collections (PFCs). However, it is difficult to popularize the procedure in Korea because of restrictions on insurance claims regarding the use of endoscopic accessories, as well as the lack of standardized Korean clinical practice guidelines. The Korean Society of Gastrointestinal Endoscopy (KSGE) appointed a Task Force to develope medical guidelines by referring to the manual for clinical practice guidelines development prepared by the National Evidence-Based Healthcare Collaborating Agency. Previous studies on PFCs were searched, and certain studies were selected with the help of experts. Then, a set of key questions was selected, and treatment guidelines were systematically reviewed. Answers to these questions and recommendations were selected via peer review. This guideline discusses endoscopic management of PFCs and makes recommendations on Indications for the procedure, pre-procedural preparations, optimal approach for drainage, procedural considerations (e.g., types of stent, advantages and disadvantages of plastic and metal stents, and accessories), adverse events of endoscopic intervention, and procedural quality issues. This guideline was reviewed by external experts and suggests best practices recommended based on the evidence available at the time of preparation. This will be revised as necessary to address advances and changes in technology and evidence obtained in clinical practice and future studies.
-
Citations
Citations to this article as recorded by- Pancreatic Pseudocyst after Fully Covered Self-expandable Metallic Stent Placement: A Case Report
Mitsuhito Koizumi, Sho Ishikawa, Kaori Marui, Masahito Kokubu, Yusuke Okujima, Yuki Numata, Yoshiki Imamura, Teru Kumagi, Yoichi Hiasa
Internal Medicine.2024;[Epub] CrossRef - Neutrophil Gelatinase-Associated Lipocalin for the Differentiation of Mucinous Pancreatic Cystic Lesions
Miruna Patricia Olar, Maria Iacobescu, Sorana D. Bolboacă, Cristina Pojoga, Ofelia Moșteanu, Radu Seicean, Ioana Rusu, Oana Banc, Cristina Adela Iuga, Andrada Seicean
International Journal of Molecular Sciences.2024; 25(6): 3224. CrossRef - Comparative outcome of single versus two double-pigtail stents for endoscopic drainage of pancreatic fluid collections with minimal necrosis: a retrospective analysis
S Giri, S Bhrugumalla, S Gangadhar, S Angadi
Acta Gastro Enterologica Belgica.2024; 87(1): 1. CrossRef - Use of an endoscopic powered debridement device for treatment of post-surgical fatty pancreatic necrosis
Judy Daboul, Shiab Mussad, Anna Cecilia Amaral, Waleed K. Hussain, Peter J. Lee, Samuel Han
Clinical Endoscopy.2024; 57(3): 412. CrossRef - Endoscopic ultrasound-guided drainage for local complications related to pancreatitis
Hyung Ku Chon, Seong-Hun Kim
International Journal of Gastrointestinal Intervention.2023; 12(1): 7. CrossRef - A preferable modality for the differentiation of peripancreatic fluid collections: Endoscopic ultrasound
Ning Xu, Longsong Li, Danqi Zhao, Zixin Wang, Xueting Wang, Runzi Wang, Yanbo Zeng, Lei Zhang, Ning Zhong, Ying Lv, Enqiang Linghu, Ningli Chai
Endoscopic Ultrasound.2022; 11(4): 291. CrossRef - Disconnected pancreatic duct syndrome in acute pancreatitis
A.V. Fedorov, V.N. Ektov, M.A. Khodorkovsky
Khirurgiya. Zhurnal im. N.I. Pirogova.2022; (8): 83. CrossRef - Single balloon enteroscopy-guided endoscopic retrograde pancreatography for the treatment of a symptomatic pancreatic pseudocyst complicated by pancreaticojejunostomy stricture: A case report
Eunae Cho, Chang-Hwan Park, Seo Yeon Cho
Medicine.2022; 101(43): e31293. CrossRef
- Pancreatic Pseudocyst after Fully Covered Self-expandable Metallic Stent Placement: A Case Report
- 4,744 View
- 218 Download
- 7 Web of Science
- 8 Crossref

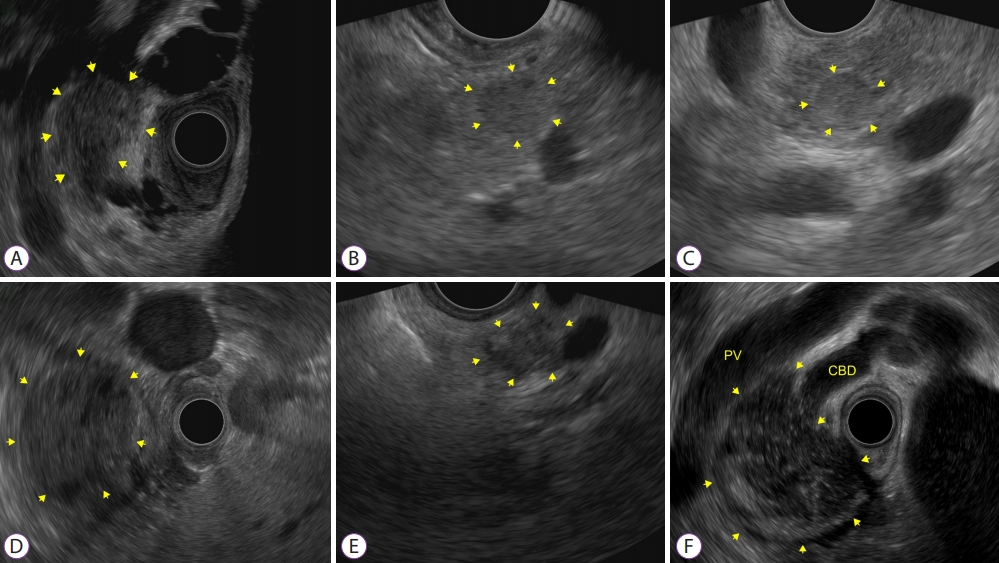
- Clinical and Technical Guideline for Endoscopic Ultrasound (EUS)-Guided Tissue Acquisition of Pancreatic Solid Tumor: Korean Society of Gastrointestinal Endoscopy (KSGE)
- Moon Jae Chung, Se Woo Park, Seong-Hun Kim, Chang Min Cho, Jun-Ho Choi, Eun Kwang Choi, Tae Hoon Lee, Eunae Cho, Jun Kyu Lee, Tae Jun Song, Jae Min Lee, Jun Hyuk Son, Jin Suk Park, Chi Hyuk Oh, Dong-Ah Park, Jeong-Sik Byeon, Soo Teik Lee, Ho Gak Kim, Hoon Jai Chun, Ho Soon Choi, Chan Guk Park, Joo Young Cho
- Clin Endosc 2021;54(2):161-181. Published online March 24, 2021
- DOI: https://doi.org/10.5946/ce.2021.069
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic ultrasound (EUS)-guided tissue acquisition of pancreatic solid tumor requires a strict recommendation for its proper use in clinical practice because of its technical difficulty and invasiveness. The Korean Society of Gastrointestinal Endoscopy (KSGE) appointed a Task Force to draft clinical practice guidelines for EUS-guided tissue acquisition of pancreatic solid tumor. The strength of recommendation and the level of evidence for each statement were graded according to the Minds Handbook for Clinical Practice Guideline Development 2014. The committee, comprising a development panel of 16 endosonographers and an expert on guideline development methodology, developed 12 evidence-based recommendations in 8 categories intended to help physicians make evidence-based clinical judgments with regard to the diagnosis of pancreatic solid tumor. This clinical practice guideline discusses EUS-guided sampling in pancreatic solid tumor and makes recommendations on circumstances that warrant its use, technical issues related to maximizing the diagnostic yield (e.g., needle type, needle diameter, adequate number of needle passes, sample obtaining techniques, and methods of specimen processing), adverse events of EUS-guided tissue acquisition, and learning-related issues. This guideline was reviewed by external experts and suggests best practices recommended based on the evidence available at the time of preparation. This guideline may not be applicable for all clinical situations and should be interpreted in light of specific situations and the availability of resources. It will be revised as necessary to cover progress and changes in technology and evidence from clinical practice.
-
Citations
Citations to this article as recorded by- Role of Endoscopic Ultrasound in the Management of Pancreatic Cancer
Balaji Musunuri, Shiran Shetty
Indian Journal of Surgical Oncology.2024; 15(S2): 269. CrossRef - Endoscopic ultrasound-guided tissue acquisition for personalized treatment in pancreatic adenocarcinoma
Sang Myung Woo
Clinical Endoscopy.2023; 56(2): 183. CrossRef - Pancreatic duct lavage cytology combined with a cell-block method for patients with possible pancreatic ductal adenocarcinomas, including pancreatic carcinoma in situ
Hiroaki Kusunose, Shinsuke Koshita, Yoshihide Kanno, Takahisa Ogawa, Toshitaka Sakai, Keisuke Yonamine, Kazuaki Miyamoto, Fumisato Kozakai, Hideyuki Anan, Kazuki Endo, Haruka Okano, Masaya Oikawa, Takashi Tsuchiya, Takashi Sawai, Yutaka Noda, Kei Ito
Clinical Endoscopy.2023; 56(3): 353. CrossRef - Anesthesia care provider sedation versus conscious sedation for endoscopic ultrasound–guided tissue acquisition: a retrospective cohort study
Sneha Shaha, Yinglin Gao, Jiahao Peng, Kendrick Che, John J. Kim, Wasseem Skef
Clinical Endoscopy.2023; 56(5): 658. CrossRef - Endoscopic ultrasound-guided tissue acquisition and gene panel testing for pancreatic cancer
Kentaro SUDO, Emiri KITA, Akiko TSUJIMOTO, Kazuyoshi NAKAMURA, Akiko ODAKA, Makiko ITAMI, Sana YOKOI, Hiroshi ISHII
Suizo.2022; 37(1): 8. CrossRef - Impact of rapid on-site evaluation on diagnostic accuracy of EUS-guided fine-needle aspiration of solid pancreatic lesions: experience from a single center
Irem Guvendir, Itir Ebru Zemheri, Kamil Ozdil
BMC Gastroenterology.2022;[Epub] CrossRef - Endoscopic Ultrasound Guided Fine Needle Aspiration and Biopsy for Pancreatic Disease
Kwang Hyuck Lee
The Korean Journal of Pancreas and Biliary Tract.2021; 26(4): 241. CrossRef
- Role of Endoscopic Ultrasound in the Management of Pancreatic Cancer
- 7,226 View
- 279 Download
- 4 Web of Science
- 7 Crossref

-
Diode Laser—Can It Replace the Electrical Current Used in Endoscopic Submucosal Dissection?

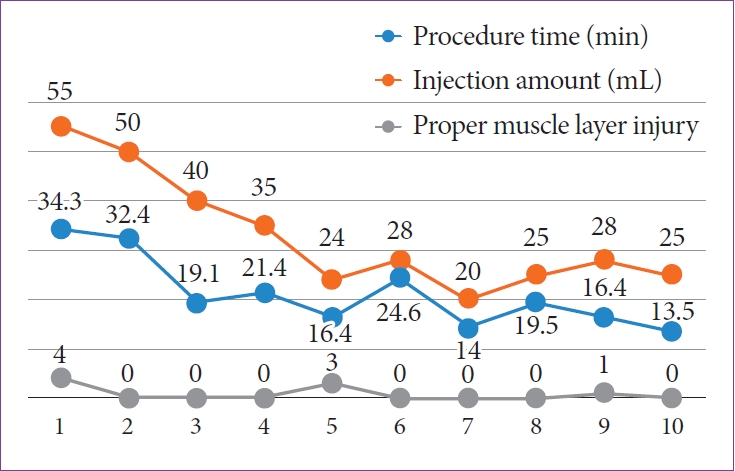
- Yunho Jung, Gwang Ho Baik, Weon Jin Ko, Bong Min Ko, Seong Hwan Kim, Jin Seok Jang, Jae-Young Jang, Wan-Sik Lee, Young Kwan Cho, Sun Gyo Lim, Hee Seok Moon, In Kyung Yoo, Joo Young Cho
- Clin Endosc 2021;54(4):555-562. Published online January 13, 2021
- DOI: https://doi.org/10.5946/ce.2020.229
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: A new medical fiber-guided diode laser system (FDLS) is expected to offer high-precision cutting with simultaneous hemostasis. Thus, this study aimed to evaluate the feasibility of using the 1,940-nm FDLS to perform endoscopic submucosal dissection (ESD) in the gastrointestinal tract of an animal model.
Methods
In this prospective animal pilot study, gastric and colorectal ESD using the FDLS was performed in ex vivo and in vivo porcine models. The completeness of en bloc resection, the procedure time, intraprocedural bleeding, histological injuries to the muscularis propria (MP) layer, and perforation were assessed.
Results
The en bloc resection and perforation rates in the ex vivo study were 100% (10/10) and 10% (1/10), respectively; those in the in vivo study were 100% (4/4) and 0% for gastric ESD and 100% (4/4) and 25% (1/4) for rectal ESD, respectively. Deep MP layer injuries tended to occur more frequently in the rectal than in the gastric ESD cases, and no intraprocedural bleeding occurred in either group.
Conclusions
The 1,940-nm FDLS was capable of yielding high en bloc resection rates without intraprocedural bleeding during gastric and colorectal ESD in animal models. -
Citations
Citations to this article as recorded by- Use of Diode Laser in Hysteroscopy for the Management of Intrauterine Pathology: A Systematic Review
Andrea Etrusco, Giovanni Buzzaccarini, Antonio Simone Laganà, Vito Chiantera, Salvatore Giovanni Vitale, Stefano Angioni, Maurizio Nicola D’Alterio, Luigi Nappi, Felice Sorrentino, Amerigo Vitagliano, Tommaso Difonzo, Gaetano Riemma, Liliana Mereu, Alessa
Diagnostics.2024; 14(3): 327. CrossRef - Recent advances in endoscopic management of gastric neoplasms
Hira Imad Cheema, Benjamin Tharian, Sumant Inamdar, Mauricio Garcia-Saenz-de-Sicilia, Cem Cengiz
World Journal of Gastrointestinal Endoscopy.2023; 15(5): 319. CrossRef - Safety and efficacy of dual emission endoscopic laser treatment in patients with upper or lower gastrointestinal vascular lesions causing chronic anemia: results from the first multicenter cohort study
Gian Eugenio Tontini, Lorenzo Dioscoridi, Alessandro Rimondi, Paolo Cantù, Flaminia Cavallaro, Aurora Giannetti, Luca Elli, Luca Pastorelli, Francesco Pugliese, Massimiliano Mutignani, Maurizio Vecchi
Endoscopy International Open.2022; 10(04): E386. CrossRef
- Use of Diode Laser in Hysteroscopy for the Management of Intrauterine Pathology: A Systematic Review
- 3,882 View
- 110 Download
- 3 Web of Science
- 3 Crossref

- Clinical Practice Guideline for the Management of Antithrombotic Agents in Patients Undergoing Gastrointestinal Endoscopy
- Hyun Lim, Eun Jeong Gong, Byung-Hoon Min, Seung Joo Kang, Cheol Min Shin, Jeong-Sik Byeon, Miyoung Choi, Chan Guk Park, Joo Young Cho, Soo Teik Lee, Ho Gak Kim, Hoon Jai Chun
- Clin Endosc 2020;53(6):663-677. Published online November 26, 2020
- DOI: https://doi.org/10.5946/ce.2020.192
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Antithrombotic agents, including antiplatelet agents and anticoagulants, are increasingly used in South Korea. The management of patients using antithrombotic agents and requiring gastrointestinal endoscopy is an important clinical challenge. Although clinical practice guidelines (CPGs) for the management of patients receiving antithrombotic agents and undergoing gastrointestinal endoscopy have been developed in the Unites States, Europe, and Asia Pacific region, it is uncertain whether these guidelines can be adopted in South Korea. After reviewing current CPGs, we identified unmet needs and recognized significant discrepancies in the clinical practice among regions. This is the first CPG in Korea providing information that may assist endoscopists in the management of patients on antithrombotic agents who require diagnostic or elective therapeutic endoscopy. This guideline was developed through the adaptation process as an evidence-based method, with four guidelines retrieved by systematic review. Eligible guidelines were evaluated according to the Appraisal of Guidelines for Research and Evaluation II process, and 13 statements were established using a grading system. This guideline was reviewed by external experts before an official. It will be revised as necessary to cover changes in technology, evidence, or other aspects of clinical practice.
-
Citations
Citations to this article as recorded by- Clinical Practice Guideline for Percutaneous Endoscopic Gastrostomy
Chung Hyun Tae, Ju Yup Lee, Moon Kyung Joo, Chan Hyuk Park, Eun Jeong Gong, Cheol Min Shin, Hyun Lim, Hyuk Soon Choi, Miyoung Choi, Sang Hoon Kim, Chul-Hyun Lim, Jeong-Sik Byeon, Ki-Nam Shim, Geun Am Song, Moon Sung Lee, Jong-Jae Park, Oh Young Lee
Gut and Liver.2024; 18(1): 10. CrossRef - A systematic critical appraisal of clinical practice guidelines of antithrombotic agents in gastrointestinal endoscopy using the AGREE II tool
Denisse Camille Dayto, Wojciech Blonski, Tea Reljic, Farina Klocksieben, Jeffrey Gill, Rene D. Gomez‐Esquivel, Brijesh Patel, Pushpak Taunk, Andrew Sephien, Camille Thelin, Ambuj Kumar
Journal of Gastroenterology and Hepatology.2024; 39(5): 818. CrossRef - The Impact of Sedation on Cardio-Cerebrovascular Adverse Events after Surveillance Esophagogastroduodenoscopy in Patients with Gastric Cancer: A Nationwide Population-Based Cohort Study
Sang Yoon Kim, Jun Kyu Lee, Kwang Hyuck Lee, Jae-Young Jang, Byung-Wook Kim
Gut and Liver.2024; 18(2): 245. CrossRef - International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
Seung Joo Kang, Chung Hyun Tae, Chang Seok Bang, Cheol Min Shin, Young-Hoon Jeong, Miyoung Choi, Joo Ha Hwang, Yutaka Saito, Philip Wai Yan Chiu, Rungsun Rerknimitr, Christopher Khor, Vu Van Khien, Kee Don Choi, Ki-Nam Shim, Geun Am Song, Oh Young Lee
Clinical Endoscopy.2024; 57(2): 141. CrossRef - Top tips on the management of antithrombotic agents in the periendoscopic period
Alberto Tringali
Gastrointestinal Endoscopy.2024; 99(6): 1021. CrossRef - Assessment of delayed bleeding after endoscopic submucosal dissection of early-stage gastrointestinal tumors in patients receiving direct oral anticoagulants
Mitsushige Sugimoto, Masaki Murata, Takashi Kawai
World Journal of Gastroenterology.2023; 29(19): 2916. CrossRef - Clinical practice guidelines for percutaneous endoscopic gastrostomy
Chung Hyun Tae, Ju Yup Lee, Moon Kyung Joo, Chan Hyuk Park, Eun Jeong Gong, Cheol Min Shin, Hyun Lim, Hyuk Soon Choi, Miyoung Choi, Sang Hoon Kim, Chul-Hyun Lim, Jeong-Sik Byeon, Ki-Nam Shim, Geun Am Song, Moon Sung Lee, Jong-Jae Park, Oh Young Lee
Clinical Endoscopy.2023; 56(4): 391. CrossRef - Clinical Practice Guideline for Percutaneous Endoscopic Gastrostomy
Chung Hyun Tae, Ju Yup Lee, Moon Kyung Joo, Chan Hyuk Park, Eun Jeong Gong, Cheol Min Shin, Hyun Lim, Hyuk Soon Choi, Miyoung Choi, Sang Hoon Kim, Chul-Hyun Lim, Jeong-Sik Byeon, Ki-Nam Shim, Geun Am Song, Moon Sung Lee, Jong-Jae Park, Oh Young Lee
The Korean Journal of Gastroenterology.2023; 82(3): 107. CrossRef - Predicting the Bleeding Risk for Patients on Anticoagulant Therapy Prior to Gastric Endoscopic Submucosal Dissection
Jie-Hyun Kim
Journal of Gastric Cancer.2022; 22(1): 1. CrossRef - Utility of a deep learning model and a clinical model for predicting bleeding after endoscopic submucosal dissection in patients with early gastric cancer
Ji Eun Na, Yeong Chan Lee, Tae Jun Kim, Hyuk Lee, Hong-Hee Won, Yang Won Min, Byung-Hoon Min, Jun Haeng Lee, Poong-Lyul Rhee, Jae J Kim
World Journal of Gastroenterology.2022; 28(24): 2721. CrossRef
- Clinical Practice Guideline for Percutaneous Endoscopic Gastrostomy
- 12,886 View
- 945 Download
- 9 Web of Science
- 10 Crossref

- Endoscopic Treatment for Gastrointestinal Stromal Tumors in the Upper Gastrointestinal Tract
- In Kyung Yoo, Joo Young Cho
- Clin Endosc 2020;53(4):383-384. Published online July 3, 2020
- DOI: https://doi.org/10.5946/ce.2020.122
-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- Exploration of a new method for Photoshop-assisted endoscopic ultrasound to distinguish gastrointestinal stromal tumor and leiomyoma
Ying Zhao, Zeyu Wang, Jiageng Tian, Yadi Ren, Man Li
Scandinavian Journal of Gastroenterology.2023; 58(3): 291. CrossRef - On the Track of New Endoscopic Alternatives for the Treatment of Selected Gastric GISTs—A Pilot Study
Artur Raiter, Katarzyna M. Pawlak, Katarzyna Kozłowska-Petriczko, Jan Petriczko, Joanna Szełemej, Anna Wiechowska-Kozłowska
Medicina.2021; 57(6): 625. CrossRef
- Exploration of a new method for Photoshop-assisted endoscopic ultrasound to distinguish gastrointestinal stromal tumor and leiomyoma
- 3,546 View
- 88 Download
- 3 Web of Science
- 2 Crossref

-
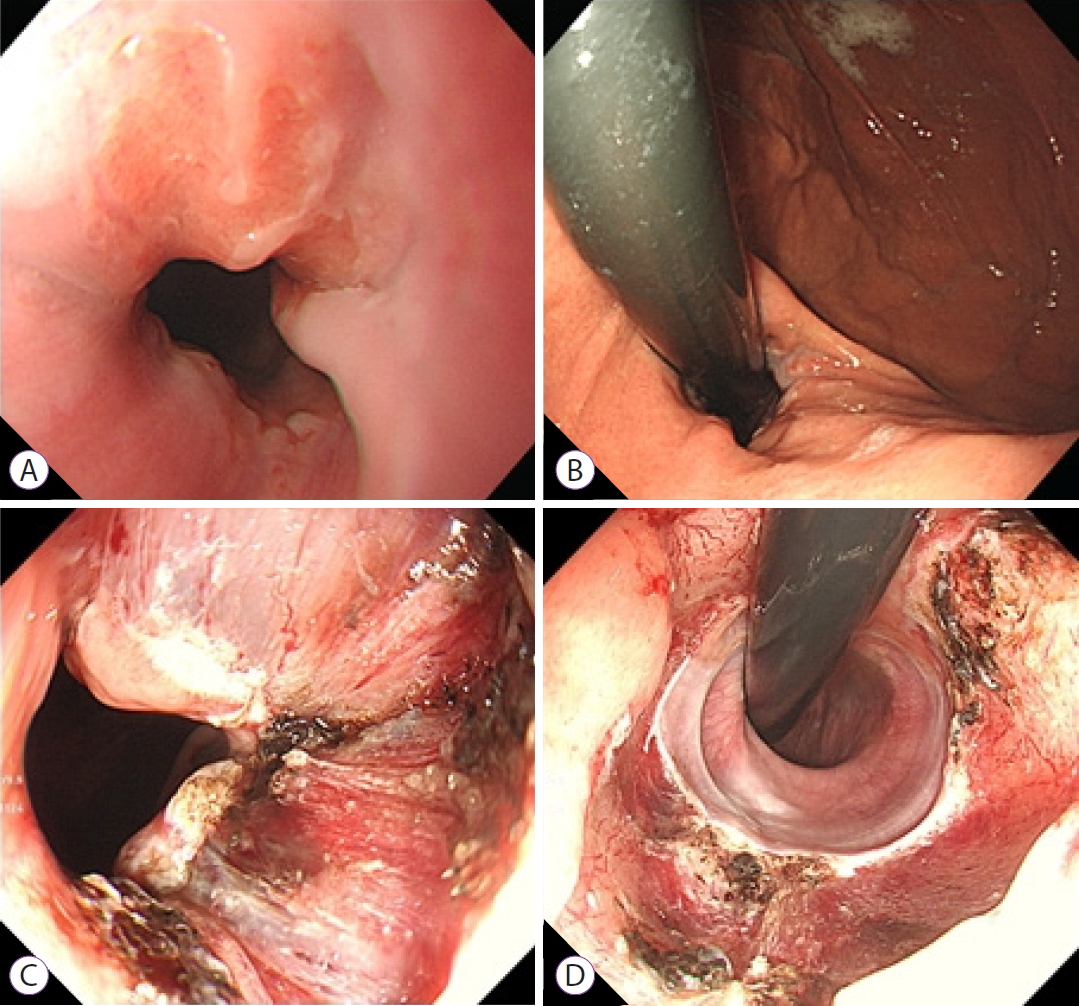
Confocal Laser Endomicroscopic Findings of Refractory Erosive Reflux Disease versus Non-Erosive Reflux Disease with Anti-Reflux Mucosectomy: An in vivo and ex vivo Study

- Eunju Jeong, In Kyung Yoo, Abdullah Özgür Yeniova, Dong Keon Yon, Joo Young Cho
- Clin Endosc 2021;54(1):55-63. Published online May 7, 2020
- DOI: https://doi.org/10.5946/ce.2020.040
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: To date, there is no standard tool to diagnose gastroesophageal reflux disease (GERD). Typically, GERD is a non-erosive reflux disease (NERD) that does not present endoscopic abnormalities. Confocal laser endomicroscopy (CLE) has been shown to be an effective tool to identify and diagnose GERD. We aimed to investigate the cellular and vascular changes in vivo and ex vivo through CLE in patients with GERD.
Methods
Patients with refractory GERD who underwent mucosectomy were recruited. The distal esophagus was observed in vivo using CLE. Mucosectomy tissue was stained with acriflavine and CLE image was obtained ex vivo. We compared cellular and vascular changes in CLE between erosive reflux disease (ERD), NERD, and a control group.
Results
Eleven patients who underwent anti-reflux mucosectomy and five control patients were enrolled in the study. Patients with ERD and NERD presented greater dilated intercellular space than patients in the control group on CLE image. The diameter, number, and cross-sectional area of the intra-papillary capillary loops (IPCLs) were significantly larger in the ERD group than in the NERD group. The irregular shape of the IPCLs were observed in both patients with ERD and NERD.
Conclusions
The irregular shape of the IPCLs were significantly correlated with a positive diagnosis of GERD. CLE may diagnose NERD with high sensitivity and accuracy. -
Citations
Citations to this article as recorded by- Anti-reflux mucosal resection for treatment of refractory gastro-oesophageal reflux disease: Efficacy and impact on perioperative indicators
Xing-Feng Ge, Xian Zhu, Fei Min, Jian-Wei Shen
World Chinese Journal of Digestology.2023; 31(4): 157. CrossRef
- Anti-reflux mucosal resection for treatment of refractory gastro-oesophageal reflux disease: Efficacy and impact on perioperative indicators
- 6,379 View
- 190 Download
- 1 Crossref

-
Hybrid Peroral Endoscopic Myotomy for Achalasia with Prior Treatment Failure

- In Kyung Yoo, Abdullah OzgurYeniova, Joo Young Cho
- Clin Endosc 2021;54(1):127-130. Published online April 2, 2020
- DOI: https://doi.org/10.5946/ce.2020.013

-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Achalasia is a neurodegenerative motility disorder caused by enteric neuron damage in the lower esophageal sphincter. Peroral endoscopic myotomy (POEM) is a standard treatment method for achalasia. Previous treatment modalities may affect the outcome of POEM as they cause submucosal fibrosis. We report a new technique called “hybrid POEM” for the treatment of patients with achalasia who had been previously treated with pneumatic balloon dilatation. We performed two techniques of POEM simultaneously, the standard POEM for the upper part of the submucosal tunnel and open POEM for the stenotic part of the esophagogastric junction. We dissected the mucosa and submucosa, and performed myotomy simultaneously. We overcame submucosal fibrosis of the esophagogastric junction, which was caused by the previous hybrid POEM treatment. The risks of mucosal incision and technical challenge of submucosal tunneling for the fibrotic area may be reduced by hybrid POEM.
- 4,057 View
- 133 Download

- Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer
- Chan Hyuk Park, Dong-Hoon Yang, Jong Wook Kim, Jie-Hyun Kim, Ji Hyun Kim, Yang Won Min, Si Hyung Lee, Jung Ho Bae, Hyunsoo Chung, Kee Don Choi, Jun Chul Park, Hyuk Lee, Min-Seob Kwak, Bun Kim, Hyun Jung Lee, Hye Seung Lee, Miyoung Choi, Dong-Ah Park, Jong Yeul Lee, Jeong-Sik Byeon, Chan Guk Park, Joo Young Cho, Soo Teik Lee, Hoon Jai Chun
- Clin Endosc 2020;53(2):142-166. Published online March 30, 2020
- DOI: https://doi.org/10.5946/ce.2020.032
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Although surgery was the standard treatment for early gastrointestinal cancers, endoscopic resection is now a standard treatment for early gastrointestinal cancers without regional lymph node metastasis. High-definition white light endoscopy, chromoendoscopy, and image-enhanced endoscopy such as narrow band imaging are performed to assess the edge and depth of early gastrointestinal cancers for delineation of resection boundaries and prediction of the possibility of lymph node metastasis before the decision of endoscopic resection. Endoscopic mucosal resection and/or endoscopic submucosal dissection can be performed to remove early gastrointestinal cancers completely by en bloc fashion. Histopathological evaluation should be carefully made to investigate the presence of risk factors for lymph node metastasis such as depth of cancer invasion and lymphovascular invasion. Additional treatment such as radical surgery with regional lymphadenectomy should be considered if the endoscopically resected specimen shows risk factors for lymph node metastasis. This is the first Korean clinical practice guideline for endoscopic resection of early gastrointestinal cancer. This guideline was developed by using mainly de novo methods and encompasses endoscopic management of superficial esophageal squamous cell carcinoma, early gastric cancer, and early colorectal cancer. This guideline will be revised as new data on early gastrointestinal cancer are collected.
-
Citations
Citations to this article as recorded by- Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study
Hae Won Yoo, Su Jin Hong, Shin Hee Kim
Gastroenterology.2024; 166(2): 313. CrossRef - A Modified eCura System to Stratify the Risk of Lymph Node Metastasis in Undifferentiated-Type Early Gastric Cancer After Endoscopic Resection
Hyo-Joon Yang, Hyuk Lee, Tae Jun Kim, Da Hyun Jung, Kee Don Choi, Ji Yong Ahn, Wan Sik Lee, Seong Woo Jeon, Jie-Hyun Kim, Gwang Ha Kim, Jae Myung Park, Sang Gyun Kim, Woon Geon Shin, Young-Il Kim, Il Ju Choi
Journal of Gastric Cancer.2024; 24(2): 172. CrossRef - Management after non-curative endoscopic resection of T1 rectal cancer
Hao Dang, Daan A. Verhoeven, Jurjen J. Boonstra, Monique E. van Leerdam
Best Practice & Research Clinical Gastroenterology.2024; 68: 101895. CrossRef - Tumor size discrepancy between endoscopic and pathological evaluations in colorectal endoscopic submucosal dissection
Takeshi Onda, Osamu Goto, Toshiaki Otsuka, Yoshiaki Hayasaka, Shun Nakagome, Tsugumi Habu, Yumiko Ishikawa, Kumiko Kirita, Eriko Koizumi, Hiroto Noda, Kazutoshi Higuchi, Jun Omori, Naohiko Akimoto, Katsuhiko Iwakiri
World Journal of Gastrointestinal Endoscopy.2024; 16(3): 136. CrossRef - Nomograms and prognosis for superficial esophageal squamous cell carcinoma
Hong Tao Lin, Ahmed Abdelbaki, Somashekar G Krishna
World Journal of Gastroenterology.2024; 30(10): 1291. CrossRef - A new clinical model for predicting lymph node metastasis in T1 colorectal cancer
Kai Wang, Hui He, Yanyun Lin, Yanhong Zhang, Junguo Chen, Jiancong Hu, Xiaosheng He
International Journal of Colorectal Disease.2024;[Epub] CrossRef - The role of endoluminal surgery in a colorectal surgical practice. A global view
Ilker Ozgur, Fevzi Cengiz
Seminars in Colon and Rectal Surgery.2024; 35(2): 101023. CrossRef - Enhanced multi-class pathology lesion detection in gastric neoplasms using deep learning-based approach and validation
Byeong Soo Kim, Bokyung Kim, Minwoo Cho, Hyunsoo Chung, Ji Kon Ryu, Sungwan Kim
Scientific Reports.2024;[Epub] CrossRef - Innovations in dedicated PET instrumentation: from the operating room to specimen imaging
Hossein Arabi, Abdollah Saberi Manesh, Habib Zaidi
Physics in Medicine & Biology.2024; 69(11): 11TR03. CrossRef - Venous invasion and lymphatic invasion are correlated with the postoperative prognosis of pancreatic neuroendocrine neoplasm
Sho Kiritani, Junichi Arita, Yuichiro Mihara, Rihito Nagata, Akihiko Ichida, Yoshikuni Kawaguchi, Takeaki Ishizawa, Nobuhisa Akamatsu, Junichi Kaneko, Kiyoshi Hasegawa
Surgery.2023; 173(2): 365. CrossRef - Long-term outcomes after endoscopic versus surgical resection of T1 colorectal carcinoma
Hyun Jin Bae, Hoyeon Ju, Han Hee Lee, Jinsu Kim, Bo-In Lee, Sung Hak Lee, Daeyoun David Won, Yoon Suk Lee, In Kyu Lee, Young-Seok Cho
Surgical Endoscopy.2023; 37(2): 1231. CrossRef - Resection speed of endoscopic submucosal dissection according to the location of gastric neoplasia: a learning curve using cumulative sum analysis
Jun-Hyung Cho, So-Young Jin, Suyeon Park
Surgical Endoscopy.2023; 37(4): 2969. CrossRef - Endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma: long-term follow-up in a Western center
Andreas Probst, Alanna Ebigbo, Stefan Eser, Carola Fleischmann, Tina Schaller, Bruno Märkl, Stefan Schiele, Bernd Geissler, Gernot Müller, Helmut Messmann
Clinical Endoscopy.2023; 56(1): 55. CrossRef - A Randomized Controlled Trial of Fibrin Glue to Prevent Bleeding After Gastric Endoscopic Submucosal Dissection
Hyun Deok Lee, Eunwoo Lee, Sang Gyun Kim, Cheol Min Shin, Jun Chul Park, Kee Don Choi, Seokyung Hahn, Soo-Jeong Cho
American Journal of Gastroenterology.2023; 118(5): 892. CrossRef - Endoscopic Resection of Undifferentiated Early Gastric Cancer
Yuichiro Hirai, Seiichiro Abe, Mai Ego Makiguchi, Masau Sekiguchi, Satoru Nonaka, Haruhisa Suzuki, Shigetaka Yoshinaga, Yutaka Saito
Journal of Gastric Cancer.2023; 23(1): 146. CrossRef - Pre-procedure oral administration of pronase improves efficacy of lugol chromoendoscopy in esophageal squamous cell carcinoma screening: a prospective, double-blinded, randomized, controlled trial
Xin Zhao, Meng Guo, Shaohua Zhu, Linhui Zhang, Tao Dong, Hui Luo, Weihua Yu, Jiangyi Zhu, Xiaotong Fan, Ying Han, Zhiguo Liu
Surgical Endoscopy.2023; 37(6): 4421. CrossRef - Endoscopic advances in the management of gastric cancer and premalignant gastric conditions
Erica Park, Makoto Nishimura, Priya Simoes
World Journal of Gastrointestinal Endoscopy.2023; 15(3): 114. CrossRef - Comparative Cost Analysis Between Endoscopic Resection and Surgery for Submucosal Colorectal Cancer
Soo Min Noh, Sung Wook Hwang, Sang Hyoung Park, Dong-Hoon Yang, Byong Duk Ye, In Ja Park, Seok-Byung Lim, Jeong-Sik Byeon
Diseases of the Colon & Rectum.2023; 66(5): 723. CrossRef - Descriptive Analysis of Gastric Cancer Mortality in Korea, 2000-2020
Tung Hoang, Hyeongtaek Woo, Sooyoung Cho, Jeeyoo Lee, Sayada Zartasha Kazmi, Aesun Shin
Cancer Research and Treatment.2023; 55(2): 603. CrossRef - Endoscopically injectable and self‐crosslinkable hydrogel‐mediated stem cell transplantation for alleviating esophageal stricture after endoscopic submucosal dissection
Hyunsoo Chung, Soohwan An, Seung Yeop Han, Jihoon Jeon, Seung‐Woo Cho, Yong Chan Lee
Bioengineering & Translational Medicine.2023;[Epub] CrossRef - Deep learning-based clinical decision support system for gastric neoplasms in real-time endoscopy: development and validation study
Eun Jeong Gong, Chang Seok Bang, Jae Jun Lee, Gwang Ho Baik, Hyun Lim, Jae Hoon Jeong, Sung Won Choi, Joonhee Cho, Deok Yeol Kim, Kang Bin Lee, Seung-Il Shin, Dick Sigmund, Byeong In Moon, Sung Chul Park, Sang Hoon Lee, Ki Bae Bang, Dae-Soon Son
Endoscopy.2023; 55(08): 701. CrossRef - A 6-year nationwide population-based study on the current status of gastric endoscopic resection in Korea using administrative data
Jae Yong Park, Mi-Sook Kim, Beom Jin Kim, Jae Gyu Kim
Scientific Reports.2023;[Epub] CrossRef - Efficacy and Safety of ClearCut™ Knife H-type in Endoscopic Submucosal Dissection for Gastric Neoplasms: A Multicenter, Randomized Trial
Eun Jeong Gong, Hyun Lim, Sang Jin Lee, Do Hoon Kim
Journal of Gastric Cancer.2023; 23(3): 451. CrossRef - Endoscopic resection for local residual or recurrent cancer after definitive chemoradiotherapy or radiotherapy for esophageal squamous cell carcinoma
Yasuhiro Tani, Ryu Ishihara, Noriko Matsuura, Yuki Okubo, Yushi Kawakami, Hirohisa Sakurai, Takahiko Nakamura, Katsunori Matsueda, Muneaki Miyake, Satoki Shichijo, Akira Maekawa, Takashi Kanesaka, Sachiko Yamamoto, Yoji Takeuchi, Koji Higashino, Noriya Ue
Scientific Reports.2023;[Epub] CrossRef - Modified underwater endoscopic mucosal resection for intermediate-sized sessile colorectal polyps
Dong Hyun Kim, Seon-Young Park, Hye-Su You, Yong-Wook Jung, Young-Eun Joo, Dae-Seong Myung, Hyun-Soo Kim, Nah Ihm Kim, Seong-Jung Kim, Jae Kyun Ju
Frontiers in Medicine.2023;[Epub] CrossRef - Clinical Application of the Kyoto Classification of Gastritis
Gwang Ha Kim
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2023; 23(2): 89. CrossRef - Endoscopic Resection for Gastric Adenocarcinoma of the Fundic Gland Type: A Case Series
Hwa Jin Lee, Gwang Ha Kim, Dong Chan Joo, Moon Won Lee, Bong Eun Lee, Kyungbin Kim
The Korean Journal of Gastroenterology.2023; 81(6): 259. CrossRef - The optimal interval of surveillance gastroscopy after endoscopic resection for gastric neoplasia: a multicenter cohort study
Younghee Choe, Byung-Wook Kim, Tae Ho Kim, Jun-Won Chung, Jongwon Kim, Soo-Young Na, Joon Sung Kim
Surgical Endoscopy.2023; 37(10): 7556. CrossRef - External Validation of the eCura System for Undifferentiated-Type Early Gastric Cancer with Noncurative Endoscopic Resection
Hyo-Joon Yang, Young-Il Kim, Ji Yong Ahn, Kee Don Choi, Sang Gyun Kim, Seong Woo Jeon, Jie-Hyun Kim, Sung Kwan Shin, Hyuk Lee, Wan Sik Lee, Gwang Ha Kim, Jae Myung Park, Woon Geon Shin, Il Ju Choi
Gut and Liver.2023; 17(4): 537. CrossRef - Outcomes of the Conventional versus Pocket-Creation Method for Endoscopic Submucosal Dissection of Gastric Body Tumors Using a Dual Knife: A Retrospective Study
Sang Pyo Lee, Hyun Joo Jang, Sea Hyub Kae, Jae Gon Lee
Gut and Liver.2023; 17(4): 547. CrossRef - LEARNING CURVE IN ESOPHAGEAL ENDOSCOPIC SUBMUCOSAL DISSECTION BY WESTERN ENDOSCOSPISTS TRAINED IN JAPAN: EXPERIENCE IN LATIN AMERICA
Josué ALIAGA RAMOS, Naohisa YOSHIDA, Rafiz ABDUL RANI, Vitor N ARANTES
Arquivos de Gastroenterologia.2023; 60(2): 208. CrossRef - Diagnostic Performance of Endoscopic Ultrasonography with Water-Filled Balloon Method for Superficial Esophageal Squamous Cell Carcinoma
Yugo Suzuki, Kosuke Nomura, Daisuke Kikuchi, Toshiro Iizuka, Mako Koseki, Yusuke Kawai, Takayuki Okamura, Yorinari Ochiai, Junnosuke Hayasaka, Yutaka Mitsunaga, Hiroyuki Odagiri, Satoshi Yamashita, Akira Matsui, Kenichi Ohashi, Shu Hoteya
Digestive Diseases and Sciences.2023; 68(10): 3974. CrossRef - Should All Undifferentiated Early Gastric Cancer Patients Undergoing Noncurative Endoscopic Resection Be Sent to the Operating Room?
Jung-Wook Kim, Albert C. Kim
Gut and Liver.2023; 17(5): 665. CrossRef - Comparing endoscopic mucosal resection with endoscopic submucosal dissection in colorectal adenoma and tumors: Meta-analysis and system review
Nian Wang, Lei Shu, Song Liu, Lin Yang, Tao Bai, Zhaohong Shi, Xinghuang Liu, Paolo Aurello
PLOS ONE.2023; 18(9): e0291916. CrossRef - Weighing the benefits of lymphadenectomy in early-stage colorectal cancer
Seung Min Baik, Ryung-Ah Lee
Annals of Surgical Treatment and Research.2023; 105(5): 245. CrossRef - Endoscopic treatment of colorectal polyps and early colorectal cancer
Yunho Jung
Journal of the Korean Medical Association.2023; 66(11): 642. CrossRef - Endoscopic submucosal dissection for early gastric cancer: It is time to consider the quality of its outcomes
Gwang Ha Kim
World Journal of Gastroenterology.2023; 29(43): 5800. CrossRef - A Retrospective Multicenter Study of Risk Factors, Stratification, and Prognosis of Lymph Node Metastasis in T1 and T2 Colorectal Cancer
Eui Myung Kim, Il Tae Son, Byung Chun Kim, Jun Ho Park, Byung Mo Kang, Jong Wan Kim
Journal of Clinical Medicine.2023; 12(24): 7744. CrossRef - A nomogram for predicting the risk of postoperative fever in elderly patients undergoing endoscopic submucosal dissection of the upper gastrointestinal tract
Zhixiang Xu, Jing Zhuang, Xin Zhu, Jun Yao
Medicine.2023; 102(50): e36438. CrossRef - Usage trends of colorectal endoscopic submucosal dissection according to hospital types based on nationwide claims data
Ji Eun Na, Bohyoung Kim, Sung Hoon Jung, Arum Choi, Sukil Kim, Tae-Oh Kim
Medicine.2023; 102(43): e35514. CrossRef - Long-term outcomes of endoscopic resection followed by additional surgery after non-curative resection in undifferentiated-type early gastric cancer: a nationwide multi-center study
Jie-Hyun Kim, Young-Il Kim, Ji Yong Ahn, Woon Geon Shin, Hyo-Joon Yang, Su Youn Nam, Byung-Hoon Min, Jae-Young Jang, Joo Hyun Lim, Wan Sik Lee, Bong Eun Lee, Moon Kyung Joo, Jae Myung Park, Hang Lak Lee, Tae-Geun Gweon, Moo In Park, Jeongmin Choi, Chung H
Surgical Endoscopy.2022; 36(3): 1847. CrossRef - A Simple Risk Scoring System for Predicting the Occurrence of Aspiration Pneumonia After Gastric Endoscopic Submucosal Dissection
Kyemyung Park, Na Young Kim, Ki Jun Kim, Chaerim Oh, Dongwoo Chae, So Yeon Kim
Anesthesia & Analgesia.2022; 134(1): 114. CrossRef - Long-term outcomes of endoscopic mucosal resection for early-stage esophageal adenocarcinoma
Kesha Oza, Tejasvi Peesay, Benjamin Greenspun, John E. Carroll, Shervin Shafa, Jay C. Zeck, Nadim G. Haddad, Marc Margolis, Puja Gaur Khaitan
Surgical Endoscopy.2022; 36(7): 5136. CrossRef - Long-Term Outcomes and Prognostic Factors of Superficial Esophageal Cancer in Patients Aged ≥ 65 Years
Jin Won Chang, Da Hyun Jung, Cheal Wung Huh, Jun Chul Park, Sung Kwan Shin, Sang Kil Lee, Yong Chan Lee
Frontiers in Medicine.2022;[Epub] CrossRef - Tumor Location as a Prognostic Factor in T1 Colorectal Cancer
Katsuro Ichimasa, Shin-ei Kudo, Yuta Kouyama, Kenichi Mochizuki, Yuki Takashina, Masashi Misawa, Yuichi Mori, Takemasa Hayashi, Kunihiko Wakamura, Hideyuki Miyachi
Journal of the Anus, Rectum and Colon.2022; 6(1): 9. CrossRef - Artificial Intelligence for Detecting and Delineating Margins of Early ESCC Under WLI Endoscopy
Wei Liu, Xianglei Yuan, Linjie Guo, Feng Pan, Chuncheng Wu, Zhongshang Sun, Feng Tian, Cong Yuan, Wanhong Zhang, Shuai Bai, Jing Feng, Yanxing Hu, Bing Hu
Clinical and Translational Gastroenterology.2022; 13(1): e00433. CrossRef - Machine Learning Model to Stratify the Risk of Lymph Node Metastasis for Early Gastric Cancer: A Single-Center Cohort Study
Ji-Eun Na, Yeong-Chan Lee, Tae-Jun Kim, Hyuk Lee, Hong-Hee Won, Yang-Won Min, Byung-Hoon Min, Jun-Haeng Lee, Poong-Lyul Rhee, Jae J. Kim
Cancers.2022; 14(5): 1121. CrossRef - Chinese consensus on prevention of colorectal neoplasia (2021, Shanghai)
Journal of Digestive Diseases.2022; 23(2): 58. CrossRef - Advances in the application of regenerative medicine in prevention of post-endoscopic submucosal dissection for esophageal stenosis
Jiaxin Wang, Yan Zhao, Peng Li, Shutian Zhang
Journal of Translational Internal Medicine.2022; 10(1): 28. CrossRef - Prolonged ischemia of the ileum and colon after surgical mucosectomy explains contraction and failure of “mucus free” bladder augmentation
Dániel Urbán, Gabriella Varga, Dániel Érces, Mahmoud Marei Marei, Raimondo Cervellione, David Keene, Anju Goyal, Tamás Cserni
Journal of Pediatric Urology.2022; 18(4): 500.e1. CrossRef - Safety and efficacy of prophylactic gastric open peroral endoscopic myotomy for prevention of post‐ESD stenosis: A case series (with video)
Won Dong Lee, Jae Sun Song, Byung Sun Kim, Min A. Yang, Young Jae Lee, Gum Mo Jung, Ji Woong Kim, Yong Keun Cho, Jin Woong Cho
Journal of Digestive Diseases.2022; 23(4): 220. CrossRef - Therapeutic approach to non-curative resection after endoscopic treatment in early gastric cancer
Eun Jeong Gong, Chang Seok Bang
Journal of the Korean Medical Association.2022; 65(5): 284. CrossRef - Endoscopic treatment for early gastric cancer
Ji Yong Ahn
Journal of the Korean Medical Association.2022; 65(5): 276. CrossRef - Endoscopic diagnosis of early gastric cancer
Dong Chan Joo, Gwang Ha Kim
Journal of the Korean Medical Association.2022; 65(5): 267. CrossRef - Current status of the gastric cancer screening program in Korea
Young-Il Kim, Il Ju Choi
Journal of the Korean Medical Association.2022; 65(5): 250. CrossRef - Colorectális polypok ellátása
Szabolcs Ábrahám, Illés Tóth, Dániel Váczi, György Lázár
Magyar Sebészet.2022; 75(2): 155. CrossRef - Utility of a deep learning model and a clinical model for predicting bleeding after endoscopic submucosal dissection in patients with early gastric cancer
Ji Eun Na, Yeong Chan Lee, Tae Jun Kim, Hyuk Lee, Hong-Hee Won, Yang Won Min, Byung-Hoon Min, Jun Haeng Lee, Poong-Lyul Rhee, Jae J Kim
World Journal of Gastroenterology.2022; 28(24): 2721. CrossRef - Rare primary rectal mucosa-associated lymphoid tissue lymphoma with curative resection by endoscopic submucosal dissection: A case report and review of literature
Yan Tao, Qiong Nan, Zi Lei, Ying-Lei Miao, Jun-Kun Niu
World Journal of Clinical Cases.2022; 10(21): 7599. CrossRef - Prevention of stricture after endoscopic submucosal dissection for esophageal cancer: intralesional steroid infusion using a spray tube
Jong Yeul Lee
Clinical Endoscopy.2022; 55(4): 516. CrossRef - Paneth Cell Carcinoma of the Stomach
Jun Wan Kim, Gwang Ha Kim, Kyung Bin Kim
The Korean Journal of Gastroenterology.2022; 80(1): 34. CrossRef - Composite scoring system and optimal tumor budding cut-off number for estimating lymph node metastasis in submucosal colorectal cancer
Jeong-ki Kim, Ye-Young Rhee, Jeong Mo Bae, Jung Ho Kim, Seong-Joon Koh, Hyun Jung Lee, Jong Pil Im, Min Jung Kim, Seung-Bum Ryoo, Seung-Yong Jeong, Kyu Joo Park, Ji Won Park, Gyeong Hoon Kang
BMC Cancer.2022;[Epub] CrossRef - Multidisciplinary Treatment Strategy for Early Colon Cancer: A Review-An English Version
Gyung Mo Son, Su Bum Park, Tae Un Kim, Byung-Soo Park, In Young Lee, Joo-Young Na, Dong Hoon Shin, Sang Bo Oh, Sung Hwan Cho, Hyun Sung Kim, Hyung Wook Kim
Journal of the Anus, Rectum and Colon.2022; 6(4): 203. CrossRef - Extragastric Metastasis of Early Gastric Cancer After Endoscopic Submucosal Dissection With Lymphovascular Invasion and Negative Resected Margins
Han Myung Lee, Yoonjin Kwak, Hyunsoo Chung, Sang Gyun Kim, Soo-Jeong Cho
Journal of Gastric Cancer.2022; 22(4): 339. CrossRef - Comparison between a novel core knife and the conventional IT knife 2 for endoscopic submucosal dissection of gastric mucosal lesions
Myeongsoon Park, Jin Wook Lee, Dong Woo Shin, Jungseok Kim, Yoo Jin Lee, Ju Yup Lee, Kwang Bum Cho
Clinical Endoscopy.2022; 55(6): 767. CrossRef - Need for careful endoscopic evaluation of large gastric neoplasms before endoscopic submucosal dissection
Seung Woo Lee
Clinical Endoscopy.2022; 55(6): 753. CrossRef - Regression of gastric endoscopic submucosal dissection induced polypoid nodular scar after Helicobacter pylori eradication: A case report
Byung Chul Jin, Ae Ri Ahn, Seong-Hun Kim, Seung Young Seo
World Journal of Clinical Cases.2022; 10(34): 12793. CrossRef - Simultaneous analysis of tumor-infiltrating immune cells density, tumor budding status, and presence of lymphoid follicles in CRC tissue
Adam R. Markowski, Anna J. Markowska, Wiktoria Ustymowicz, Anna Pryczynicz, Katarzyna Guzińska-Ustymowicz
Scientific Reports.2022;[Epub] CrossRef - Role of Endoscopy in Management of Upper Gastrointestinal Cancers
Jeff Liang, Yi Jiang, Yazan Abboud, Srinivas Gaddam
Diseases.2022; 11(1): 3. CrossRef - Association between severe hepatic steatosis examined by Fibroscan and the risk of high-risk colorectal neoplasia
Kwang Woo Kim, Hyoun Woo Kang, Hosun Yoo, Yukyung Jun, Hyun Jung Lee, Jong Pil Im, Ji Won Kim, Joo Sung Kim, Seong-Joon Koh, Yong Jin Jung, Atsushi Hosui
PLOS ONE.2022; 17(12): e0279242. CrossRef - Long-term outcomes and clinical safety of expanded indication early gastric cancer treated with endoscopic submucosal dissection versus surgical resection: a meta-analysis
Xing Xu, Guoliang Zheng, Na Gao, Zhichao Zheng
BMJ Open.2022; 12(12): e055406. CrossRef - Long-Term Safety of Delayed Surgery After Upfront Endoscopic Resection for Early Gastric Cancer: A Propensity Matched Study
Ji Eun Na, Yeong Gi Kim, Tae Jun Kim, Hyuk Lee, Yang Won Min, Byung-Hoon Min, Jun Haeng Lee, Seon Yeong Baek, Min Su Park, Poong-Lyul Rhee, Jae J. Kim
Annals of Surgical Oncology.2021; 28(1): 106. CrossRef - Incidence rates, risk factors, and outcomes of aspiration pneumonia after gastric endoscopic submucosal dissection: A systematic review and meta‐analysis
Dong Tang, Fuxiang Yuan, Xiaoying Ma, Haixia Qu, Yuan Li, Weiwei Zhang, Huan Ma, Haiping Liu, Yan Yang, Lin Xu, Yuqiang Gao, Shuhui Zhan
Journal of Gastroenterology and Hepatology.2021; 36(6): 1457. CrossRef - Clinical feasibility and oncologic safety of primary endoscopic submucosal dissection for clinical submucosal invasive early gastric cancer
Ji Eun Na, Hyuk Lee, Yang Won Min, Byung-Hoon Min, Jun Haeng Lee, Poong-Lyul Rhee, Kyoung-Mee Kim, Jae J. Kim
Journal of Cancer Research and Clinical Oncology.2021; 147(10): 3051. CrossRef - Endoscopic resections for superficial esophageal squamous cell epithelial neoplasia: focus on histological discrepancies between biopsy and resected specimens
Lang Yang, Hua Jin, Xiao-li Xie, Yang-tian Cao, Zhen-hua Liu, Na Li, Peng Jin, Yu-qi He, Jian-qiu Sheng
BMC Gastroenterology.2021;[Epub] CrossRef - Atypical Scar Patterns after Gastric Endoscopic Submucosal Dissection
Bomin Kim, Beom Jin Kim, Hong Jip Yoon, Hyunsuk Lee, Jae Yong Park, Chang Hwan Choi, Jae Gyu Kim
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2021; 21(1): 72. CrossRef - Endoscopic Submucosal Dissection versus Surgery for Undifferentiated-Type Early Gastric Cancer: A Systematic Review and Meta-Analysis
Cheal-Wung Huh, Dae Won Ma, Byung-Wook Kim, Joon Sung Kim, Seung Jae Lee
Clinical Endoscopy.2021; 54(2): 202. CrossRef - Role of Endoscopic Ultrasound in Selecting Superficial Esophageal Cancers for Endoscopic Resection
Jinju Choi, Hyunsoo Chung, Ayoung Lee, Jue Lie Kim, Soo-Jeong Cho, Sang Gyun Kim
The Annals of Thoracic Surgery.2021; 111(5): 1689. CrossRef - Recent advances in early esophageal cancer: diagnosis and treatment based on endoscopy
Hang Yang, Bing Hu
Postgraduate Medicine.2021; 133(6): 665. CrossRef - Endoscopic Resection of Gastric Cancer
Ga Hee Kim, Hwoon-Yong Jung
Gastrointestinal Endoscopy Clinics of North America.2021; 31(3): 563. CrossRef - Gastric Mucosa-Associated Lymphoid Tissue Lymphomas Diagnosed by Jumbo Biopsy Using Endoscopic Submucosal Dissection: A Case Report
Jian Han, Jun Wang, Hua-ping Xie
Frontiers in Medicine.2021;[Epub] CrossRef - Considerations for Endoscopic Treatment of Undifferentiated-type Early Gastric Cancer
Kyoungwon Jung
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2021; 21(2): 103. CrossRef - Papillary Adenocarcinoma
Tae-Se Kim, Byung-Hoon Min
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2021; 21(2): 122. CrossRef - Early gastrointestinal cancer: The application of artificial intelligence
Hang Yang, Bing Hu
Artificial Intelligence in Gastrointestinal Endoscopy.2021; 2(4): 185. CrossRef - Successful Endoscopic Resection of Primary Rectal Mucosa-Associated Lymphoid Tissue Lymphoma by Endoscopic Submucosal Dissection: A Case Report
Jian Han, Zhe Zhu, Chao Zhang, Hua-ping Xie
Frontiers in Medicine.2021;[Epub] CrossRef - Assessment of the Diagnostic Performance of Endoscopic Ultrasonography After Conventional Endoscopy for the Evaluation of Esophageal Squamous Cell Carcinoma Invasion Depth
Ryu Ishihara, Junki Mizusawa, Ryoji Kushima, Noriko Matsuura, Tomonori Yano, Tomoko Kataoka, Haruhiko Fukuda, Noboru Hanaoka, Toshiyuki Yoshio, Seiichiro Abe, Yoshinobu Yamamoto, Shinji Nagata, Hiroyuki Ono, Masashi Tamaoki, Naohiro Yoshida, Kohei Takizaw
JAMA Network Open.2021; 4(9): e2125317. CrossRef - Variation in Diagnosis, Treatment, and Outcome of Esophageal Cancer in a Regionalized Care System in Ontario, Canada
Steven Habbous, Olga Yermakhanova, Katharina Forster, Claire M. B. Holloway, Gail Darling
JAMA Network Open.2021; 4(9): e2126090. CrossRef - Risk Stratification of T1 Colorectal Cancer Metastasis to Lymph Nodes: Current Status and Perspective
Katsuro Ichimasa, Shin-ei Kudo, Hideyuki Miyachi, Yuta Kouyama, Masashi Misawa, Yuichi Mori
Gut and Liver.2021; 15(6): 818. CrossRef - Close Observation versus Additional Surgery after Noncurative Endoscopic Resection of Esophageal Squamous Cell Carcinoma
Byeong Geun Song, Ga Hee Kim, Charles J. Cho, Hyeong Ryul Kim, Yang Won Min, Hyuk Lee, Byung-Hoon Min, Ho June Song, Yong-Hee Kim, Jun Haeng Lee, Hwoon-Yong Jung, Jae Ill Zo, Young Mog Shim
Digestive Surgery.2021; 38(3): 247. CrossRef
- Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study
- 15,875 View
- 1,123 Download
- 88 Web of Science
- 88 Crossref

- The Role of Dual Red Imaging in Gastric Endoscopic Submucosal Dissection
- In Kyung Yoo, Joo Young Cho
- Clin Endosc 2020;53(1):1-2. Published online January 30, 2020
- DOI: https://doi.org/10.5946/ce.2020.018
-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- Near infrared imaging system for preventing blood vision obstruction in endoscopy
Meng-Huang Wu, Jason C. Hsu, Jin-Sung Kim, Tsung-Jen Huang, Yi-Hung Huang, Hon Pan Yiu, Ching-Yu Lee, Jowy Tani, Cheng-Chun Chang
Optics Express.2023; 31(26): 43877. CrossRef - Clinically Available Optical Imaging Technologies in Endoscopic Lesion Detection: Current Status and Future Perspective
Zhongyu He, Peng Wang, Yuelong Liang, Zuoming Fu, Xuesong Ye, Aiping Liu
Journal of Healthcare Engineering.2021; 2021: 1. CrossRef
- Near infrared imaging system for preventing blood vision obstruction in endoscopy
- 3,690 View
- 112 Download
- 2 Web of Science
- 2 Crossref

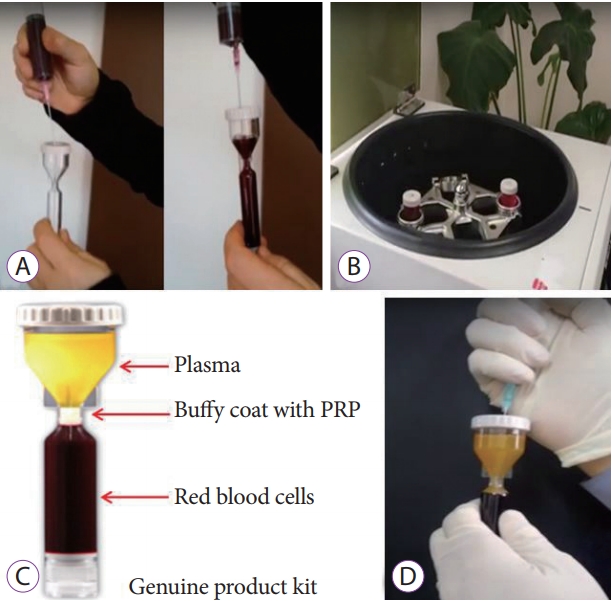
- Effectiveness of Autologous Platelet-Rich Plasma for the Healing of Ulcers after Endoscopic Submucosal Dissection
- Eunju Jeong, In kyung Yoo, Ozlem Ozer Cakir, Hee Kyung Kim, Won Hee Kim, Sung Pyo Hong, Joo Young Cho
- Clin Endosc 2019;52(5):472-478. Published online May 17, 2019
- DOI: https://doi.org/10.5946/ce.2018.152
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Platelet-rich plasma (PRP) has been used for wound healing in various medical fields. The aim of this study was to evaluate the clinical efficacy and safety of local PRP injections after endoscopic submucosal dissection (ESD).
Methods
Patients were non-randomly divided into the following two groups: (1) control group in which patients were administered only an intravenous proton pump inhibitor (PPI), and (2) a study group in which patients were administered an intravenous PPI and a topical PRP injection. We assessed the reduction in the ulcer area and stage of the ulcer after the procedure (24 hours, 48 hours, and 28 days after endoscopic surgery).
Results
We enrolled 7 study and 7 control patients. In the study group, the rate of ulcer reduction was 59% compared to 52% in the control group (p=0.372), 28 days after ESD. There were 5 patients in the S stage and 2 patients in the H stage in the study group compared to no patient in the S stage and 7 patients in the H stage in the control group (p=0.05), 28 days after ESD. There were no serious complications in either group.
Conclusions
The local injection of PRP is a safe and effective procedure for ulcer healing after ESD. -
Citations
Citations to this article as recorded by- Clinical efficacy of blood derivatives on wound healing: A systematic review and network meta‐analysis
Yanhong Wu, Guang Peng, Yuzhi Wang, Jianwu Chen, Bin Zhang, Jianbing Tang, Biao Cheng
International Wound Journal.2024;[Epub] CrossRef - Endoscopic Shielding With Platelet-rich Plasma After Resection Of Large Colorectal Lesions
Vicente Lorenzo-Zúñiga, Vicente Moreno de Vega, Ramón Bartolí
Surgical Laparoscopy, Endoscopy & Percutaneous Techniques.2021; 31(3): 376. CrossRef - The Additive Effect of Platelet-Rich Plasma in the Treatment of Actively Bleeding Peptic Ulcer
Waseem M. Seleem, Amr Shaaban Hanafy
Clinical Endoscopy.2021; 54(6): 864. CrossRef - Endless Challenges in Overcoming Complications Associated with Endoscopic Submucosal Dissection
Satoshi Ono, Shun Ito, Kenji Ogata
Clinical Endoscopy.2019; 52(5): 395. CrossRef
- Clinical efficacy of blood derivatives on wound healing: A systematic review and network meta‐analysis
- 6,534 View
- 139 Download
- 4 Web of Science
- 4 Crossref

- Endoscopic Submucosal Dissection Followed by Concurrent Chemoradiotherapy in Patients with Early Esophageal Cancer with a High Risk of Lymph Node Metastasis
- Hee Kyung Kim, Weon Jin Ko, Chang-Il Kwon, Ga Won Song, In Kyun Yoo, Ji Hyun Song, Hak Su Kim, Joo Young Cho
- Clin Endosc 2019;52(5):502-505. Published online May 14, 2019
- DOI: https://doi.org/10.5946/ce.2018.176
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic submucosal dissection is recommended as an alternative therapy for early esophageal cancer. However, achieving curative resection in this procedure remains controversial since precise prediction of lymph node metastasis can be difficult. Here, we present the preliminary results of endoscopic submucosal dissection followed by concurrent chemoradiotherapy for early esophageal cancer with a high risk of lymph node metastasis. From May 2006 to January 2014, six patients underwent concurrent chemoradiotherapy after endoscopic submucosal dissection with a median follow-up period of 63 months. No complications were encountered during concurrent chemoradiotherapy. Although local recurrence did not occur in all patients, two patients were diagnosed with metachronous cancer. Overall, the survival rate was 100%. Thus, endoscopic submucosal dissection followed by concurrent chemoradiotherapy may be a feasible treatment for early esophageal cancer in patients with a high risk of lymph node metastasis. Future prospective large-scale studies are warranted to confirm our results.
-
Citations
Citations to this article as recorded by- Unveiling Therapeutic Targets for Esophageal Cancer: A Comprehensive Review
Rakesh Acharya, Ananya Mahapatra, Henu Kumar Verma, L. V. K. S. Bhaskar
Current Oncology.2023; 30(11): 9542. CrossRef - Editorial “Discrepancy Between the Clinical and Final Pathological Findings of Lymph Node Metastasis in Superficial Esophageal Cancer”
Rian M. Hasson, Joseph D. Phillips
Annals of Surgical Oncology.2019; 26(9): 2662. CrossRef
- Unveiling Therapeutic Targets for Esophageal Cancer: A Comprehensive Review
- 4,348 View
- 92 Download
- 1 Web of Science
- 2 Crossref

- Introduction to Endoscopic Submucosal Surgery
- Weon Jin Ko, Joo Young Cho
- Clin Endosc 2018;51(1):8-12. Published online January 23, 2018
- DOI: https://doi.org/10.5946/ce.2017.154
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - The concept of using natural orifices to reduce the complications of surgery, Natural Orifices Transluminal Endoscopic Surgery, has also been applied to therapeutic endoscopy. Endoscopic submucosal surgery (ESS) provides more treatment options for various gastrointestinal diseases than traditional therapeutic endoscopy by using the submucosal layer as a working space. ESS has been performed in various fields ranging from transluminal peritoneoscopy to peroral endoscopic myotomy. With further advances in technology, ESS will be increasingly useful for diagnosis and treatment of gastrointestinal diseases.
-
Citations
Citations to this article as recorded by- Design and validation of performance-oriented injectable chitosan thermosensitive hydrogels for endoscopic submucosal dissection
Jia Liu, Panxianzhi Ni, Yi Wang, Zhengkui Zhou, Junlin Li, Tianxu Chen, Tun Yuan, Jie Liang, Yujiang Fan, Jing Shan, Xiaobin Sun, Xingdong Zhang
Biomaterials Advances.2023; 146: 213286. CrossRef - Comparison of peroral endoscopic myotomy between de-novo achalasia and achalasia with prior treatment
Abdullah Ozgur Yeniova, In kyung Yoo, Eunju Jeong, Joo Young Cho
Surgical Endoscopy.2021; 35(1): 200. CrossRef - Tunnel endoscopic interventions in esophageal diseases
E. A. Drobyazgin, Yu. V. Chikinev, D. A. Arkhipov, N. I. Mit’ko, M. N. Chekanov, E. I. Vereshchagin, I. V. Peshkova, A. S. Polyakevich
Experimental and Clinical Gastroenterology.2021; 1(6): 75. CrossRef
- Design and validation of performance-oriented injectable chitosan thermosensitive hydrogels for endoscopic submucosal dissection
- 5,361 View
- 157 Download
- 3 Web of Science
- 3 Crossref

- Efficacy of the Over-the-Scope Clip System for Treatment of Gastrointestinal Fistulas, Leaks, and Perforations: A Korean Multi-Center Study
- Hang Lak Lee, Joo Young Cho, Jun-Hyung Cho, Jong Jae Park, Chan Gyoo Kim, Seong Hwan Kim, Joung-Ho Han
- Clin Endosc 2018;51(1):61-65. Published online August 29, 2017
- DOI: https://doi.org/10.5946/ce.2017.027
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Currently, a new over-the-scope clip (OTSC) system has been introduced. This system has been used for gastrointestinal perforations and fistulas in other countries. The aim of our study is to examine the therapeutic success rate of endoscopic treatment using the OTSC system in Korea.
Methods
This was a multicenter prospective study. A total of seven endoscopists at seven centers performed this procedure.
Results
A total of 19 patients were included, with gastrointestinal leakages from anastomosis sites, fistulas, or esophageal perforations due to Boerhaave’s syndrome. Among these, there were three gastrojejunostomy sites, three esophagojejunostomy sites, four esophagogastrostomy sites, one esophagocolonostomy site, one jejuno-jejunal site, two endoscopic full thickness resection site closures, one Boerhaave’s syndrome, two esophago-bronchial fistulas, one gastrocolonic fistula, and one colonopseudocyst fistula. The size of the leakage ranged from 5 to 30 mm. The median procedure time was 16 min. All cases were technically successful. Complete closure of the leak was achieved in 14 of 19 patients using OTSC alone.
Conclusions
The OTSC system is a safe and effective method for the management of gastrointestinal leakage, especially in cases of anastomotic leakage after surgery. -
Citations
Citations to this article as recorded by- Bariatric surgery and reproduction-implications for gynecology and obstetrics
Isaac A. Babarinsa, Mohammed Bashir, Husham AbdelRahman Ahmed, Badreldeen Ahmed, Justin C. Konje
Best Practice & Research Clinical Obstetrics & Gynaecology.2023; 90: 102382. CrossRef - Current status in endoscopic management of upper gastrointestinal perforations, leaks and fistulas
Shannon Melissa Chan, Kitty Kit Ying Auyeung, Siu Fung Lam, Philip Wai Yan Chiu, Anthony Yuen Bun Teoh
Digestive Endoscopy.2022; 34(1): 43. CrossRef - Endoscopic vacuum therapy (EVT) for acute esophageal perforation: Could it replace surgery?
Petros Stathopoulos, Malte Zumblick, Sabine Wächter, Leif Schiffmann, Thomas M. Gress, Detlef Bartsch, Guido Seitz, Ulrike W. Denzer
Endoscopy International Open.2022; 10(05): E686. CrossRef - Acquired Benign Tracheoesophageal Fistula
Hasnain S. Bawaadam, Matthew Russell, Yaron B. Gesthalter
Journal of Bronchology & Interventional Pulmonology.2022; 29(3): e38. CrossRef - Exclusión pilórica con dispositivo Ovesco (over-thescope) en caso de fístula yeyunal en obstrucción duodenal de etiología maligna
Raul Eduardo Pinilla Morales, Helena Facundo Navia, Elio Fabio Sánchez Cortés, Ivette C. Jiménez Lafourie, Álvaro Eduardo Sánchez Hernández, Luis Carlos Llorente Portillo
Revista colombiana de Gastroenterología.2022; 37(3): 320. CrossRef - Endoscopic management of leaks and fistulas after bariatric surgery: a systematic review and meta-analysis
Pawel Rogalski, Agnieszka Swidnicka-Siergiejko, Justyna Wasielica-Berger, Damian Zienkiewicz, Barbara Wieckowska, Eugeniusz Wroblewski, Andrzej Baniukiewicz, Magdalena Rogalska-Plonska, Grzegorz Siergiejko, Andrzej Dabrowski, Jaroslaw Daniluk
Surgical Endoscopy.2021; 35(3): 1067. CrossRef - Endoscopic management of gastro‐bronchial fistula following two‐stage esophagectomy using over‐the‐scope‐clip (OTSC): Case series
Chih Y. Tan, Htet A. Kyaw, Neda Farhangmehr, Cheuk‐Bong Tang, Naga V. Jayanthi
Advances in Digestive Medicine.2021; 8(2): 84. CrossRef - Over-the-Scope Clip Closure of Persistent Gastrocutaneous Fistula After Percutaneous Endoscopic Gastrostomy Tube Removal: A Report of Two Cases
Shigenori Masaki, Keishi Yamada
Cureus.2021;[Epub] CrossRef - Over‐the‐scope clip: a novel approach to the management of a colorectal anastomotic leak
Stephanie G. Jordan, Gregory J. Nolan
ANZ Journal of Surgery.2021; 91(11): 2534. CrossRef - Conservative treatment of patients with small bowel fistula
A.V. Vodyasov, D.M. Kopaliani, P.A. Yartsev, O.Kh. Kaloeva
Khirurgiya. Zhurnal im. N.I. Pirogova.2021; (4): 78. CrossRef - An Approach to Accelerate Healing and Shorten the Hospital Stay of Patients With Anastomotic Leakage After Esophagectomy: An Explorative Study of Systematic Endoscopic Intervention
LeQi Zhong, JiuDi Zhong, ZiHui Tan, YiTong Wei, XiaoDong Su, ZheSheng Wen, TieHua Rong, Yi Hu, KongJia Luo
Frontiers in Oncology.2021;[Epub] CrossRef - AGA Clinical Practice Update on Endoscopic Management of Perforations in Gastrointestinal Tract: Expert Review
Jeffrey H. Lee, Prashant Kedia, Stavros N. Stavropoulos, David Carr-Locke
Clinical Gastroenterology and Hepatology.2021; 19(11): 2252. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Diagnostic challenge and surgical management of Boerhaave’s syndrome: a case series
Jiayue Wang, Degang Wang, Jianjiao Chen
Journal of Medical Case Reports.2021;[Epub] CrossRef - Over-the-scope clip management of non-acute, full-thickness gastrointestinal defects
David J. Morrell, Joshua S. Winder, Ansh Johri, Salvatore Docimo, Ryan M. Juza, Samantha R. Witte, Vamsi V. Alli, Eric M. Pauli
Surgical Endoscopy.2020; 34(6): 2690. CrossRef - Use of the Over the Scope Clip to Close Perforations and Fistulas
Panida Piyachaturawat, Parit Mekaroonkamol, Rungsun Rerknimitr
Gastrointestinal Endoscopy Clinics of North America.2020; 30(1): 25. CrossRef - Therapie der Ösophagusleckagen
Jutta Weber-Eibel
Journal für Gastroenterologische und Hepatologische Erkrankungen.2020; 18(1): 8. CrossRef - Successful Closure of a Benign Refractory Tracheoesophageal Fistula Using an Over-the-Scope Clip after Failed Esophageal Stent Placement and Surgical Management
Nonthalee Pausawasdi, Chotirot Angkurawaranon, Tanyaporn Chantarojanasiri, Arunchai Chang, Wanchai Wongkornrat, Somchai Leelakusolvong, Asada Methasate
Clinical Endoscopy.2020; 53(3): 361. CrossRef - Clinical efficacy of the over-the-scope clip device: A systematic review
Nicholas Bartell, Krystle Bittner, Vivek Kaul, Truptesh H Kothari, Shivangi Kothari
World Journal of Gastroenterology.2020; 26(24): 3495. CrossRef - Endoscopic devices and techniques for the management of bariatric surgical adverse events (with videos)
Allison R. Schulman, Rabindra R. Watson, Barham K. Abu Dayyeh, Manoop S. Bhutani, Vinay Chandrasekhara, Pichamol Jirapinyo, Kumar Krishnan, Nikhil A. Kumta, Joshua Melson, Rahul Pannala, Mansour A. Parsi, Guru Trikudanathan, Arvind J. Trindade, John T. Ma
Gastrointestinal Endoscopy.2020; 92(3): 492. CrossRef - Gastrointestinal tract injuries after thermal ablative therapies for hepatocellular carcinoma: A case report and review of the literature
Teresa Marzia Rogger, Andrea Michielan, Sandro Sferrazza, Cecilia Pravadelli, Luisa Moser, Flora Agugiaro, Giovanni Vettori, Sonia Seligmann, Elettra Merola, Marcello Maida, Francesco Antonio Ciarleglio, Alberto Brolese, Giovanni de Pretis
World Journal of Gastroenterology.2020; 26(35): 5375. CrossRef - Over‐the‐scope clip system: A review of 1517 cases over 9 years
Hideki Kobara, Hirohito Mori, Noriko Nishiyama, Shintaro Fujihara, Keiichi Okano, Yasuyuki Suzuki, Tsutomu Masaki
Journal of Gastroenterology and Hepatology.2019; 34(1): 22. CrossRef - Recent advancements in the minimally invasive management of esophageal perforation, leaks, and fistulae
Shirin Siddiqi, Dean P. Schraufnagel, Hafiz Umair Siddiqui, Michael J. Javorski, Adam Mace, Abdulrhman S. Elnaggar, Haytham Elgharably, Patrick R. Vargo, Robert Steffen, Saad M. Hasan, Siva Raja
Expert Review of Medical Devices.2019; 16(3): 197. CrossRef - Diagnosis and endoscopic treatment of esophageal leakage: a systematic review
Bram D. Vermeulen, Peter D. Siersema
Techniques in Gastrointestinal Endoscopy.2019; 21(2): 58. CrossRef - Management of esophagojejunal anastomosis leakage after total gastrectomy
Pablo Priego, Pietro Giordano, Marta Cuadrado, Araceli Ballestero, Julio Galindo, Eduardo Lobo
European Surgery.2018; 50(6): 262. CrossRef - Endoluminal Therapies for Esophageal Perforations and Leaks
Jeffrey R. Watkins, Alexander S. Farivar
Thoracic Surgery Clinics.2018; 28(4): 541. CrossRef - Esophageal leaks: I thought that glue was not effective
Ignacio Fernández-Urién, Juan Vila
Endoscopy International Open.2018; 06(09): E1100. CrossRef
- Bariatric surgery and reproduction-implications for gynecology and obstetrics
- 7,581 View
- 324 Download
- 26 Web of Science
- 27 Crossref

- Evaluation and Endoscopic Management of Esophageal Submucosal Tumor
- Weon Jin Ko, Ga Won Song, Joo Young Cho
- Clin Endosc 2017;50(3):250-253. Published online November 7, 2016
- DOI: https://doi.org/10.5946/ce.2016.109
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Submucosal tumors (SMTs) originate from tissues that constitute the submucosal layer and muscularis propria, and are covered by normal mucosa. Esophageal SMTs are rare, accounting for <1% of all esophageal tumors. However, the recent widespread use of endoscopy has led to a rapid increase in incidental detection of SMTs in Korea. Esophageal SMTs are benign in ≥90% of cases, but the possibility of malignancies such as gastrointestinal stromal tumor and malignant leiomyosarcoma still exists. Therefore, patients undergo resection in the presence of symptoms or the possibility of a malignant tumor. For resection of esophageal SMTs, surgical resection was the only option available in case of possible malignancy, but minimally invasive surgery by endoscopic resection is becoming more preferable to surgical resection with the development of endoscopic ultrasonography, endoscopic techniques, and other devices.
-
Citations
Citations to this article as recorded by- Chest pain in a patient with suicidal history
Chien-Ming Chiang, Hsueh-Chien Chiang, Jui-Wen Kang
Frontline Gastroenterology.2024; : flgastro-2023-102617. CrossRef - Cholangiocarcinoma With Rare Esophageal Metastasis
Mana Matsuoka, Katsumasa Kobayashi, Yukito Okura, Takahito Nozaka, Ayako Sato, Masato Yauchi, Taichi Matsumoto, Yohei Furumoto, Takao Horiuchi, Toru Asano
ACG Case Reports Journal.2022; 9(1): e00717. CrossRef - Natural History of Asymptomatic Esophageal Subepithelial Tumors of 30 mm or Less in Size
Seokin Kang, Do Hoon Kim, Yuri Kim, Dongsub Jeon, Hee Kyong Na, Jeong Hoon Lee, Ji Yong Ahn, Kee Wook Jung, Kee Don Choi, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung
Journal of Korean Medical Science.2022;[Epub] CrossRef - A Submucosal Tumor-like Lesion of the Cervical Esophagus Similar to the Tonsillar Structures of Waldeyer’s Ring: A Case Report
Shibo Song, Xiaolong Feng, Xudong Liu, Guiqi Wang, Liyan Xue
Medicina.2022; 58(12): 1804. CrossRef - Clinical study of submucosal tunneling endoscopic resection and endoscopic submucosal dissection in the treatment of submucosal tumor originating from the muscularis propria layer of the esophagus
Yue Zhang, Jing Wen, Shuxian Zhang, Xuyang Liang, Ling Ren, Lu Wang, Yunliang Sun, Shouying Li, Kun Wang, Shengxiang Lv, Xiao Qiao
Medicine.2022; 101(51): e32380. CrossRef - Role of endoscopic ultrasound in anticancer therapy: Current evidence and future perspectives
Andre Bratanic, Dorotea Bozic, Antonio Mestrovic, Dinko Martinovic, Marko Kumric, Tina Ticinovic Kurir, Josko Bozic
World Journal of Gastrointestinal Oncology.2021; 13(12): 1863. CrossRef - Left atrial appendage thrombus detected by transesophageal examination with linear endoscopic ultrasound
Kenji Ikezawa, Minoru Shigekawa, Kaoruko Sengoku, Teppei Yoshioka, Ryotaro Sakamori, Yasushi Sakata, Tetsuo Takehara
Clinical Case Reports.2019; 7(7): 1327. CrossRef - A Potentially Malignant Giant Esophageal Paraganglioma
Tammi Arbel Rubinstein, Gennady Kouniavsky, Bibi Kanengisser Pines, Efraim Idelevich, Li or Lazar, Amir Elami, Ilan Bar, Guy Pines
The Annals of Thoracic Surgery.2019; 108(6): e349. CrossRef - Strategy for esophageal non-epithelial tumors based on a retrospective analysis of a single facility
Tomoaki Aoki, Tetsu Nakamura, Taro Oshikiri, Hiroshi Hasegawa, Masashi Yamamoto, Yoshiko Matsuda, Shingo Kanaji, Kimihiro Yamashita, Takeru Matsuda, Yasuo Sumi, Satoshi Suzuki, Yoshihiro Kakeji
Esophagus.2018; 15(4): 286. CrossRef - Esophageal Squamous Cell Carcinoma Presenting as a Subepithelial Tumor
Soon Young Kim, Sang Kil Lee, Hyang Joo Ryu
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2017; 17(3): 144. CrossRef
- Chest pain in a patient with suicidal history
- 7,832 View
- 271 Download
- 11 Web of Science
- 10 Crossref

- Current Techniques for Treating Gastrointestinal Stromal Tumors in the Upper Gastrointestinal Tract
- Weon Jin Ko, Joo Young Cho
- Clin Endosc 2016;49(3):226-228. Published online May 23, 2016
- DOI: https://doi.org/10.5946/ce.2016.061
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Most gastrointestinal stromal tumors (GISTs) arise from the proper muscle layer of the upper gastrointestinal (GI) tract and have a low malignant potential. They are sometimes accompanied by symptoms, but in most cases are detected by chance. Endoscopic surgery of subepithelial tumors in the upper GI tract has been actively performed, and its merits include the need for fewer medical devices compared with other surgical procedures and post-resection organ preservation. However, because endoscopic procedures are still limited to small or pilot studies, a multidisciplinary approach combining laparoscopy and endoscopy is needed for more effective and pathologically acceptable management of GISTs. Many new endoscopic surgeries have been developed, and this review describes the current status of and the new approaches for endoscopic surgery of GISTs in the upper GI tract.
-
Citations
Citations to this article as recorded by- Endoscopic resection of gastric gastrointestinal stromal tumor using clip-and-cut endoscopic full-thickness resection: a single-center, retrospective cohort in Korea
Yuri Kim, Ji Yong Ahn, Hwoon-Yong Jung, Seokin Kang, Ho June Song, Kee Don Choi, Do Hoon Kim, Jeong Hoon Lee, Hee Kyong Na, Young Soo Park
Clinical Endoscopy.2024; 57(3): 350. CrossRef - New data on the types of sulfide copper-nickel ores of the Kharaelakh trough and the main patterns of their distribution
I. O. Krylov, I. I. Nikulin
Moscow University Bulletin. Series 4. Geology.2023; (3): 98. CrossRef - Advances in endoscopic resection techniques of small gastric tumors originating from the muscularis propria
Suliman Khan, Xiaona Cui, Safyan Nasir, Shoaib Mohammad Rafiq, Bo Qin, Qian Bai
Frontiers in Oncology.2022;[Epub] CrossRef - New data on the composition of plagioclase on the western flank of the Oktyabrsky deposit according to infrared spectroscopy
I. O. Krylov, I. I. Nikulin, A. A. Samsonov, D. M. Korshunov, D. I. Vildanov
Moscow University Bulletin. Series 4. Geology.2022; (2): 27. CrossRef - A modified endoscopic full thickness resection for gastric subepithelial tumors from muscularis propria layer: Novel method
Jung Min Lee, In Kyung Yoo, Sung Pyo Hong, Joo Young Cho, Young Kwan Cho
Journal of Gastroenterology and Hepatology.2021; 36(9): 2558. CrossRef - Endoskopische Vollwandresektion im oberen Gastrointestinaltrakt – erste Erfahrungen
T. Heuer, C. D. Gerharz, M. Banysch, G. M. Kaiser, M. Hornstein, E. Kasim
Der Gastroenterologe.2020; 15(5): 403. CrossRef - Endoscopic full‐thickness resection for gastrointestinal submucosal tumors
Ming‐Yan Cai, Francisco Martin Carreras‐Presas, Ping‐Hong Zhou
Digestive Endoscopy.2018; 30(S1): 17. CrossRef - The fourth space surgery: endoscopic subserosal dissection for upper gastrointestinal subepithelial tumors originating from the muscularis propria layer
Fei Liu, Song Zhang, Wei Ren, Tian Yang, Ying Lv, Tingsheng Ling, Xiaoping Zou, Lei Wang
Surgical Endoscopy.2018; 32(5): 2575. CrossRef - Tumor rupture of gastric gastrointestinal stromal tumors during endoscopic resection: a risk factor for peritoneal metastasis?
Shiyi Song, Wei Ren, Yi Wang, Shu Zhang, Song Zhang, Fei Liu, Qiang Cai, Guifang Xu, Xiaoping Zou, Lei Wang
Endoscopy International Open.2018; 06(08): E950. CrossRef
- Endoscopic resection of gastric gastrointestinal stromal tumor using clip-and-cut endoscopic full-thickness resection: a single-center, retrospective cohort in Korea
- 6,426 View
- 108 Download
- 10 Web of Science
- 9 Crossref

- Double-Scope Peroral Endoscopic Myotomy (POEM) for Esophageal Achalasia: The First Trial of a New Double-Scope POEM
- Hee Jin Hong, Ga Won Song, Weon Jin Ko, Won Hee Kim, Ki Baik Hahm, Sung Pyo Hong, Joo Young Cho
- Clin Endosc 2016;49(4):383-386. Published online March 15, 2016
- DOI: https://doi.org/10.5946/ce.2015.108
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - With the accumulation of clinical trials demonstrating its efficacy and safety, peroral endoscopic myotomy (POEM) has emerged as a less invasive treatment option for esophageal achalasia compared with laparoscopic Heller myotomy. However, the difficulty in determining the exact extent of myotomy, a critical factor associated with the success and safety of the procedure, remains a limitation. Although the various endoscopic landmarks and ancillary techniques have been applied, none of these has been proven sufficient. As a solution for this limitation, the double-scope POEM technique with a second endoscope to assure the exact length of the submucosal tunnel has been applied since 2014. Before double-scope POEM was introduced, the second endoscope was applied only to confirm the accuracy of the procedure. In the present study, we performed double-scope POEM in the treatment of esophageal achalasia through a novel procedure of simultaneous application of the second endoscope to assist in the conventional POEM procedure.
-
Citations
Citations to this article as recorded by- Peroral Endoscopic Myotomy (POEM) in Children: A State of the Art Review
Ali A. Mencin, Amrita Sethi, Monique T. Barakat, Diana G. Lerner
Journal of Pediatric Gastroenterology & Nutrition.2022; 75(3): 231. CrossRef - Per-oral endoscopic myotomy (POEM) for a sigmoid type of achalasia: short-term outcomes and changes in the esophageal angle
Shota Maruyama, Yusuke Taniyama, Tadashi Sakurai, Makoto Hikage, Chiaki Sato, Kai Takaya, Takuro Konno, Takeshi Naitoh, Michiaki Unno, Takashi Kamei
Surgical Endoscopy.2020; 34(9): 4124. CrossRef - Characteristics of a Subset of Achalasia With Normal Integrated Relaxation Pressure
Eunju Kim, In Kyung Yoo, Dong Keon Yon, Joo Young Cho, Sung Pyo Hong
Journal of Neurogastroenterology and Motility.2020; 26(2): 274. CrossRef - Feasibility of using an led-probe in third-space endoscopy: a clinical study
Oscar Víctor Hernández Mondragón, Raúl Zamarripa Mottú, Omar Solórzano Pineda, Raúl Alberto Gutierrez Aguilar, Luís Fernando García Contreras
BMC Gastroenterology.2020;[Epub] CrossRef - 2007–2019: a “Third”-Space Odyssey in the Endoscopic Management of Gastrointestinal Tract Diseases
Anastassios C. Manolakis, Haruhiro Inoue, Akiko Ueno, Yuto Shimamura
Current Treatment Options in Gastroenterology.2019; 17(2): 202. CrossRef - Treatment of Achalasia with Per-Oral Endoscopic Myotomy: Analysis of 50 Consecutive Patients
Erica D. Kane, David J. Desilets, Donna Wilson, Marc Leduc, Vikram Budhraja, John R. Romanelli
Journal of Laparoendoscopic & Advanced Surgical Techniques.2018; 28(5): 514. CrossRef - Two penetrating vessels as a novel indicator of the appropriate distal end of peroral endoscopic myotomy
Shinwa Tanaka, Fumiaki Kawara, Takashi Toyonaga, Haruhiro Inoue, Robert Bechara, Namiko Hoshi, Hirohumi Abe, Yoshiko Ohara, Tsukasa Ishida, Yoshinori Morita, Eiji Umegaki
Digestive Endoscopy.2018; 30(2): 206. CrossRef
- Peroral Endoscopic Myotomy (POEM) in Children: A State of the Art Review
- 7,731 View
- 109 Download
- 9 Web of Science
- 7 Crossref

- Image Quality Analysis of Various Gastrointestinal Endoscopes: Why Image Quality Is a Prerequisite for Proper Diagnostic and Therapeutic Endoscopy
- Weon Jin Ko, Pyeong An, Kwang Hyun Ko, Ki Baik Hahm, Sung Pyo Hong, Joo Young Cho
- Clin Endosc 2015;48(5):374-379. Published online September 30, 2015
- DOI: https://doi.org/10.5946/ce.2015.48.5.374
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Arising from human curiosity in terms of the desire to look within the human body, endoscopy has undergone significant advances in modern medicine. Direct visualization of the gastrointestinal (GI) tract by traditional endoscopy was first introduced over 50 years ago, after which fairly rapid advancement from rigid esophagogastric scopes to flexible scopes and high definition videoscopes has occurred. In an effort towards early detection of precancerous lesions in the GI tract, several high-technology imaging scopes have been developed, including narrow band imaging, autofocus imaging, magnified endoscopy, and confocal microendoscopy. However, these modern developments have resulted in fundamental imaging technology being skewed towards red-green-blue and this technology has obscured the advantages of other endoscope techniques. In this review article, we have described the importance of image quality analysis using a survey to consider the diversity of endoscope system selection in order to better achieve diagnostic and therapeutic goals. The ultimate aims can be achieved through the adoption of modern endoscopy systems that obtain high image quality.
-
Citations
Citations to this article as recorded by- Colonoscopy Quality, Innovation, and the Assessment of New Technology
Sanjay R.V. Gadi, Sriya S. Muralidharan, Jeremy R. Glissen Brown
Techniques and Innovations in Gastrointestinal Endoscopy.2024; 26(2): 177. CrossRef - Endoscopy image enhancement method by generalized imaging defect models based adversarial training
Wenjie Li, Jingfan Fan, Yating Li, Pengcheng Hao, Yucong Lin, Tianyu Fu, Danni Ai, Hong Song, Jian Yang
Physics in Medicine & Biology.2022; 67(9): 095016. CrossRef - Reduced detection rate of artificial intelligence in images obtained from untrained endoscope models and improvement using domain adaptation algorithm
Junseok Park, Youngbae Hwang, Hyun Gun Kim, Joon Seong Lee, Jin-Oh Kim, Tae Hee Lee, Seong Ran Jeon, Su Jin Hong, Bong Min Ko, Seokmin Kim
Frontiers in Medicine.2022;[Epub] CrossRef - Diagnosis of Early Gastric Cancer Using Image-enhanced Endoscopy
Weon Jin Ko
The Korean Journal of Medicine.2017; 92(3): 264. CrossRef
- Colonoscopy Quality, Innovation, and the Assessment of New Technology
- 8,135 View
- 104 Download
- 5 Web of Science
- 4 Crossref

- Is the Endoscopic Grasp-and-Traction Device Useful for Endoscopic Submucosal Dissection in Treating Early Gastric Cancer?
- Joo Young Cho
- Clin Endosc 2015;48(3):181-182. Published online May 29, 2015
- DOI: https://doi.org/10.5946/ce.2015.48.3.181
- 5,064 View
- 61 Download

- Endoscopic Treatment of Various Gastrointestinal Tract Defects with an Over-the-Scope Clip: Case Series from a Tertiary Referral Hospital
- Woong Cheul Lee, Weon Jin Ko, Jun-Hyung Cho, Tae Hee Lee, Seong Ran Jeon, Hyun Gun Kim, Joo Young Cho
- Clin Endosc 2014;47(2):178-182. Published online March 31, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.2.178
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Recently, increasingly invasive therapeutic endoscopic procedures and more complex gastrointestinal surgeries such as endoscopic mucosal resection, endoscopic submucosal dissection, and novel laparoscopic approaches have resulted in endoscopists being confronted more frequently with perforations, fistulas, and anastomotic leakages, for which nonsurgical closure is desired. In this article, we present our experiences with the use of over-the-scope clip (OTSC) for natural orifice transluminal endoscopic surgery (NOTES) closure, prevention of perforation, anastomotic leakages, and fistula closures. The OTSC is a valuable device for closing intestinal perforations and fistulas, for NOTES closure, and for the prevention of perforation after the excision of a tumor from the proper muscle layer. Furthermore, it seems to be quite safe to perform, even by endoscopists with little experience of the technique.
-
Citations
Citations to this article as recorded by- Experimental Evaluation of the Optimal Suture Pattern With a Flexible Endoscopic Suturing System
Peter Halvax, Michele Diana, Yoshihiro Nagao, Jacques Marescaux, Lee Swanström
Surgical Innovation.2017; 24(3): 201. CrossRef - Management of non-acute gastrointestinal defects using the over-the-scope clips (OTSCs): a retrospective single-institution experience
Joshua S. Winder, Afif N. Kulaylat, Jane R. Schubart, Hassan M. Hal, Eric M. Pauli
Surgical Endoscopy.2016; 30(6): 2251. CrossRef - Early endoscopic closure of colocutaneous fistula adjacent to unmatured low colorectal anastomosis with the Over-The-Scope Clip (OTSC)
Constantinos Avgoustou, K. Paraskeva
Hellenic Journal of Surgery.2016; 88(3): 193. CrossRef - Endoscopic Closure for Full-Thickness Gastrointestinal Defects: Available Applications and Emerging Innovations
Nobuyoshi Takeshita, Khek Yu Ho
Clinical Endoscopy.2016; 49(5): 438. CrossRef
- Experimental Evaluation of the Optimal Suture Pattern With a Flexible Endoscopic Suturing System
- 6,116 View
- 58 Download
- 3 Web of Science
- 4 Crossref

- Evidence-Based Recommendations on Upper Gastrointestinal Tract Stenting: A Report from the Stent Study Group of the Korean Society of Gastrointestinal Endoscopy
- Sam Ryong Jee, Joo Young Cho, Kyung Ho Kim, Sang Gyun Kim, Jun-Hyung Cho, The Stent Study Group of the Korean Society of Gastrointestinal Endoscopy
- Clin Endosc 2013;46(4):342-354. Published online July 31, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.4.342
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Endoscopic stents have evolved dramatically over the past 20 years. With the introduction of uncovered self-expanding metal stents in the early 1990s, they are primarily used to palliate symptoms of malignant obstruction in patients with inoperable gastrointestinal (GI) cancer. At present, stents have emerged as an effective, safe, and less invasive alternative for the treatment of malignant GI obstruction. Clinical decisions about stent placement should be made based on the exact understanding of the patient's condition. These recommendations based on a critical review of the available data and expert consensus are made for the purpose of providing endoscopists with information about stent placement. These can be helpful for management of patients with inoperable cancer or various nonmalignant conditions in the upper GI tract.
-
Citations
Citations to this article as recorded by- Endoscopic Procedures for Upper Gastrointestinal Tract Lesions and a Brief Review of Literature
Selim Doğan, Ekrem Çakar, Bünyamin Gürbulak, Şükrü Çolak, Hasan Bektaş, Cihad Tatar
Istanbul Medical Journal.2022; 23(2): 154. CrossRef - Endoscopic Treatment of a Twisted Small Bowel Obstruction after Laparoscopic Proximal Gastrectomy with Double Tract Reconstruction
Ki Bum Park, Seong Woo Jeon
The Korean Journal of Gastroenterology.2020; 75(5): 296. CrossRef - Temporary self-expandable metallic stent placement in post-gastrectomy complications
Hyun Jin Oh, Chul-Hyun Lim, Seung Bae Yoon, Han Hee Lee, Jin Su Kim, Yu Kyung Cho, Jae Myung Park, Myung-Gyu Choi
Gastric Cancer.2019; 22(1): 231. CrossRef - Peptic Ulcer-related Stenosis
Cheol Woong Choi
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2019; 19(1): 10. CrossRef - Clinical Feasibility and Safety of Endoscopic Self-Expandable Metal Stent Placement for Upper Gastrointestinal Pathologies
Bünyamin Gürbulak, Esin Kabul Gürbulak, Hasan Bektaş, İsmail Ethem Akgün, Hızır Yakup Akyildiz, Özgür Segmen, Fevzi Celayir, Muharrem Battal, Kenan Büyükaşık
International Surgery.2018; 103(11-12): 605. CrossRef - Endoscopic management of complications of self-expandable metal stents for treatment of malignant esophageal stenosis and tracheoesophageal fistulas
Renáta Bor, Anna Fábián, Anita Bálint, Klaudia Farkas, Mónika Szűcs, Ágnes Milassin, László Czakó, Mariann Rutka, Tamás Molnár, Zoltán Szepes
Therapeutic Advances in Gastroenterology.2017; 10(8): 599. CrossRef - Gastroduodenal Outlet Obstruction and Palliative Self-Expandable Metal Stenting: A Dual-Centre Experience
Nik S. Ding, Sina Alexander, Michael P. Swan, Christopher Hair, Patrick Wilson, Emma Clarebrough, David Devonshire
Journal of Oncology.2013; 2013: 1. CrossRef
- Endoscopic Procedures for Upper Gastrointestinal Tract Lesions and a Brief Review of Literature
- 7,902 View
- 52 Download
- 7 Crossref

- Endoscopic Resection of Hypopharyngeal Squamous Cell Carcinoma
- Gene Hyun Bok, Won Young Cho, Joo Young Cho, So Young Jin, Ji Ho Ahn, Chang Gyun Chun, Tae Hee Lee, Hyun Gun Kim
- Clin Endosc 2013;46(2):189-192. Published online March 31, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.2.189
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Hypopharyngeal cancers are often diagnosed at an advanced stage and have a poor prognosis. Even when they are diagnosed at an operable stage, surgery often results in substantial morbidity and decreased patients' quality of life. Although the endoscopic diagnosis of early hypopharyngeal cancer is difficult, recent developments in advanced imaging endoscopy have enabled easier diagnosis of these lesions. Endoscopic resection of early hypopharyngeal cancer is a potential minimally invasive treatment that can preserve the function and quality of life of patients. Reports of this procedure are limited, however. We report a case of hypopharygeal cancer treated with endoscopic resection.
-
Citations
Citations to this article as recorded by- Identify metabolism-related genes IDO1, ALDH2, NCOA2, SLC7A5, SLC3A2, LDHB, and HPRT1 as potential prognostic markers and correlate with immune infiltrates in head and neck squamous cell carcinoma
Ce Li, Shuai Chen, Wenming Jia, Wenming Li, Dongmin Wei, Shengda Cao, Ye Qian, Rui Guan, Heng Liu, Dapeng Lei
Frontiers in Immunology.2022;[Epub] CrossRef - Endoscopic resection for superficial hypopharyngeal/laryngeal cancer and clinical pathway options
Wei‐Chen Huang, Li‐Hsiang Cheng, Tien‐Yu Huang, Yu‐Lueng Shih, Wei‐Kuo Chang, Tsai‐Yuan Hsieh, Peng‐Jen Chen
Advances in Digestive Medicine.2019; 6(3): 123. CrossRef - MG132 reverse the malignant characteristics of hypopharyngeal cancer
JUKE MA, LIANG YU, JIAJUN TIAN, YAKUI MU, ZHENGHUA LV, JIDONG ZOU, JIANFENG LI, HAIBO WANG, WEI XU
Molecular Medicine Reports.2014; 9(6): 2587. CrossRef
- Identify metabolism-related genes IDO1, ALDH2, NCOA2, SLC7A5, SLC3A2, LDHB, and HPRT1 as potential prognostic markers and correlate with immune infiltrates in head and neck squamous cell carcinoma
- 5,741 View
- 49 Download
- 3 Crossref

- Endoscopic Ultrasound Elastography for the Pancreas in Korea: A Preliminary Single Center Study
- Tae Hee Lee, Young Deok Cho, Sang-Woo Cha, Joo Young Cho, Jae Young Jang, Soung Won Jeong, Hyun Jong Choi, Jong Ho Moon
- Clin Endosc 2013;46(2):172-177. Published online March 31, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.2.172
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Background/Aims Endoscopic ultrasound elastography (EUS-EG) has been widely used for the evaluation of pancreatic cancer in the Western world. To date, there is very little experience with EUS-EG in Korea. We described the results of comparison between normal pancreas and pancreatic cancer in Korea.
Methods The present study was performed at a tertiary hospital on 35 subjects comprising 20 with normal pancreas (control group) and 15 with pancreatic cancer (disease group). We compared the EUS-EG performance of the two groups.
Results The pancreas in the control group showed a mean elasticity value of 0.53% (95% confidence interval [CI], 0.45 to 0.61). The elasticity value was higher than that previously reported from Western country (0.47%; 95% CI, 0.38 to 0.57). In the disease group, the mean elasticity value of pancreatic lesions was 0.02% (95% CI, 0.01 to 0.02). The mean elasticity value of the disease group was significantly lower than that of the control group (
p <0.0001).Conclusions EUS-EG could be a highly sensitive diagnostic modality for pancreatic cancer in Korea with little EUS-EG experience. We also provided the reference range of elasticity value of normal pancreas, which might be valuable in the interpretation of pancreatic elasticity data for Korean adults.
-
Citations
Citations to this article as recorded by- Present status of ultrasound elastography for the diagnosis of pancreatic tumors: review of the literature
Takamichi KUWAHARA, Kazuo HARA, Nobumasa MIZUNO, Shin HABA, Nozomi OKUNO
Choonpa Igaku.2022; 49(3): 275. CrossRef - Recent Advances in Endosonography—Elastography: Literature Review
Akira Yamamiya, Atsushi Irisawa, Koki Hoshi, Akane Yamabe, Naoya Izawa, Kazunori Nagashima, Takahito Minaguchi, Masamichi Yamaura, Yoshitsugu Yoshida, Ken Kashima, Yasuhito Kunogi, Fumi Sakuma, Keiichi Tominaga, Makoto Iijima, Kenichi Goda
Journal of Clinical Medicine.2021; 10(16): 3739. CrossRef - Pancreatic cancer in 2021: What you need to know to win
Valeria Tonini, Manuel Zanni
World Journal of Gastroenterology.2021; 27(35): 5851. CrossRef - Endoscopic ultrasonography elastography in the diagnosis of intrapancreatic ectopic spleen: A case report
Nan Ge, Si-Yu Sun
World Journal of Clinical Cases.2020; 8(9): 1729. CrossRef - Present status of ultrasound elastography for the diagnosis of pancreatic tumors: review of the literature
Takamichi Kuwahara, Kazuo Hara, Nobumasa Mizuno, Shin Haba, Nozomi Okuno
Journal of Medical Ultrasonics.2020; 47(3): 413. CrossRef - Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer
Masayuki Kitano, Takeichi Yoshida, Masahiro Itonaga, Takashi Tamura, Keiichi Hatamaru, Yasunobu Yamashita
Journal of Gastroenterology.2019; 54(1): 19. CrossRef - Early Detection of Pancreatic Cancer: Opportunities and Challenges
Aatur D. Singhi, Eugene J. Koay, Suresh T. Chari, Anirban Maitra
Gastroenterology.2019; 156(7): 2024. CrossRef - Diagnostic Efficacy of Endoscopic Ultrasound Elastography in Differentiating Solid Pancreatic Lesions: A Single-Center Experience
Ahmed Youssef Altonbary, Hazem Hakim, Ahmed Mohamed El-Shamy
Clinical Endoscopy.2019; 52(4): 360. CrossRef - Elastography of the Pancreas, Current View
Christoph F. Dietrich, Michael Hocke
Clinical Endoscopy.2019; 52(6): 533. CrossRef - Endoscopic ultrasound elastography in the diagnosis of pancreatic masses: A meta-analysis
Binglan Zhang, Fuping Zhu, Pan Li, Shishi Yu, Yajing Zhao, Minmin Li
Pancreatology.2018; 18(7): 833. CrossRef - Endoscopic ultrasound (EUS) elastography and strain ratio, could it help in differentiating malignant from benign pancreatic lesions?
Hussein Hassan Okasha, Reem Ezzat Mahdy, Shaimaa Elkholy, Mohamed Sayed Hassan, Ahmed Nabil El-Mazny, Kareem Essam Eldin Hadad, Moustafa Saeed, Mohamed El-Nady, Osama Soliman Elbalky, Asem Ashraf, Amr Abo El-Magd, Abeer Awad
Medicine.2018; 97(36): e11689. CrossRef - Differentiation of Pancreatic Masses via Endoscopic Ultrasound Strain Ratio Elastography Using Adjacent Pancreatic Tissue as the Reference
Nadan Rustemović, Mirjana Kalauz, Katja Grubelić Ravić, Hrvoje Iveković, Branko Bilić, Zvonimir Ostojić, Dalibor Opačić, Iva Ledinsky, Matea Majerović, Ana Višnjić
Pancreas.2017; 46(3): 347. CrossRef - Endoscopic ultrasound elastography for solid pancreatic lesions
Tanyaporn Chantarojanasiri, Pradermchai Kongkam
World Journal of Gastrointestinal Endoscopy.2017; 9(10): 506. CrossRef - New Imaging Techniques
Julio Iglesias-García, Jose Lariño-Noia, Juan Enrique Domínguez-Muñoz
Gastrointestinal Endoscopy Clinics of North America.2017; 27(4): 551. CrossRef - Maximizing the endosonography: The role of contrast harmonics, elastography and confocal endomicroscopy
Andrada Seicean, Ofelia Mosteanu, Radu Seicean
World Journal of Gastroenterology.2017; 23(1): 25. CrossRef - Prospective cohort study comparing transient EUS guided elastography to EUS-FNA for the diagnosis of solid pancreatic mass lesions
J. Mayerle, G. Beyer, P. Simon, E.J. Dickson, R.C. Carter, F. Duthie, M.M. Lerch, C.J. McKay
Pancreatology.2016; 16(1): 110. CrossRef - Elasticity of the tibial nerve assessed by sonoelastography was reduced before the development of neuropathy and further deterioration associated with the severity of neuropathy in patients with type 2 diabetes
Fukashi Ishibashi, Miki Taniguchi, Rie Kojima, Asami Kawasaki, Aiko Kosaka, Harumi Uetake
Journal of Diabetes Investigation.2016; 7(3): 404. CrossRef - Endoscopic Ultrasound Elastography for Pancreatic Cancer Diagnosis: A Step Forward?
Woo Jin Lee
Clinical Endoscopy.2013; 46(2): 116. CrossRef
- Present status of ultrasound elastography for the diagnosis of pancreatic tumors: review of the literature
- 6,268 View
- 48 Download
- 18 Crossref

- Peroral Endoscopic Myotomy for Treatment of Achalasia: Initial Results of a Korean Study
- Byung Hoo Lee, Kwang Yeun Shim, Su Jin Hong, Gene Hyun Bok, Jun-Hyung Cho, Tae Hee Lee, Joo Young Cho
- Clin Endosc 2013;46(2):161-167. Published online March 31, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.2.161
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Background/Aims Achalasia is a rare esophageal motility disorder. Recently, a novel endoscopic technique, peroral endoscopic myotomy (POEM), was introduced as an alternative treatment for achalasia. We report the results and short term outcomes of POEM for patients with achalasia.
Methods POEM was performed in 13 patients with achalasia. The procedure consisted of creating a submucosal tunnel followed by endoscopic myotomy of circular muscle bundles. The mucosal entry was closed by conventional hemostatic clips. A validated clinical symptom score (Eckardt score) and high resolution manometry were used to evaluate the outcomes.
Results Both the clinical score of achalasia, as well as the resting lower esophageal sphincter (LES) pressure, were significantly reduced after POEM. Mean posttreatment Eckardt score was 0.4±0.7, compared to 6.4±1.9 prior to the treatment (
p =0.001). The mean pretreatment and posttreatment LES pressure was 30.3 and 15.3 mm Hg, respectively (p =0.007). Following POEM, symptomatic relief from dysphagia without reflux symptoms was observed in all patients (13/13). No serious complications related to POEM were encountered.Conclusions Based upon our initial experience, the authors believe that POEM is a feasible, safe, and effective treatment and may possibly substitute established treatments of refractory achalasia.
-
Citations
Citations to this article as recorded by- Achalasia: Current therapeutic options
Sebastien Rolland, William Paterson, Robert Bechara
Neurogastroenterology & Motility.2023;[Epub] CrossRef - Long versus short peroral endoscopic myotomy for the treatment of achalasia: results of a non-inferiority randomised controlled trial
Pietro Familiari, Federica Borrelli de Andreis, Rosario Landi, Francesca Mangiola, Ivo Boskoski, Andrea Tringali, Vincenzo Perri, Guido Costamagna
Gut.2023; 72(8): 1442. CrossRef - Efficacy and Safety of Peroral Endoscopic Myotomy (POEM) in Achalasia: An Updated Meta-analysis
Afshin Khaiser, Muhammad Baig, David Forcione, Matthew Bechtold, Srinivas R. Puli
Middle East Journal of Digestive Diseases.2023; 15(4): 235. CrossRef - Type II achalasia is associated with a comparably favorable outcome following per oral endoscopic myotomy
Yutaka Tomizawa, Nadim Mahmud, Kevin Dasher, Joseph R Triggs, Monica Saumoy, Gary W Falk, Gregory G Ginsberg
Diseases of the Esophagus.2021;[Epub] CrossRef - Miotomía endoscópica peroral para el tratamiento de acalasia y otros trastornos motores del esófago. Resultados a corto y mediano plazo en un centro de referencia en México
O.V. Hernández-Mondragón, O.M. Solórzano-Pineda, M.A. González-Martínez, J.M. Blancas-Valencia, C. Caballero-Luengas
Revista de Gastroenterología de México.2019; 84(1): 1. CrossRef - Peroral endoscopic myotomy for the treatment of achalasia and other esophageal motor disorders: Short-term and medium-term results at a Mexican tertiary care center
O.V. Hernández-Mondragón, O.M. Solórzano-Pineda, M.A. González-Martínez, J.M. Blancas-Valencia, C. Caballero-Luengas
Revista de Gastroenterología de México (English Edition).2019; 84(1): 1. CrossRef - Bridging the Gap between Advancements in the Evolution of Diagnosis and Treatment towards Better Outcomes in Achalasia
Seng-Kee Chuah, Chee-Sang Lim, Chih-Ming Liang, Hung-I Lu, Keng-Liang Wu, Chi-Sin Changchien, Wei-Chen Tai
BioMed Research International.2019; 2019: 1. CrossRef - Preliminary study of 1940 nm thulium laser usage in peroral endoscopic myotomy for achalasia
J Liu, Y Jiao, Y Niu, L Yu, M Ji, S Zhang
Diseases of the Esophagus.2018;[Epub] CrossRef - POEM in the Treatment of Esophageal Disorders
Nasim Parsa, Mouen A. Khashab
Current Treatment Options in Gastroenterology.2018; 16(1): 27. CrossRef - Current Status of Peroral Endoscopic Myotomy
Young Kwan Cho, Seong Hwan Kim
Clinical Endoscopy.2018; 51(1): 13. CrossRef - Safety and efficacy of POEM for treatment of achalasia: a systematic review of the literature
Oscar M. Crespin, Louis W. C. Liu, Ambica Parmar, Timothy D. Jackson, Jemila Hamid, Eran Shlomovitz, Allan Okrainec
Surgical Endoscopy.2017; 31(5): 2187. CrossRef - Early adverse events of per-oral endoscopic myotomy
Yuki B. Werner, Daniel von Renteln, Tania Noder, Guido Schachschal, Ulrike W. Denzer, Stefan Groth, Jan F. Nast, Jan F. Kersten, Martin Petzoldt, Gerhard Adam, Oliver Mann, Alessandro Repici, Cesare Hassan, Thomas Rösch
Gastrointestinal Endoscopy.2017; 85(4): 708. CrossRef - A multicenter international registry of redo per-oral endoscopic myotomy (POEM) after failed POEM
Amy Tyberg, Stefan Seewald, Reem Z. Sharaiha, Guadalupe Martinez, Amit P. Desai, Nikhil A. Kumta, Arnon Lambroza, Amrita Sethi, Kevin M. Reavis, Ketisha DeRoche, Monica Gaidhane, Michael Talbot, Payal Saxena, Felipe Zamarripa, Maximilien Barret, Nicholas
Gastrointestinal Endoscopy.2017; 85(6): 1208. CrossRef - Long-term outcomes of peroral endoscopic myotomy for patients with achalasia: a retrospective single-center study
H. Guo, H. Yang, X. Zhang, L. Wang, Y. Lv, X. Zou, T. Ling
Diseases of the Esophagus.2017; 30(5): 1. CrossRef - Per-Oral Esophageal Myotomy
Eric S. Hungness, Juaquito M. Jorge
Advances in Surgery.2017; 51(1): 193. CrossRef - Comparison of the Outcomes of Peroral Endoscopic Myotomy for Achalasia According to Manometric Subtype
Won Hee Kim, Joo Young Cho, Weon Jin Ko, Sung Pyo Hong, Ki Baik Hahm, Jun-Hyung Cho, Tae Hee Lee, Su Jin Hong
Gut and Liver.2017; 11(5): 642. CrossRef - Peroral endoscopic myotomy for the treatment of esophageal achalasia: systematic review and pooled analysis
K. Patel, N. Abbassi-Ghadi, S. Markar, S. Kumar, P. Jethwa, G. Zaninotto
Diseases of the Esophagus.2016; 29(7): 807. CrossRef - Gastroesophageal reflux disease after peroral endoscopic myotomy: Analysis of clinical, procedural and functional factors, associated with gastroesophageal reflux disease and esophagitis
Pietro Familiari, Santi Greco, Giovanni Gigante, Anna Calì, Ivo Boškoski, Graziano Onder, Vincenzo Perri, Guido Costamagna
Digestive Endoscopy.2016; 28(1): 33. CrossRef - Peroral Endoscopic Myotomy for Esophageal Achalasia
Pietro Familiari, Giovanni Gigante, Michele Marchese, Ivo Boskoski, Andrea Tringali, Vincenzo Perri, Guido Costamagna
Annals of Surgery.2016; 263(1): 82. CrossRef - Surgery or Peroral Esophageal Myotomy for Achalasia
Luigi Marano, Giovanni Pallabazzer, Biagio Solito, Stefano Santi, Alessio Pigazzi, Raffaele De Luca, Francesco Giuseppe Biondo, Alessandro Spaziani, Maurizio Longaroni, Natale Di Martino, Virginia Boccardi, Alberto Patriti
Medicine.2016; 95(10): e3001. CrossRef - Prospective evaluation of CT esophagram findings after peroral endoscopic myotomy
Davinderbir Pannu, Dennis Yang, Patricia L. Abbitt, Peter V. Draganov
Gastrointestinal Endoscopy.2016; 84(3): 408. CrossRef - Clinical response to peroral endoscopic myotomy in patients with idiopathic achalasia at a minimum follow-up of 2 years
Yuki B Werner, Guido Costamagna, Lee L Swanström, Daniel von Renteln, Pietro Familiari, Ahmed M Sharata, Tania Noder, Guido Schachschal, Jan F Kersten, Thomas Rösch
Gut.2016; 65(6): 899. CrossRef - Double-Scope Peroral Endoscopic Myotomy (POEM) for Esophageal Achalasia: The First Trial of a New Double-Scope POEM
Hee Jin Hong, Ga Won Song, Weon Jin Ko, Won Hee Kim, Ki Baik Hahm, Sung Pyo Hong, Joo Young Cho
Clinical Endoscopy.2016; 49(4): 383. CrossRef - Achalasia: from diagnosis to management
Michael F. Vaezi, Valter N. Felix, Roberto Penagini, Aurelio Mauro, Eduardo Guimarães Hourneaux de Moura, Leonardo Zorrón Cheng Tao Pu, Jan Martínek, Erwin Rieder
Annals of the New York Academy of Sciences.2016; 1381(1): 34. CrossRef - Complications of submucosal endoscopy
Jean-Michel Gonzalez, Alban Benezech, Marc Barthet
Best Practice & Research Clinical Gastroenterology.2016; 30(5): 783. CrossRef - Update on the endoscopic treatments for achalasia
Dushant S Uppal, Andrew Y Wang
World Journal of Gastroenterology.2016; 22(39): 8670. CrossRef - Gestion des complications de l’endoscopie interventionnelle œsophagienne
C. Lorenceau-Savale, G. Rahmi
Acta Endoscopica.2015; 45(3): 90. CrossRef - Greater curvature myotomy is a safe and effective modified technique in per-oral endoscopic myotomy (with videos)
Manabu Onimaru, Haruhiro Inoue, Haruo Ikeda, Chiaki Sato, Hiroki Sato, Chainarong Phalanusitthepha, Esperanza Grace Santi, Kevin L. Grimes, Hiroaki Ito, Shin-ei Kudo
Gastrointestinal Endoscopy.2015; 81(6): 1370. CrossRef - Peroral Esophageal Myotomy Versus Laparoscopic Heller's Myotomy for Achalasia: A Meta-analysis
Mingtian Wei, Tinghan Yang, Xuyang Yang, Ziqiang Wang, Zongguang Zhou
Journal of Laparoendoscopic & Advanced Surgical Techniques.2015; 25(2): 123. CrossRef - Efficacy of peroral endoscopic myotomy (POEM) in the treatment of achalasia: a systematic review and meta-analysis
Rupjyoti Talukdar, Haruhiro Inoue, D. Nageshwar Reddy
Surgical Endoscopy.2015; 29(11): 3030. CrossRef - Is Peroral Endoscopic Myotomy Effective for the Treatment of Spastic Esophageal Disorders Refractory to Medical Therapy?
Jae Pil Han
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2015; 15(2): 143. CrossRef - The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on peroral endoscopic myotomy
Vinay Chandrasekhara, David Desilets, Gary W. Falk, Haruhiro Inoue, John R. Romanelli, Thomas J. Savides, Stavros N. Stavropoulos, Lee L. Swanstrom
Gastrointestinal Endoscopy.2015; 81(5): 1087. CrossRef - Peroral Endoscopic Myotomy for Treating Achalasia and Esophageal Motility Disorders
Young Hoon Youn, Hitomi Minami, Philip Wai Yan Chiu, Hyojin Park
Journal of Neurogastroenterology and Motility.2015; 22(1): 14. CrossRef - Effect of Peroral Endoscopic Myotomy on Esoph ageal Motor Function
Su Jin Hong
Journal of Neurogastroenterology and Motility.2015; 22(1): 1. CrossRef - Achalasia: current treatment options
Pietro Familiari, Santi Greco, Ance Volkanovska, Giovanni Gigante, Anna Cali, Ivo Boškoski, Guido Costamagna
Expert Review of Gastroenterology & Hepatology.2015; 9(8): 1101. CrossRef - Systematic review and meta‐analysis: Efficacy and safety of POEM for achalasia
Lavinia A Barbieri, Cesare Hassan, Riccardo Rosati, Uberto Fumagalli Romario, Loredana Correale, Alessandro Repici
United European Gastroenterology Journal.2015; 3(4): 325. CrossRef - Per-oral endoscopic myotomy white paper summary
Stavros N. Stavropoulos, David J. Desilets, Karl-Hermann Fuchs, Christopher J. Gostout, Gregory Haber, Haruhiro Inoue, Michael L. Kochman, Rani Modayil, Thomas Savides, Daniel J. Scott, Lee L. Swanstrom, Melina C. Vassiliou
Surgical Endoscopy.2014; 28(7): 2005. CrossRef - Peroral endoscopic myotomy for achalasia
A. J. Bredenoord, T. Rösch, P. Fockens
Neurogastroenterology & Motility.2014; 26(1): 3. CrossRef - Peroral Endoscopic Myotomy: Establishing a New Program
Nikhil A. Kumta, Shivani Mehta, Prashant Kedia, Kristen Weaver, Reem Z. Sharaiha, Norio Fukami, Hitomi Minami, Fernando Casas, Monica Gaidhane, Arnon Lambroza, Michel Kahaleh
Clinical Endoscopy.2014; 47(5): 389. CrossRef - Introduction of the per-oral endoscopic myotomy technique to pediatric surgical practice
Stephanie Chao, Michael Russo, Robert Wright, Homero Rivas, James Wall
Journal of Pediatric Surgery Case Reports.2014; 2(6): 313. CrossRef - International Digestive Endoscopy Network 2014: Turnpike to the Future
Eun Young Kim, Kwang An Kwon, Il Ju Choi, Ji Kon Ryu, Ki Baik Hahm
Clinical Endoscopy.2014; 47(5): 371. CrossRef - Per-oral endoscopic myotomy white paper summary
Stavros N. Stavropoulos, David J. Desilets, Karl-Hermann Fuchs, Christopher J. Gostout, Gregory Haber, Haruhiro Inoue, Michael L. Kochman, Rani Modayil, Thomas Savides, Daniel J. Scott, Lee L. Swanstrom, Melina C. Vassiliou
Gastrointestinal Endoscopy.2014; 80(1): 1. CrossRef - Effectiveness of peroral endoscopic myotomy in the treatment of achalasia: A pilot trial in Chinese Han population with a minimum of one‐year follow‐up
Ting Sheng Ling, Hui Min Guo, Tian Yang, Chun Yan Peng, Xiao Ping Zou, Rui Hua Shi
Journal of Digestive Diseases.2014; 15(7): 352. CrossRef - Jackhammer Esophagus Treated by a Peroral Endoscopic Myotomy
Weon Jin Ko, Byoung Moo Lee, Won Young Park, Jin Nyoung Kim, Jun-Hyung Cho, Tae Hee Lee, Su Jin Hong, Joo Young Cho
The Korean Journal of Gastroenterology.2014; 64(6): 370. CrossRef - Effect of peroral endoscopic myotomy in achalasia patients with failure of prior pneumatic dilation: A prospective case–control study
Tingsheng Ling, Huimin Guo, Xiaoping Zou
Journal of Gastroenterology and Hepatology.2014; 29(8): 1609. CrossRef - Medical and Endoscopic Management of Achalasia
Jae Pil Han, Su Jin Hong
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2014; 14(2): 82. CrossRef - EndoFLIP system for the intraoperative evaluation of peroral endoscopic myotomy
Pietro Familiari, Giovanni Gigante, Michele Marchese, Ivo Boskoski, Vincenzo Bove, Andrea Tringali, Vincenzo Perri, Graziano Onder, Guido Costamagna
United European Gastroenterology Journal.2014; 2(2): 77. CrossRef - Per Oral Endoscopic Myotomy (POEM): Review of Current Techniques and Outcomes (Including Postoperative Reflux)
David Friedel, Rani Modayil, Stavros N. Stavropoulos
Current Surgery Reports.2013; 1(4): 203. CrossRef - Peroral Endoscopic Myotomy for the Treatment of Achalasia: An Analysis
Dennis Yang, Mihir S. Wagh
Diagnostic and Therapeutic Endoscopy.2013; 2013: 1. CrossRef - Therapeutic flexible endoscopy replacing surgery: Part 3—Peroral esophageal myotomy
Ezra N. Teitelbaum, Eric S. Hungness
Techniques in Gastrointestinal Endoscopy.2013; 15(4): 211. CrossRef - Highlights of International Digestive Endoscopy Network 2013
Kwang An Kwon, Il Ju Choi, Eun Young Kim, Seok Ho Dong, Ki Baik Hahm
Clinical Endoscopy.2013; 46(5): 425. CrossRef - Perorale endoskopische Myotomie zur Therapie der Achalasie
B.H.A. von Rahden, J. Filser, S. Reimer, H. Inoue, C.-T. Germer
Der Chirurg.2013;[Epub] CrossRef
- Achalasia: Current therapeutic options
- 10,830 View
- 75 Download
- 52 Crossref

- Intra-Abdominal Tuberculous Lymphadenitis Diagnosed Using an Endoscopic Ultrasonography-Guided ProCore Needle Biopsy
- Tae Hee Lee, Joo Young Cho, Gene Hyun Bok, Won Young Cho, So Young Jin
- Clin Endosc 2013;46(1):77-80. Published online January 31, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.1.77
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Intra-abdominal tuberculous lymphadenitis can mimic a variety of other abdominal disorders such as pancreatic cancer, metastatic lymph nodes, or lymphoma, which can make a proper diagnosis difficult. A correct diagnosis of intra-abdominal tuberculous lymphadenitis can lead to appropriate management. Endoscopic ultrasonography (EUS)-guided needle biopsy may be the procedure of choice for tissue acquisition when onsite cytopathology examination is unavailable because it is essential to obtain sufficient material suitable for the examination using an ancillary method, such as flow cytometry, molecular diagnosis, cytogenetics, or microbiological culture. We report a case of intra-abdominal tuberculous lymphadenitis diagnosed using an EUS-guided, 22-gauge histology new needle biopsy without an onsite cytopathology examination.
-
Citations
Citations to this article as recorded by- Coeliac lymph node abscess: A case report of a rare manifestation of extrapulmonary tuberculosis
Asitha Goonetilleke, Malith Nandasena, Nilesh Fernandopulle, Anne Thushara Matthias
SAGE Open Medical Case Reports.2024;[Epub] CrossRef - Current role of endoscopic ultrasound for gastrointestinal and abdominal tuberculosis
Hasan Maulahela, Achmad Fauzi, Kaka Renaldi, Qorina P Srisantoso, Amirah Jasmine
JGH Open.2022; 6(11): 745. CrossRef - Tuberculous Lymphadenitis Mimicking Gastric Subepithelial Tumor Diagnosed Using Endoscopic Ultrasound-guided Fine-needle Aspiration
Sung Bum Kim, Tae Nyeun Kim, Kook Hyun Kim
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2018; 18(1): 65. CrossRef - Gastric Tuberculosis Presenting as a Subepithelial Mass: A Rare Cause of Gastrointestinal Bleeding
Tae Un Kim, Su Jin Kim, Hwaseong Ryu, Jin Hyeok Kim, Hee Seok Jeong, Jieun Roh, Jeong A Yeom, Byung Soo Park, Dong Il Kim, Ki Hyun Kim
The Korean Journal of Gastroenterology.2018; 72(6): 304. CrossRef - Ultrasonography in the Assessment of Lymph Node Disease
Hans-Peter Weskott, Sanshan Yin
Ultrasound Clinics.2014; 9(3): 351. CrossRef - Minimally Invasive Mediastinal Staging of Non–Small-Cell Lung Cancer: Emphasis on Ultrasonography-Guided Fine-Needle Aspiration
Cynthia L. Harris, Eric M. Toloza, Jason B. Klapman, Shivakumar Vignesh, Kathryn Rodriguez, Frank J. Kaszuba
Cancer Control.2014; 21(1): 15. CrossRef
- Coeliac lymph node abscess: A case report of a rare manifestation of extrapulmonary tuberculosis
- 5,661 View
- 60 Download
- 3 Web of Science
- 6 Crossref

-
ESD Hands-on Course Using
Ex Vivo andIn Vivo Models in South Korea - Gene Hyun Bok, Joo Young Cho
- Clin Endosc 2012;45(4):358-361. Published online November 30, 2012
- DOI: https://doi.org/10.5946/ce.2012.45.4.358
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Endoscopic submucosal dissection (ESD) is an established treatment for gastric neoplasias especially in regions with a high volume of gastric cancer. Although ESD has many advantages over endoscopic mucosal resection, ESD is technically more difficult and can result in severe complications. Therefore establishment of an effective training system is required to help endoscopists climb the ESD learning curve. Although a standard training system for ESD remains to be established, some centers are incorporating
ex vivo and/orin vivo animal models to provide a safe and effective means of ESD training. However, it is unknown if these animal models are more effective than other programs. Moreover the efficacy of the animal model may vary according to socio-economic status and the volume of gastric cancer. In this article we introduce the basic and advanced ESD training model using theex vivo andin vivo animal model from South Korea and review the associated literature from other regions.-
Citations
Citations to this article as recorded by- A Steep Early Learning Curve for Endoscopic Submucosal Dissection in the Live Porcine Model
Ricardo Küttner-Magalhães, Mário Dinis-Ribeiro, Marco J. Bruno, Ricardo Marcos-Pinto, Carla Rolanda, Arjun D. Koch
Digestive Diseases.2022; 40(6): 816. CrossRef - Risk Factors Indicating Difficulty During Gastric Endoscopic Submucosal Dissection for Inexperienced Endoscopists: A Retrospective Study
Kensuke Higuchi, Atsushi Katagiri, Shinya Nakatani, Kazuo Kikuchi, Takahisa Fujiwara, Toshihiko Gocho, Kazuya Inoki, Kenichi Konda, Fuyuhiko Yamamura, Hitoshi Yoshida
Cureus.2022;[Epub] CrossRef - Clinical practice guideline for endoscopic resection of early gastrointestinal cancer
Chan Hyuk Park, Dong-Hoon Yang, Jong Wook Kim, Jie-Hyun Kim, Ji Hyun Kim, Yang Won Min, Si Hyung Lee, Jung Ho Bae, Hyunsoo Chung, Kee Don Choi, Jun Chul Park, Hyuk Lee, Min-Seob Kwak, Bun Kim, Hyun Jung Lee, Hye Seung Lee, Miyoung Choi, Dong-Ah Park, Jong
Intestinal Research.2021; 19(2): 127. CrossRef - Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer
Chan Hyuk Park, Dong-Hoon Yang, Jong Wook Kim, Jie-Hyun Kim, Ji Hyun Kim, Yang Won Min, Si Hyung Lee, Jung Ho Bae, Hyunsoo Chung, Kee Don Choi, Jun Chul Park, Hyuk Lee, Min-Seob Kwak, Bun Kim, Hyun Jung Lee, Hye Seung Lee, Miyoung Choi, Dong-Ah Park, Jong
Clinical Endoscopy.2020; 53(2): 142. CrossRef - Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer
Chan Hyuk Park, Dong-Hoon Yang, Jong Wook Kim, Jie-Hyun Kim, Ji Hyun Kim, Yang Won Min, Si Hyung Lee, Jung Ho Bae, Hyunsoo Chung, Kee Don Choi, Jun Chul Park, Hyuk Lee, Min-Seob Kwak, Bun Kim, Hyun Jung Lee, Hye Seung Lee, Miyoung Choi, Dong-Ah Park, Jong
The Korean Journal of Gastroenterology.2020; 75(5): 264. CrossRef - Current status and trend in training for endoscopic submucosal dissection: A nationwide survey in Korea
Jae Gon Lee, Chan Hyuk Park, Hyunsoo Chung, Jun Chul Park, Do Hoon Kim, Bo-In Lee, Jeong-Sik Byeon, Hwoon-Yong Jung, Richard Bruce Mink
PLOS ONE.2020; 15(5): e0232691. CrossRef - Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer
Chan Hyuk Park, Dong-Hoon Yang, Jong Wook Kim, Jie-Hyun Kim, Ji Hyun Kim, Yang Won Min, Si Hyung Lee, Jung Ho Bae, Hyunsoo Chung, Kee Don Choi, Jun Chul Park, Hyuk Lee, Min-Seob Kwak, Bun Kim, Hyun Jung Lee, Hye Seung Lee, Miyoung Choi, Dong-Ah Park, Jong
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2020; 20(2): 117. CrossRef - A Template for Curriculum Development to Teach Complex Surgical or Endoscopic Techniques With Logistical Challenges
Karen J Dickinson, Brian J Dunkin, Barbara L Bass, Aman B Ali, J. Joseph Nguyen-Lee, Stephanie Zajac
Journal of Surgical Education.2020; 77(6): 1511. CrossRef - Training in endoscopic mucosal resection and endoscopic submucosal dissection: Face, content and expert validity of the live porcine model
Ricardo Küttner-Magalhães, Mário Dinis-Ribeiro, Marco J Bruno, Ricardo Marcos-Pinto, Carla Rolanda, Arjun D Koch
United European Gastroenterology Journal.2018; 6(4): 547. CrossRef - Endoscopic Submucosal Dissection (ESD) and Related Techniques as Precursors of “New Notes” Resection Methods for Gastric Neoplasms
Osamu Goto, Hiroya Takeuchi, Yuko Kitagawa, Naohisa Yahagi
Gastrointestinal Endoscopy Clinics of North America.2016; 26(2): 313. CrossRef - Simulator training in gastrointestinal endoscopy – From basic training to advanced endoscopic procedures
S.E. van der Wiel, R. Küttner Magalhães, Carla Rolanda Rocha Gonçalves, M. Dinis-Ribeiro, M.J. Bruno, A.D. Koch
Best Practice & Research Clinical Gastroenterology.2016; 30(3): 375. CrossRef - Short-Term Outcomes of Endoscopic Submucosal Dissection in Patients with Early Gastric Cancer: A Prospective Multicenter Cohort Study
ll Ju Choi, Na Rae Lee, Sang Gyun Kim, Wan Sik Lee, Seun Ja Park, Jae J. Kim, Jun Haeng Lee, Jin-Won Kwon, Seung-Hee Park, Ji Hye You, Ji Hyun Kim, Chul-Hyun Lim, Joo Young Cho, Gwang Ha Kim, Yong Chan Lee, Hwoon-Yong Jung, Ji Young Kim, Hoon Jai Chun, Sa
Gut and Liver.2016; 10(5): 739. CrossRef - ENDOSCOPIC GASTRIC SUBMUCOSAL DISSECTION: experimental comparative protocol between standard technique and Hybrid-Knife(r)
Ernesto Quaresma MENDONÇA, Lucas Snioka ZURETTI, Thiago PANZANI, Marianny SULBARAN, Christiano Makoto SAKAI, Paulo SAKAI
Arquivos de Gastroenterologia.2016; 53(3): 192. CrossRef - Disección endoscópica submucosa: curva de aprendizaje en modelos porcinos
Victor Efrén Gallardo Cabrera, Oscar Hernández Mondragón, Dulce María Rascón Martínez, Gerardo Blanco Velasco, Roberto Ramos González, Amina Evelyn Tun Abraham, Juan Manuel Blancas Valencia
Endoscopia.2015; 27(3): 109. CrossRef - Endoscopic submucosal dissection training with ex vivo human gastric remnants
David V. Pham, Anand Shah, Frank J. Borao, Steven Gorcey
Surgical Endoscopy.2014; 28(1): 222. CrossRef - International Digestive Endoscopy Network 2014: Turnpike to the Future
Eun Young Kim, Kwang An Kwon, Il Ju Choi, Ji Kon Ryu, Ki Baik Hahm
Clinical Endoscopy.2014; 47(5): 371. CrossRef - ESD training: A challenging path to excellence
Alberto Herreros de Tejada
World Journal of Gastrointestinal Endoscopy.2014; 6(4): 112. CrossRef
- A Steep Early Learning Curve for Endoscopic Submucosal Dissection in the Live Porcine Model
- 5,725 View
- 65 Download
- 17 Crossref

- Successful Treatment of Early Gastric Cancer Adjacent to a Fundal Varix by Endoscopic Submucosal Dissection and Endoscopic Cyanoacrylate Therapy
- Yeon Soo Kim, Won Young Cho, Joo Young Cho, So Young Jin
- Clin Endosc 2012;45(2):169-173. Published online June 30, 2012
- DOI: https://doi.org/10.5946/ce.2012.45.2.169
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Endoscopic submucosal dissection (ESD) was developed for the
en bloc resection of large early gastrointestinal neoplasms. A disadvantage of ESD is its technical difficulty, which requires advanced skills and is associated with a higher rate of complications. Endoscopic variceal obturation (EVO) using cyanoacrylate has emerged as the initial treatment of choice for acute gastric variceal bleeding. This procedure achieves hemostasis in 90% of cases. A 52-year-old patient with Child A alcoholic liver cirrhosis presented with early gastric cancer in the cardia and type 1 isolated gastric varices in the fundus. The two lesions were so close together that treatment was not easy. The lesions were managed successfully with a combination of ESD and EVO using cyanoacrylate.-
Citations
Citations to this article as recorded by- Successful endoscopic submucosal dissection for early gastric cancer adjacent to gastric cardia varix
Ko Watanabe, Takuto Hikichi, Jun Nakamura, Tadayuki Takagi, Rei Suzuki, Mitsuru Sugimoto, Yuichi Waragai, Hitomi Kikuchi, Naoki Konno, Hiroyuki Asama, Mika Takasumi, Hiroshi Watanabe, Katsutoshi Obara, Hiromasa Ohira
FUKUSHIMA JOURNAL OF MEDICAL SCIENCE.2016; 62(2): 101. CrossRef - Injection and Cautery Methods for Nonvariceal Bleeding Control
Cristina Bucci, Gianluca Rotondano, Riccardo Marmo
Gastrointestinal Endoscopy Clinics of North America.2015; 25(3): 509. CrossRef - Hyaluronic acid solution injection for upper and lower gastrointestinal bleeding after failed conventional endoscopic therapy
Jin Wook Lee, Hyung Hun Kim
Digestive Endoscopy.2014; 26(2): 285. CrossRef - Observable Laryngopharyngeal Lesions during the Upper Gastrointestinal Endoscopy
Kyung Sik Park
Clinical Endoscopy.2013; 46(3): 224. CrossRef
- Successful endoscopic submucosal dissection for early gastric cancer adjacent to gastric cardia varix
- 7,328 View
- 69 Download
- 4 Crossref

- Submucosal Endoscopy, a New Era of Pure Natural Orifice Translumenal Endoscopic Surgery (NOTES)
- Suck-Ho Lee, Won Young Cho, Joo Young Cho
- Clin Endosc 2012;45(1):4-10. Published online March 31, 2012
- DOI: https://doi.org/10.5946/ce.2012.45.1.4
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Natural orifice translumenal endoscopic surgery (NOTES) involves the intentional perforation of the viscera with an endoscope to access the abdominal cavity and perform an intraabdominal operation. In a brief time period, NOTES has been shown to be feasible in laboratory animal and human studies. Easy access to the peritoneal cavity and complete gastric closure should be secured before NOTES can be recommended as an acceptable alternative in clinical practice. The concept of submucosal endoscopy has been introduced as a solution to overcome these two primary barriers to human NOTES application. Its offset entry/exit access method effectively prevents contamination and allows the rapid closure of the entry site with a simple mucosal apposition. In addition, it could be used as an endoscopic working space for various submucosal conditions. Herein, the detailed procedures, laboratory results and human application of the submucosal endoscopy will be reviewed.
-
Citations
Citations to this article as recorded by- Peroral Endoscopic Myotomy for the Management of Esophageal Diverticula: Tunneling Forward
Matt Pelton, Michel Kahaleh, Amy Tyberg
Techniques and Innovations in Gastrointestinal Endoscopy.2024; 26(1): 56. CrossRef - Editorial: Recent updates in advanced gastrointestinal endoscopy
Abhilash Perisetti, Benjamin Tharian, Tony C. Tham, Hemant Goyal
Frontiers in Medicine.2023;[Epub] CrossRef - Preparation and Patient Evaluation for Safe Gastrointestinal Endoscopy
Seong Hee Kang, Jong Jin Hyun
Clinical Endoscopy.2013; 46(3): 212. CrossRef - International Digestive Endoscopy Network 2012: A Patchwork of Networks for the Future
Kwang An Kwon, Il Ju Choi, Eun Young Kim, Seok Ho Dong, Ki Baik Hahm
Clinical Endoscopy.2012; 45(3): 209. CrossRef
- Peroral Endoscopic Myotomy for the Management of Esophageal Diverticula: Tunneling Forward
- 7,167 View
- 56 Download
- 4 Crossref


 KSGE
KSGE


 First
First Prev
Prev