Previous issues
- Page Path
- HOME > Browse Articles > Previous issues
Commentarys
- Usefulness of an Overtube Device in Gastrointestinal Endoscopy
- Seung Han Kim
- Clin Endosc 2019;52(3):203-204. Published online May 30, 2019
- DOI: https://doi.org/10.5946/ce.2019.085
-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- Endoscopic Management of a Long-Duration Esophageal Food Impaction: A Case Report
Santiago Philibert-Rosas, Israel Podolsky Rapoport
Cureus.2024;[Epub] CrossRef - Usefulness of cricoid pressure in patients with poor gastric wall extension as a result of eructation in upper gastrointestinal endoscopy
Toshiki Horii, Hisatomo Ikehara, Chika Kusano
Digestive Endoscopy.2019;[Epub] CrossRef
- Endoscopic Management of a Long-Duration Esophageal Food Impaction: A Case Report
- 4,624 View
- 98 Download
- 3 Web of Science
- 2 Crossref

- Commentary on “Efficacy of Endoscopic Submucosal Dissection of Esophageal Neoplasms under General Anesthesia”
- Soo In Choi, Jun Chul Park
- Clin Endosc 2019;52(3):205-206. Published online May 23, 2019
- DOI: https://doi.org/10.5946/ce.2019.093
- 4,232 View
- 74 Download

- The Most Common Cause of Lower Gastrointestinal Bleeding without Other Symptoms in Children is Colonic Polyp: Is Total Colonoscopy Needed?
- Yeoun Joo Lee, Jae Hong Park
- Clin Endosc 2019;52(3):207-208. Published online May 24, 2019
- DOI: https://doi.org/10.5946/ce.2019.084
-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- Practice Patterns of Colorectal Polypectomy in Pediatric Endoscopic Specialists in South Korea: A Nationwide Survey Study
Yoon Lee, Sujin Choi, Ben Kang
Pediatric Gastroenterology, Hepatology & Nutrition.2023; 26(1): 15. CrossRef - Associations of Polyp Characteristics in Children and Adolescents Presenting with Less Than Five Colorectal Polyps: A Full Colonoscopy Is Still Required
Ju Young Kim, Yu Bin Kim, Sujin Choi, Yoo Min Lee, Hyun Jin Kim, Soon Chul Kim, Hyo-Jeong Jang, So Yoon Choi, Dae Yong Yi, Yoon Lee, You Jin Choi, Yunkoo Kang, Kyung Jae Lee, Suk Jin Hong, Jun Hyun Hwang, Sanggyu Kwak, Byung-Ho Choe, Ben Kang
Gut and Liver.2023; 17(3): 441. CrossRef - Disease patterns among Saudi children undergoing colonoscopy for lower gastrointestinal bleeding: Single tertiary care center experience
Sami Alrashidi, Tarig AlAmery, Abdullah Alshanbary, Eman Aljohani, Salman M Bashir, Bader Alsaleem, Ali Asery, Abdulrahman Al-Hussaini
Saudi Journal of Gastroenterology.2023; 29(6): 388. CrossRef - Adenovirus is prevalent in juvenile polyps and correlates with low vitamin D receptor expression
Lingling Wang, Hongmei Guo, Jingwen Li, Susu He, Guang Yang, Erguang Li
Pediatric Research.2022; 91(7): 1703. CrossRef - Potential Utility of Fecal Calprotectin in Discriminating Colorectal Polyps From Other Major Etiologies in Children Presenting With Isolated Hematochezia
Yu Bin Kim, Ju Young Kim, Sujin Choi, Hyun Jin Kim, Yoo Min Lee, Yoon Lee, Hyo-Jeong Jang, Eun Hye Lee, Kyung Jae Lee, Soon Chul Kim, So Yoon Choi, Yunkoo Kang, Dae Yong Yi, You Jin Choi, Byung-Ho Choe, Ben Kang
Journal of Korean Medical Science.2022;[Epub] CrossRef - Colon polyps in children
A. L. Ionov, M. V. Pichugina, A. V. Myzin, V. A. Luka, T. D. Kostomarova, Ya. P. Sulavko
Koloproktologia.2022; 21(2): 64. CrossRef
- Practice Patterns of Colorectal Polypectomy in Pediatric Endoscopic Specialists in South Korea: A Nationwide Survey Study
- 3,950 View
- 90 Download
- 5 Web of Science
- 6 Crossref

- Endoscopic Management of Acute Cholecystitis Following Metal Stent Placement for Malignant Biliary Strictures: A View from the Inside Looking in
- Sean Bhalla, Ryan Law
- Clin Endosc 2019;52(3):209-211. Published online May 23, 2019
- DOI: https://doi.org/10.5946/ce.2019.097
- 4,163 View
- 57 Download

Focused Review Series: Expanding Indication: Interventional Endoscopic Management for Pancreaticobiliary Diseaseses
- Endoscopic Ultrasound-Guided Biliary Drainage for Benign Biliary Diseases
- Yousuke Nakai, Hirofumi Kogure, Hiroyuki Isayama, Kazuhiko Koike
- Clin Endosc 2019;52(3):212-219. Published online March 14, 2019
- DOI: https://doi.org/10.5946/ce.2018.188

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Although endoscopic retrograde cholangiopancreatography (ERCP) is the first-line treatment for benign biliary diseases, this procedure is technically difficult in some conditions such as a surgically altered anatomy and gastric outlet obstruction. After a failed ERCP, a surgical or a percutaneous approach is selected as a rescue procedure; however, various endoscopic ultrasound (EUS)-guided interventions are increasingly utilized in pancreatobiliary diseases, including EUS-guided rendezvous for failed biliary cannulation, EUS-guided antegrade treatment for stone management, and EUS-guided hepaticogastrostomy for anastomotic strictures in patients with a surgically altered anatomy. There are some technical hurdles in EUS-guided interventions for benign biliary diseases owing to the difficulty in puncturing a relatively small bile duct and in subsequent guidewire manipulation, as well as the lack of dedicated devices. A recent major advancement in this field is the introduction of a 2-step approach, in which EUS-guided drainage is placed in the first session and antegrade treatment is performed in subsequent sessions. This approach allows the use of various techniques such as mechanical lithotripsy and cholangioscopy without a risk of bile leak. In summary, EUS-guided interventions are among the treatment options for benign biliary diseases; however, standardization of the procedure and development of a treatment algorithm are needed.
-
Citations
Citations to this article as recorded by- EUS-BD for calibration of benign stenosis of the bile duct in patients with altered anatomy or inaccessible papilla
Fabrice Caillol, Sébastien Godat, Alexey Solovyev, Amina Harouchi, Sarra Oumrani, Mariola Marx, Solene Hoibian, Yanis Dahel, Jean-Philippe Ratone, Marc Giovannini
Endoscopy International Open.2024; 12(03): E377. CrossRef - Safety of endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction and ascites
Tsukasa Yasuda, Kazuo Hara, Nobumasa Mizuno, Shin Haba, Takamichi Kuwahara, Nozomi Okuno, Yasuhiro Kuraishi, Takafumi Yanaidani, Sho Ishikawa, Masanori Yamada, Toshitaka Fukui
Clinical Endoscopy.2024; 57(2): 246. CrossRef - A retrospective multicenter study comparing the punctures to B2 and B3 in endoscopic ultrasound–guided hepaticogastrostomy
Masanari Sekine, Yusuke Hashimoto, Taro Shibuki, Kei Okumura, Ikuhiro Kobori, Aki Miyagaki, Yoshihiro Sasaki, Yuichi Takano, Keita Matsumoto, Hirosato Mashima
DEN Open.2023;[Epub] CrossRef - Endoscopic ultrasound guided biliary drainage in surgically altered anatomy: A comprehensive review of various approaches
Sridhar Sundaram, Aditya Kale
World Journal of Gastrointestinal Endoscopy.2023; 15(3): 122. CrossRef - BILE: A Literature Review Based Novel Clinical Classification and Treatment Algorithm of Iatrogenic Bile Duct Injuries
Dimitrios Symeonidis, Konstantinos Tepetes, George Tzovaras, Athina A. Samara, Dimitrios Zacharoulis
Journal of Clinical Medicine.2023; 12(11): 3786. CrossRef - Endoscopic Biliary Drainage in Surgically Altered Anatomy
Marco Spadaccini, Carmelo Marco Giacchetto, Matteo Fiacca, Matteo Colombo, Marta Andreozzi, Silvia Carrara, Roberta Maselli, Fabio Saccà, Alessandro De Marco, Gianluca Franchellucci, Kareem Khalaf, Glenn Koleth, Cesare Hassan, Andrea Anderloni, Alessandro
Diagnostics.2023; 13(24): 3623. CrossRef - EUS‐guided hepaticogastrostomy for hepaticojejunostomy stricture using a 22G needle and a mechanical dilator (with video)
Takeshi Ogura, Saori Ueno, Atsushi Okuda, Nobu Nishioka, Kazuhide Higuchi
Journal of Hepato-Biliary-Pancreatic Sciences.2022;[Epub] CrossRef - Combined endoscopic retrograde and endosonography-guided (CERES) cholangiography for interventional repair of transected bile ducts after cholecystectomy: treatment approaches and outcomes
Marina de Benito Sanz, Ana Y. Carbajo, Ramon Sanchez-Ocana, Carlos Chavarría, Carlos de la Serna-Higuera, Manuel Perez-Miranda
Surgical Endoscopy.2022; 36(3): 2197. CrossRef - Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline
Schalk W. van der Merwe, Roy L. J. van Wanrooij, Michiel Bronswijk, Simon Everett, Sundeep Lakhtakia, Mihai Rimbas, Tomas Hucl, Rastislav Kunda, Abdenor Badaoui, Ryan Law, Paolo G. Arcidiacono, Alberto Larghi, Marc Giovannini, Mouen A. Khashab, Kenneth F.
Endoscopy.2022; 54(02): 185. CrossRef - Practical Tips for Safe and Successful Endoscopic Ultrasound-Guided Hepaticogastrostomy: A State-of-the-Art Technical Review
Saburo Matsubara, Keito Nakagawa, Kentaro Suda, Takeshi Otsuka, Masashi Oka, Sumiko Nagoshi
Journal of Clinical Medicine.2022; 11(6): 1591. CrossRef - Clinical value of preferred endoscopic ultrasound-guided antegrade surgery in the treatment of extrahepatic bile duct malignant obstruction
Xuan Zhao, Lihong Shi, Jinchen Wang, Siming Guo, Sumin Zhu
Clinics.2022; 77: 100017. CrossRef - EUS-guided hepaticoduodenostomy for the management of postsurgical bile duct injury: An alternative to surgery (with video)
Carlos Robles-Medranda, Roberto Oleas, Martha Arevalo-Mora, Juan Alcivar-Vasquez, Raquel Del Valle
Endoscopic Ultrasound.2022; 11(5): 421. CrossRef - Current endoscopic approaches to biliary strictures
Tatsuya Sato, Yousuke Nakai, Mitsuhiro Fujishiro
Current Opinion in Gastroenterology.2022; 38(5): 450. CrossRef - Endoscopic salvage therapy after failed biliary cannulation using advanced techniques: A concise review
Yung-Kuan Tsou, Kuang-Tse Pan, Mu Hsien Lee, Cheng-Hui Lin
World Journal of Gastroenterology.2022; 28(29): 3803. CrossRef - Redo hepaticojejunostomy in the management of bilioenteric anastomotic strictures
BharathKumar Bhat, Samrat Ray, Shailendra Lalwani, Vivek Mangla, NaimishN Mehta, Amitabh Yadav, Samiran Nundy
Current Medicine Research and Practice.2022; 12(4): 162. CrossRef - Evaluating the role of endoscopic ultrasound in pancreatitis
Surinder Singh Rana
Expert Review of Gastroenterology & Hepatology.2022; 16(10): 953. CrossRef - Novel transluminal treatment protocol for hepaticojejunostomy stricture using covered self-expandable metal stent
Takeshi Ogura, Nobu Nishioka, Masanori Yamada, Tadahiro Yamada, Saori Ueno, Jyun Matsuno, Kazuya Ueshima, Yoshitaro Yamamoto, Atsushi Okuda, Kazuhide Higuchi
Surgical Endoscopy.2021; 35(1): 209. CrossRef - Effect of echoendoscope angle on success of guidewire manipulation during endoscopic ultrasound-guided hepaticogastrostomy
Takeshi Ogura, Nobu Nishioka, Saori Ueno, Tadahiro Yamada, Masanori Yamada, Akira Imoto, Akitoshi Hakoda, Kazuhide Higuchi
Endoscopy.2021; 53(04): 369. CrossRef - Transluminal antegrade biopsy using a novel forceps biopsy device for hepaticojejunostomy stricture
Takeshi Ogura, Atsushi Okuda, Nobu Nishioka, Masanori Yamada, Kazuhide Higuchi
Endoscopy.2021; 53(07): E269. CrossRef - Endoscopic treatment of hepaticojejunostomy anastomotic strictures using fully‐covered metal stents
Tatsuya Sato, Hirofumi Kogure, Yousuke Nakai, Sachiko Kanai, Kazunaga Ishigaki, Ryunosuke Hakuta, Kei Saito, Tomotaka Saito, Naminatsu Takahara, Tsuyoshi Hamada, Suguru Mizuno, Atsuo Yamada, Hiroyuki Isayama, Kazuhiko Koike
Digestive Endoscopy.2021; 33(3): 451. CrossRef - The safety and efficacy of self-expandable metallic stent placement for malignant biliary obstruction with surgically altered anatomy
Tsuyoshi Takeda, Takashi Sasaki, Takafumi Mie, Takaaki Furukawa, Ryo Kanata, Akiyoshi Kasuga, Masato Matsuyama, Masato Ozaka, Naoki Sasahira
Scandinavian Journal of Gastroenterology.2021; 56(1): 94. CrossRef - EUS-guided choledochoduodenostomy as a rescue after failed ERCP and percutaneous transhepatic biliary drainage in the management of postoperative benign biliary stricture
Aniruddha Pratap Singh, Pradev Inavolu, Shujaath Asif, D. Nageshwar Reddy, Sundeep Lakhtakia
VideoGIE.2021; 6(2): 90. CrossRef - Diagnosis and management of benign biliary strictures post liver transplantation in adults
Margaret G. Keane, John Devlin, Philip Harrison, Maen Masadeh, Mustafa A. Arain, Deepak Joshi
Transplantation Reviews.2021; 35(1): 100593. CrossRef - A unique device enabling electrohydraulic lithotripsy with an ultraslim scope for difficult stones after endoscopic ultrasound-guided biliary drainage
Hassan Atalla, Hideyuki Shiomi, Takuya Ikegawa, Takashi Kobayashi, Arata Sakai, Atsuhiro Masuda, Yuzo Kodama
Endoscopy.2021; 53(02): E52. CrossRef - EUS-guided hepaticogastrostomy as a gateway to intermittent access for biliary leak management
Michiel Bronswijk, Giuseppe Vanella, Baki Topal, Schalk Van der Merwe
Endoscopy.2021; 53(11): E427. CrossRef - Endoscopic ultrasound‐guided rendezvous with a combination of 22‐gauge needle and 0.018‐inch guidewire for acute cholangitis with Cronkhite‐Canada syndrome
Kosuke Takahashi, Ichiro Yasuda, Tatsuyuki Hanaoka
Digestive Endoscopy.2021;[Epub] CrossRef - What You Need to Know Before Performing Endoscopic Ultrasound-guided Hepaticogastrostomy
Tanyaporn Chantarojanasiri, Thawee Ratanachu-Ek, Nonthalee Pausawasdi
Clinical Endoscopy.2021; 54(3): 301. CrossRef - EUS-guided hepaticoenterostomy with using a dedicated plastic stent for the benign pancreaticobiliary diseases: A single-center study of a large case series
Yukitoshi Matsunami, Takao Itoi, Atsushi Sofuni, Takayoshi Tsuchiya, Kentaro Ishii, Reina Tanaka, Ryosuke Tonozuka, Mitsuyoshi Honjo, Shuntaro Mukai, Kazumasa Nagai, Kenjiro Yamamoto, Yasutsugu Asai, Takashi Kurosawa, Hiroyuki Kojima, Eri Joyama, Yuichi N
Endoscopic Ultrasound.2021; 10(4): 294. CrossRef - GRUPUGE PERSPECTIVE: Endoscopic Ultrasound-Guided Biliary Drainage
Nuno Nunes, Margarida Flor de Lima, Ana Caldeira, Sílvia Leite, Susana Marques, Teresa Moreira, Pedro Moutinho-Ribeiro, Miguel Bispo
GE - Portuguese Journal of Gastroenterology.2021; 28(3): 179. CrossRef - Endoscopic management of difficult common bile duct stones: Where are we now? A comprehensive review
Alberto Tringali, Deborah Costa, Alessandro Fugazza, Matteo Colombo, Kareem Khalaf, Alessandro Repici, Andrea Anderloni
World Journal of Gastroenterology.2021; 27(44): 7597. CrossRef - Initial clinical experience of a steerable access device for EUS-guided biliary drainage
Marvin Ryou, Petros C. Benias, Vivek Kumbhari
Gastrointestinal Endoscopy.2020; 91(1): 178. CrossRef - Management of Difficult Bile Duct Stones by Large Balloon, Cholangioscopy, Enteroscopy and Endosonography
Yousuke Nakai, Tatsuya Sato, Ryunosuke Hakuta, Kazunaga Ishigaki, Kei Saito, Tomotaka Saito, Naminatsu Takahara, Tsuyoshi Hamada, Suguru Mizuno, Hirofumi Kogure, Minoru Tada, Hiroyuki Isayama, Kazuhiko Koike
Gut and Liver.2020; 14(3): 297. CrossRef - Piercing technique via cholangioscopy for the reconstruction of complete anastomotic obstruction after choledochojejunostomy
Haruka Toyonaga, Tsuyoshi Hayashi, Akio Katanuma
Digestive Endoscopy.2020;[Epub] CrossRef - EUS-guided rendezvous with a steerable access needle in choledocholithiasis
Sundeep Lakhtakia, Radhika Chavan, Mohan Ramchandani, Jahangeer Basha, D. Nageshwar Reddy
VideoGIE.2020; 5(8): 359. CrossRef - Relief of biliary obstruction: choosing between endoscopic ultrasound and endoscopic retrograde cholangiopancreatography
Andrew Canakis, Todd H Baron
BMJ Open Gastroenterology.2020; 7(1): e000428. CrossRef - Treatment of Long-Limb Biliary-Enteric Anastomotic Strictures: ERCP, PTBD, or EUS?
Yousuke Nakai
Digestive Diseases and Sciences.2019; 64(9): 2379. CrossRef
- EUS-BD for calibration of benign stenosis of the bile duct in patients with altered anatomy or inaccessible papilla
- 12,815 View
- 296 Download
- 33 Web of Science
- 36 Crossref

- Endoscopic Ultrasound-Guided Biliary Drainage for Unresectable Hilar Malignant Biliary Obstruction
- Yousuke Nakai, Hirofumi Kogure, Hiroyuki Isayama, Kazuhiko Koike
- Clin Endosc 2019;52(3):220-225. Published online November 29, 2018
- DOI: https://doi.org/10.5946/ce.2018.094
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic transpapillary biliary drainage is the current standard of care for unresectable hilar malignant biliary obstruction (MBO) and bilateral metal stent placement is shown to have longer patency. However, technical and clinical failure is possible and percutaneous transhepatic biliary drainage (PTBD) is sometimes necessary. Endoscopic ultrasound-guided biliary drainage (EUS-BD) is increasingly being reported as an alternative rescue procedure to PTBD. EUS-BD has a potential advantage of not traversing the biliary stricture and internal drainage can be completed in a single session. Some approaches to bilateral biliary drainage for hilar MBO under EUS-guidance include a bridging method, hepaticoduodenostomy, and a combination of EUS-BD and transpapillary biliary drainage. The aim of this review is to summarize data on EUS-BD for hilar MBO and to clarify its advantages over the conventional approaches such as endoscopic transpapillary biliary drainage and PTBD.
-
Citations
Citations to this article as recorded by- Retrospective comparative study of new slim‐delivery and conventional large‐cell stents for stent‐in‐stent methods for hilar malignant biliary obstruction
Kazunaga Ishigaki, Rintaro Fukuda, Yousuke Nakai, Go Endo, Kohei Kurihara, Kota Ishida, Shuichi Tange, Shinya Takaoka, Yurie Tokito, Yukari Suzuki, Hiroki Oyama, Sachiko Kanai, Tatsunori Suzuki, Yukiko Ito, Tatsuya Sato, Ryunosuke Hakuta, Kei Saito, Tomot
Digestive Endoscopy.2024; 36(3): 360. CrossRef - ERCP for the initial management of malignant biliary obstruction – real world data on 596 procedures
I. M. Mikalsen, S. Breder, A. W. Medhus, T. Folseraas, L. Aabakken, K. V. Ånonsen
Scandinavian Journal of Gastroenterology.2024; 59(3): 369. CrossRef - A Novel Method of Calculating the Drained Liver Volume Using a 3D Volume Analyzer for Biliary Drainage of Unresectable Malignant Hilar Biliary Obstruction
Naoto Imagawa, Mitsuharu Fukasawa, Shinichi Takano, Satoshi Kawakami, Yoshimitsu Fukasawa, Hiroyuki Hasegawa, Natsuhiko Kuratomi, Shota Harai, Naruki Shimamura, Dai Yoshimura, Shoji Kobayashi, Takashi Yoshida, Mitsuaki Sato, Yuichiro Suzuki, Nobuyuki Enom
Digestive Diseases and Sciences.2024; 69(3): 969. CrossRef - Safety of endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction and ascites
Tsukasa Yasuda, Kazuo Hara, Nobumasa Mizuno, Shin Haba, Takamichi Kuwahara, Nozomi Okuno, Yasuhiro Kuraishi, Takafumi Yanaidani, Sho Ishikawa, Masanori Yamada, Toshitaka Fukui
Clinical Endoscopy.2024; 57(2): 246. CrossRef - A novel method of bilateral biliary decompression by EUS-guided hepaticogastrostomy with bridging stenting using the partial stent-in-stent method for reintervention of multiple metal stent failure
Hidenobu Hara, Susumu Hijioka, Yoshikuni Nagashio, Yuta Maruki, Mark Chatto, Yutaka Saito, Takuji Okusaka
VideoGIE.2024; 9(6): 286. CrossRef - Three-year evaluation of a novel, nonfluoroscopic, all-artificial model for EUS-guided biliary drainage training for the impact to practice: A prospective observational study (with videos)
Tanyaporn Chantarojanasiri, Aroon Siripun, Pradermchai Kongkam, Nonthalee Pausawasdi, Thawee Ratanachu-ek
Endoscopic Ultrasound.2023; 12(1): 96. CrossRef - Endoscopic management of benign and malignant hilar stricture
Marcella Pimpinelli, Michael Makar, Michel Kahaleh
Digestive Endoscopy.2023; 35(4): 443. CrossRef - Endoscopic and Endosonographic Palliation for Triple Obstruction Caused by Recurrent Gallbladder Cancer: A Case Report
Young Rong Kim, Chi Hyuk Oh, Min Jae Yang
The Korean Journal of Pancreas and Biliary Tract.2023; 28(1): 19. CrossRef - Unpredictable Event during EUS-Guided Hepaticojejunostomy
Abdullatif SİRİN, Mehmet ÇETİN, Salih TOKMAK
Düzce Tıp Fakültesi Dergisi.2023; 25(1): 89. CrossRef - Optimal endoscopic drainage strategy for unresectable malignant hilar biliary obstruction
Itaru Naitoh, Tadahisa Inoue
Clinical Endoscopy.2023; 56(2): 135. CrossRef - Role of radiofrequency ablation in advanced malignant hilar biliary obstruction
Mamoru Takenaka, Tae Hoon Lee
Clinical Endoscopy.2023; 56(2): 155. CrossRef - Preoperative endoscopic ultrasound-guided biliary drainage for primary drainage in obstructive jaundice
Shuntaro Mukai, Takao Itoi
Expert Review of Gastroenterology & Hepatology.2023; 17(12): 1197. CrossRef - Use of endoscopic ultrasound‐guided biliary drainage as a rescue of re‐intervention after the placement of multiple metallic stents for malignant hilar biliary obstruction
Hidetoshi Kitamura, Susumu Hijioka, Yoshikuni Nagashio, Shunsuke Sugawara, Satoshi Nara, Miyuki Sone, Minoru Esaki, Yasuaki Arai, Takuji Okusaka, Atsushi Nakajima
Journal of Hepato-Biliary-Pancreatic Sciences.2022; 29(3): 404. CrossRef - Practical Tips for Safe and Successful Endoscopic Ultrasound-Guided Hepaticogastrostomy: A State-of-the-Art Technical Review
Saburo Matsubara, Keito Nakagawa, Kentaro Suda, Takeshi Otsuka, Masashi Oka, Sumiko Nagoshi
Journal of Clinical Medicine.2022; 11(6): 1591. CrossRef - Role of ERCP in Malignant Hilar Biliary Obstruction
Tae Hoon Lee, Jong Ho Moon, Sherman Stuart
Gastrointestinal Endoscopy Clinics of North America.2022; 32(3): 427. CrossRef - Endoscopic management of perihilar cholangiocarcinoma
Hiroki Kawashima, Eizaburo Ohno, Takuya Ishikawa, Yasuyuki Mizutani, Tadashi Iida, Takeshi Yamamura, Naomi Kakushima, Kazuhiro Furukawa, Masanao Nakamura
Digestive Endoscopy.2022; 34(6): 1147. CrossRef - Combination of ERCP with endoscopic ultrasound-guided hepaticogastrostomy and hepaticoduodenostomy for biliary drainage in malignant hilar biliary obstruction
Haruka Toyonaga, Tsuyoshi Hayashi, Toshifumi Kin, Kazuki Hama, Kosuke Iwano, Risa Nakamura, Akio Katanuma
Endoscopy.2022; 54(S 02): E912. CrossRef - A case of malignant hilar biliary obstruction after total gastrectomy treated by EUS-HJS + bridging stenting
Yurie Tokito, Ryunosuke Hakuta, Hirofumi Kogure, Yousuke Nakai, Mitsuhiro Fujishiro
Progress of Digestive Endoscopy.2022; 100(1): 50. CrossRef - Current endoscopic approaches to biliary strictures
Tatsuya Sato, Yousuke Nakai, Mitsuhiro Fujishiro
Current Opinion in Gastroenterology.2022; 38(5): 450. CrossRef - Endoscopic ultrasound-guided biliary drainage in malignant hilar obstruction
Se Woo Park
International Journal of Gastrointestinal Intervention.2022; 11(3): 105. CrossRef - Comparative Study on Nosocomial Biliary Tract Infection Rate Between Biliary Stent Loaded with Radioactive 125I Seeds and Conventional Biliary Stent in the Treatment of Distal Malignant Biliary Obstruction
Jianli An, Yanchao Dong, Hongtao Niu, Yanguo Li, Xiaoyu Han, Zibo Zou, Jingpeng Wu, Ye Tian, Zhuo Chen
Surgical Laparoscopy, Endoscopy & Percutaneous Techniques.2022; 32(6): 724. CrossRef - ERCP plus endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage for malignant hilar biliary obstruction: a multicenter observational open-label study
Pradermchai Kongkam, Theerapat Orprayoon, Chaloemphon Boonmee, Passakorn Sodarat, Orathai Seabmuangsai, Chatchawan Wachiramatharuch, Yutthaya Auan-Klin, Khanh Cong Pham, Abbas Ali Tasneem, Stephen J. Kerr, Rommel Romano, Sureeporn Jangsirikul, Wiriyaporn
Endoscopy.2021; 53(01): 55. CrossRef - Feasibility of EUS-guided hepaticogastrostomy for inoperable malignant hilar biliary strictures
Jérôme Winkler, Fabrice Caillol, Jean-Philippe Ratone, Erwan Bories, Christian Pesenti, Marc Giovannini
Endoscopic Ultrasound.2021; 10(1): 51. CrossRef - Combined ERCP and transhepatic endoscopic ultrasound-guided stent placement for biliary drainage in malignant hilar obstruction: not too good to be true
Manuel Perez-Miranda
Endoscopy.2021; 53(01): 63. CrossRef - Extrahepatic cholangiocarcinoma: Current status of endoscopic approach and additional therapies
Alina Ioana Tantau, Alina Mandrutiu, Anamaria Pop, Roxana Delia Zaharie, Dana Crisan, Carmen Monica Preda, Marcel Tantau, Voicu Mercea
World Journal of Hepatology.2021; 13(2): 166. CrossRef - Efficacy of lumen-apposing metal stents or self-expandable metal stents for endoscopic ultrasound-guided choledochoduodenostomy: a systematic review and meta-analysis
Arnaldo Amato, Emanuele Sinagra, Ciro Celsa, Marco Enea, Andrea Buda, Filippo Vieceli, Lucia Scaramella, Paul Belletrutti, Alessandro Fugazza, Calogero Cammà, Franco Radaelli, Alessandro Repici, Andrea Anderloni
Endoscopy.2021; 53(10): 1037. CrossRef - What You Need to Know Before Performing Endoscopic Ultrasound-guided Hepaticogastrostomy
Tanyaporn Chantarojanasiri, Thawee Ratanachu-Ek, Nonthalee Pausawasdi
Clinical Endoscopy.2021; 54(3): 301. CrossRef - EUS-guided biliary drainage for malignant hilar biliary obstruction: A concise review
Sridhar Sundaram, Vinay Dhir
Endoscopic Ultrasound.2021; 10(3): 154. CrossRef - Proper management of inoperable malignant hilar biliary obstruction: Endoscopic retrograde cholangiopancreatography, endoscopic ultrasound, or percutaneous approach?
Tae Hoon Lee
International Journal of Gastrointestinal Intervention.2021; 10(3): 120. CrossRef - A Recent Update on Endoscopic Drainage of Advanced Malignant Hilar Obstruction
Tae Hoon Lee, Jong Ho Moon, Sang-Heum Park
The Korean Journal of Gastroenterology.2021; 78(2): 94. CrossRef - Endoscopic Biliary Drainage for Hilar Obstruction: Further Evidence But Still A Long Way To Go
Yousuke Nakai
Clinical Endoscopy.2021; 54(5): 629. CrossRef - EUS-guided antegrade metallic stent placement using the stent-in-stent method with a 6-Fr novel slim delivery system in a patient with malignant hilar biliary obstruction
Shigeyuki Suenaga, Seiji Kaino, Takanori Tsuyama, Yuko Fujimoto, Shogo Amano, Toshiyuki Uekitani, Isao Sakaida
Endoscopic Ultrasound.2021; 10(5): 387. CrossRef - Retrospective Comparative Study of Side-by-Side and Stent-in-Stent Metal Stent Placement for Hilar Malignant Biliary Obstruction
Kazunaga Ishigaki, Tsuyoshi Hamada, Yousuke Nakai, Hiroyuki Isayama, Tatsuya Sato, Ryunosuke Hakuta, Kei Saito, Tomotaka Saito, Naminatsu Takahara, Suguru Mizuno, Hirofumi Kogure, Yukiko Ito, Hiroshi Yagioka, Saburo Matsubara, Dai Akiyama, Dai Mohri, Mino
Digestive Diseases and Sciences.2020; 65(12): 3710. CrossRef - Radiologic Assessment for Endoscopic US-guided Biliary Drainage
Shunsuke Sugawara, Miyuki Sone, Shinichi Morita, Susumu Hijioka, Yasunari Sakamoto, Masahiko Kusumoto, Yasuaki Arai
RadioGraphics.2020; 40(3): 667. CrossRef - Endoscopic ultrasound‐guided bilateral biliary drainage through the mesh of the metal stents using a balloon occlusion method
Kazunari Nakahara, Ryo Morita, Fumio Itoh
Digestive Endoscopy.2020;[Epub] CrossRef - Simultaneous endoscopic ultrasound-guided hepaticogastrostomy and bridging stenting with partial stent-in-stent method
Kosuke Maehara, Susumu Hijioka, Yoshikuni Nagashio, Akihiro Ohba, Yuya Kanai, Takuji Okusaka, Yutaka Saito
Endoscopy.2020; 52(10): E381. CrossRef - Efficacy and safety of EUS biliary drainage in malignant distal and hilar biliary obstruction: A comprehensive review of literature and algorithm
Stanley Khoo, NhanDuc Tri Do, Pradermchai Kongkam
Endoscopic Ultrasound.2020; 9(6): 369. CrossRef - Acute biliary interventions
T.C. See
Clinical Radiology.2019;[Epub] CrossRef - Endoscopic ultrasound-guided biliary drainage: A change in paradigm?
En-Ling Leung Ki EL, Bertrand Napoleon
World Journal of Gastrointestinal Endoscopy.2019; 11(5): 345. CrossRef - Drainage of the right liver using EUS guidance
Fabrice Caillol, Mathieu Rouy, Christian Pesenti, Jean-Philippe Ratone, Marc Giovannini
Endoscopic Ultrasound.2019; 8(7): 50. CrossRef
- Retrospective comparative study of new slim‐delivery and conventional large‐cell stents for stent‐in‐stent methods for hilar malignant biliary obstruction
- 6,733 View
- 300 Download
- 39 Web of Science
- 40 Crossref

- Endoscopic Palliation for Biliary and Pancreatic Malignancies: Recent Advances
- Zaheer Nabi, D. Nageshwar Reddy
- Clin Endosc 2019;52(3):226-234. Published online January 22, 2019
- DOI: https://doi.org/10.5946/ce.2019.003
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Malignancies of the pancreatobiliary system are usually unresectable at the time of diagnosis. As a consequence, a majority of these cases are candidates for palliative care. With advances in chemotherapeutic agents and multidisciplinary care, the survival rate in pancreatobiliary malignancies has improved. Therefore, there is a need to provide an effective and long-lasting palliative care for these patients. Endoscopic palliation is preferred to surgery as the former is associated with equal efficacy and reduced morbidity. The main role of endoscopic palliation in the vast majority of pancreatobiliary malignancies includes biliary and enteral stenting for malignant obstructive jaundice and gastric outlet obstruction, respectively. Recent advances in endoscopic palliation appear promising in imparting long-lasting relief of symptoms. Use of radiofrequency ablation and photodynamic therapy in malignant biliary obstruction has been shown to improve the survival rates as well as the patency of biliary stents. The emergence of endoscopic ultrasound (EUS) as a therapeutic tool has enhanced the capability of minimally invasive palliation in pancreatobiliary cancers. EUS is a valuable alternative to endoscopic retrograde cholangiopancreatography for the palliation of obstructive jaundice. More recently, EUS is emerging as an effective primary modality for biliary and gastric bypass.
-
Citations
Citations to this article as recorded by- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
Seung Joo Kang, Chung Hyun Tae, Chang Seok Bang, Cheol Min Shin, Young-Hoon Jeong, Miyoung Choi, Joo Ha Hwang, Yutaka Saito, Philip Wai Yan Chiu, Rungsun Rerknimitr, Christopher Khor, Vu Van Khien, Kee Don Choi, Ki-Nam Shim, Geun Am Song, Oh Young Lee
Clinical Endoscopy.2024; 57(2): 141. CrossRef - Palliative Interventions and Best Supportive Care in Biliary Malignancy
Christine Chung, Lauren Wancata
Surgical Clinics of North America.2024;[Epub] CrossRef - Gamma-Glutamyl Transferase: A Friend against Cholestatic Itch? A Retrospective Observational Data Analysis in Patients with Extrahepatic Cholestasis
Floris W. Haijer, Cornelis B. Van Vliet, Marjolein G. J. Brusse-Keizer, Job A. M. Van der Palen, Marjo J. Kerbert-Dreteler, Jeroen J. Kolkman, Dirk Uhlmann
International Journal of Hepatology.2023; 2023: 1. CrossRef - Endoscopic and Endosonographic Palliation for Triple Obstruction Caused by Recurrent Gallbladder Cancer: A Case Report
Young Rong Kim, Chi Hyuk Oh, Min Jae Yang
The Korean Journal of Pancreas and Biliary Tract.2023; 28(1): 19. CrossRef - Endoscopic Management of Pancreatobiliary Malignancies
Dong Wook Lee, Eun Young Kim
Digestive Diseases and Sciences.2022; 67(5): 1635. CrossRef - Angle of covered self-expandable metallic stents after placement is a risk factor for recurrent biliary obstruction
Kojiro Tanoue, Hirotsugu Maruyama, Yuki Ishikawa-Kakiya, Yosuke Kinoshita, Kappei Hayashi, Masafumi Yamamura, Masaki Ominami, Yuji Nadatani, Shusei Fukunaga, Koji Otani, Shuhei Hosomi, Fumio Tanaka, Noriko Kamata, Yasuaki Nagami, Koichi Taira, Toshio Wata
World Journal of Hepatology.2022; 14(5): 993. CrossRef - Angle of covered self-expandable metallic stents after placement is a risk factor for recurrent biliary obstruction
Kojiro Tanoue, Hirotsugu Maruyama, Yuki Ishikawa-Kakiya, Yosuke Kinoshita, Kappei Hayashi, Masafumi Yamamura, Masaki Ominami, Yuji Nadatani, Shusei Fukunaga, Koji Otani, Shuhei Hosomi, Fumio Tanaka, Noriko Kamata, Yasuaki Nagami, Koichi Taira, Toshio Wata
World Journal of Hepatology.2022; 14(5): 992. CrossRef - Pancreatic Cancer: Challenges and Opportunities in Locoregional Therapies
Alaa Y. Bazeed, Candace M. Day, Sanjay Garg
Cancers.2022; 14(17): 4257. CrossRef - Endoscopic retrograde cholangiopancreatography: Current practice and future research
David J Sanders, Shivanand Bomman, Rajesh Krishnamoorthi, Richard A Kozarek
World Journal of Gastrointestinal Endoscopy.2021; 13(8): 260. CrossRef - Early malfunction of a biliary self-expandable metal stent with an antireflux valve
Sang Hoon Kim, Chi Hyuk Oh, Jae Min Lee, Seong Ji Choi, Hyuk Soon Choi, Eun Sun Kim, Bora Keum, Yoon Tae Jeen, Hoon Jai Chun, Hong Sik Lee, Chang Duck Kim
Medicine.2020; 99(16): e19750. CrossRef - Endosonography-guided Radiofrequency Ablation in Pancreatic Diseases
Giuseppe Vanella, Gabriele Capurso, Paolo G. Arcidiacono
Journal of Clinical Gastroenterology.2020; 54(7): 591. CrossRef - Efficacy and safety of radiofrequency ablation in patients with unresectable malignant biliary strictures
Martha Claudia Galindo Orozco, Angélica Hernández Guerrero, Juan Octavio Alonso Larraga, Eduardo Ramírez Solis, José Guillermo de la Mora Levy, María del Carmen Manzano Robleda
Revista Española de Enfermedades Digestivas.2020;[Epub] CrossRef
- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
- 6,347 View
- 312 Download
- 9 Web of Science
- 12 Crossref

Reviews
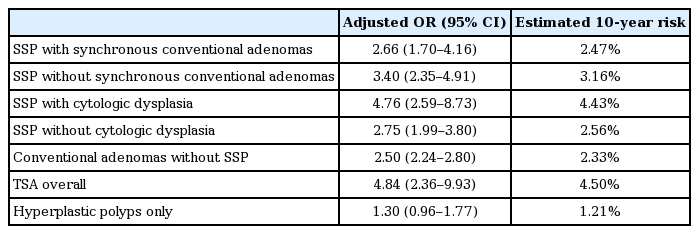
- Screening Relevance of Sessile Serrated Polyps
- Charles J. Kahi
- Clin Endosc 2019;52(3):235-238. Published online January 8, 2019
- DOI: https://doi.org/10.5946/ce.2018.112
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Conventional adenomas have historically been considered to be the only screening-relevant colorectal cancer (CRC) precursor lesion. The prevailing paradigm was that most CRCs arise along the chromosomal instability pathway, where adenomas accumulate incremental genetic alterations over time, leading eventually to malignancy. However, it is now recognized that this “conventional” pathway accounts for only about two-thirds of CRCs. The serrated pathway is responsible for most of the remainder, and is a disproportionate contributor to postcolonoscopy CRC. Hallmarks of the serrated pathway are mutations in the BRAF gene, high levels of methylation of promoter CpG islands, and the sessile serrated polyp (SSP). Accumulating evidence shows that SSPs can be considered adenoma-equivalent from the standpoint of CRC screening. SSPs have a higher prevalence than previously thought, and appear to have a relatively long dwell time similar to that of conventional adenomas. In addition, SSPs, whether sporadic or as part of the serrated polyposis syndrome, are associated with increased risk of synchronous and metachronous neoplasia. These features collectively support that SSPs are highly relevant to CRC prevention.
-
Citations
Citations to this article as recorded by- Sessile serrated lesion detection rates continue to increase: 2008–2020
Nicholas Edwardson, Prajakta Adsul, Zorisadday Gonzalez, V. Shane Pankratz, Gulshan Parasher, Kevin English, Shiraz Mishra
Endoscopy International Open.2023; 11(01): E107. CrossRef - Long-term Use of Hormone Replacement Therapy is Associated With a Lower Risk of Developing High-risk Serrated Polyps in Women
Dylan E. O’Sullivan, Yibing Ruan, Nauzer Forbes, Steven J. Heitman, Robert J. Hilsden, Joy Pader, Darren R. Brenner
Journal of Clinical Gastroenterology.2022; 56(8): 697. CrossRef - Distinct colon mucosa microbiomes associated with tubular adenomas and serrated polyps
Julio Avelar-Barragan, Lauren DeDecker, Zachary N. Lu, Bretton Coppedge, William E. Karnes, Katrine L. Whiteson
npj Biofilms and Microbiomes.2022;[Epub] CrossRef - Impact of looping on premalignant polyp detection during colonoscopy
Osamu Toyoshima, Toshihiro Nishizawa, Shuntaro Yoshida, Tatsuya Matsuno, Toru Arano, Ryo Kondo, Kazunori Kinoshita, Yuki Yasumi, Yosuke Tsuji, Mitsuhiro Fujishiro
World Journal of Gastrointestinal Endoscopy.2022; 14(11): 694. CrossRef - Cost-Effectiveness of Colorectal Cancer Surveillance in Hodgkin Lymphoma Survivors Treated with Procarbazine and/or Infradiaphragmatic Radiotherapy
Berbel L.M. Ykema, Andrea Gini, Lisanne S. Rigter, Manon C.W. Spaander, Leon M.G. Moons, Tanya M. Bisseling, Jan Paul de Boer, Wieke H.M. Verbeek, Pieternella J. Lugtenburg, Cecile P.M. Janus, Eefke J. Petersen, Judith M. Roesink, Richard W.M. van der Maa
Cancer Epidemiology, Biomarkers & Prevention.2022; 31(12): 2157. CrossRef - Serrated Lesions in Inflammatory Bowel Disease: Genotype-Phenotype Correlation
Iva Brcic, Heather Dawson, Hans Peter Gröchenig, Christoph Högenauer, Karl Kashofer
International Journal of Surgical Pathology.2021; 29(1): 46. CrossRef - Increased Sessile Serrated Adenoma Detection Rate With Mechanical New Technology Devices
Elijah Verheyen, Daniel Castaneda, Seth A. Gross, Violeta Popov
Journal of Clinical Gastroenterology.2021; 55(4): 335. CrossRef - The prevalence of sessile serrated lesion in the colorectum and its relationship to synchronous colorectal advanced neoplasia: a systemic review and meta-analysis
Sz-Iuan Shiu, Hiroshi Kashida, Yoriaki Komeda
European Journal of Gastroenterology & Hepatology.2021; 33(12): 1495. CrossRef - Detection of Microsatellite Instability in Colorectal Cancer Patients With a Plasma-Based Real-Time PCR Analysis
Namjoo Kim, Sung Min Kim, Beom Jae Lee, Byung il Choi, Hee Sook Yoon, Sang Hee Kang, Seung Han Kim, Moon Kyung Joo, Jong-Jae Park, Chungyeul Kim
Frontiers in Pharmacology.2021;[Epub] CrossRef - Biology and Therapeutic Targets of Colorectal Serrated Adenocarcinoma; Clues for a Histologically Based Treatment against an Aggressive Tumor
Begoña Alburquerque-González, Fernando F. López-Calderón, María Dolores López-Abellán, Ángel Esteban-Gil, José García-Solano, Pablo Conesa-Zamora
International Journal of Molecular Sciences.2020; 21(6): 1991. CrossRef - Computer aided detection for laterally spreading tumors and sessile serrated adenomas during colonoscopy
Guanyu Zhou, Xun Xiao, Mengtian Tu, Peixi Liu, Dan Yang, Xiaogang Liu, Renyi Zhang, Liangping Li, Shan Lei, Han Wang, Yan Song, Pu Wang, Wajid Mumtaz
PLOS ONE.2020; 15(4): e0231880. CrossRef - The Molecular Hallmarks of the Serrated Pathway in Colorectal Cancer
Fatima De Palma, Valeria D’Argenio, Jonathan Pol, Guido Kroemer, Maria Maiuri, Francesco Salvatore
Cancers.2019; 11(7): 1017. CrossRef
- Sessile serrated lesion detection rates continue to increase: 2008–2020
- 6,870 View
- 275 Download
- 12 Web of Science
- 12 Crossref

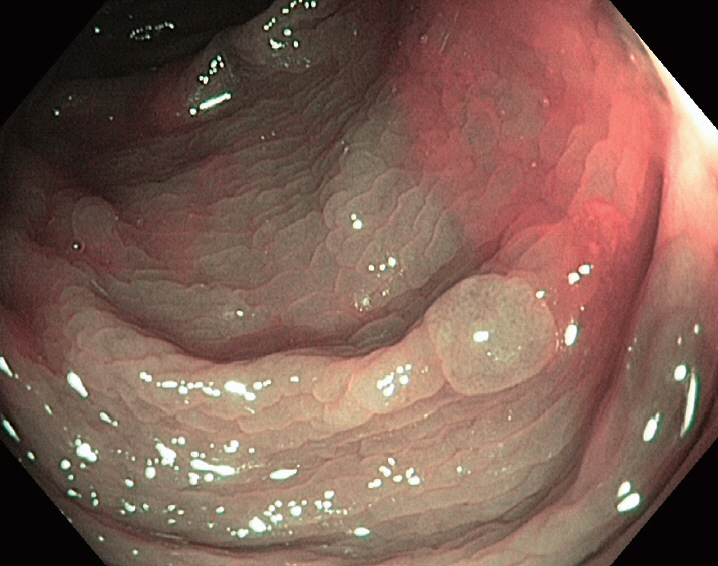
- Should We Resect and Discard Low Risk Diminutive Colon Polyps
- Pujan Kandel, Michael B. Wallace
- Clin Endosc 2019;52(3):239-246. Published online January 21, 2019
- DOI: https://doi.org/10.5946/ce.2018.136
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Diminutive colorectal polyps <5 mm are very common and almost universally benign. The current strategy of resection with histological confirmation of all colorectal polyps is costly and may increase the risk of colonoscopy. Accurate, optical diagnosis without histology can be achieved with currently available endoscopic technologies. The American Society of Gastrointestinal Endoscopy Preservation and Incorporation of Valuable endoscopic Innovations supports strategies for optical diagnosis of small non neoplastic polyps as long as two criteria are met. For hyperplastic appearing polyps <5 mm in recto-sigmoid colon, the negative predictive value should be at least 90%. For diminutive low grade adenomatous appearing polyps, a resect and discard strategy should be sufficiently accurate such that post-polypectomy surveillance recommendations based on the optical diagnosis, agree with a histologically diagnosis at least 90% of the time. Although the resect and discard as well as diagnose and leave behind approach has major benefits with regard to both safety and cost, it has yet to be used widely in practice. To fully implement such as strategy, there is a need for better-quality training, quality assurance, and patient acceptance. In the article, we will review the current state of the science on optical diagnose of colorectal polyps and its implications for colonoscopy practice.
-
Citations
Citations to this article as recorded by- The role of artificial intelligence in colonoscopy
Hyun Jae Kim, Nasim Parsa, Michael F. Byrne
Seminars in Colon and Rectal Surgery.2024; 35(1): 101007. CrossRef - Artificial intelligence for characterization of colorectal polyps: Prospective multicenter study
Glenn De Lange, Victor Prouvost, Gabriel Rahmi, Geoffroy Vanbiervliet, Catherine Le Berre, Sahar Mack, Thibaud Koessler, Emmanuel Coron
Endoscopy International Open.2024; 12(03): E413. CrossRef - Ecogastroenterology: cultivating sustainable clinical excellence in an environmentally conscious landscape
Kassem Sharif, Enrique Rodriguez de Santiago, Paula David, Arnon Afek, Ian M Gralnek, Shomron Ben-Horin, Adi Lahat
The Lancet Gastroenterology & Hepatology.2024; 9(6): 550. CrossRef - Rationalising the use of specimen pots following colorectal polypectomy: a small step towards greener endoscopy
Karl King Yong, Yun He, Hoi Ching Annie Cheung, Ramya Sriskandarajah, William Jenkins, Robert Goldin, Sabina Beg
Frontline Gastroenterology.2023; 14(4): 295. CrossRef - Measurements, Algorithms, and Presentations of Reality: Framing Interactions with AI-Enabled Decision Support
Niels van Berkel, Maura Bellio, Mikael B. Skov, Ann Blandford
ACM Transactions on Computer-Human Interaction.2023; 30(2): 1. CrossRef - Real-World Validation of a Computer-Aided Diagnosis System for Prediction of Polyp Histology in Colonoscopy: A Prospective Multicenter Study
James Weiquan Li, Clement Chun Ho Wu, Jonathan Wei Jie Lee, Raymond Liang, Gwyneth Shook Ting Soon, Lai Mun Wang, Xuan Han Koh, Calvin Jianyi Koh, Wei Da Chew, Kenneth Weicong Lin, Mann Yie Thian, Ronnie Matthew, Guowei Kim, Christopher Jen Lock Khor, Kwo
American Journal of Gastroenterology.2023; 118(8): 1353. CrossRef - The Utility of Narrow-Band Imaging International Colorectal Endoscopic Classification in Predicting the Histologies of Diminutive Colorectal Polyps Using I-Scan Optical Enhancement: A Prospective Study
Yeo Wool Kang, Jong Hoon Lee, Jong Yoon Lee
Diagnostics.2023; 13(16): 2720. CrossRef - Optical diagnosis of colorectal polyps using novel blue light imaging classification among trainee endoscopists
Christopher Koehn, Douglas K. Rex, Jimmy Allen, Umer Bhatti, Indira Bhavsar‐Burke, Viveksandeep Thoguluva Chandrasekar, Abhishek Challa, Abhiram Duvvuri, Lara Dakhoul, John Ha, Nour Hamade, S. Bradley Hicks, Claire Jansson‐Knodell, Edward Krajicek, Shanke
Digestive Endoscopy.2022; 34(1): 191. CrossRef - Too Good to Be True? Evaluation of Colonoscopy Sensitivity Assumptions Used in Policy Models
Carolyn M. Rutter, Pedro Nascimento de Lima, Jeffrey K. Lee, Jonathan Ozik
Cancer Epidemiology, Biomarkers & Prevention.2022; 31(4): 775. CrossRef - Non-optical polyp-based resect and discard strategy: A prospective clinical study
Mahsa Taghiakbari, Celia Hammar, Mira Frenn, Roupen Djinbachian, Heiko Pohl, Erik Deslandres, Simon Bouchard, Mickael Bouin, Daniel von Renteln
World Journal of Gastroenterology.2022; 28(19): 2137. CrossRef - No-Code Platform-Based Deep-Learning Models for Prediction of Colorectal Polyp Histology from White-Light Endoscopy Images: Development and Performance Verification
Eun Jeong Gong, Chang Seok Bang, Jae Jun Lee, Seung In Seo, Young Joo Yang, Gwang Ho Baik, Jong Wook Kim
Journal of Personalized Medicine.2022; 12(6): 963. CrossRef - Artificial intelligence-assisted colonoscopy: a narrative review of current data and clinical applications
JW Li, LM Wang, TL Ang
Singapore Medical Journal.2022; 63(3): 118. CrossRef - Impact of the Volume and Distribution of Training Datasets in the Development of Deep-Learning Models for the Diagnosis of Colorectal Polyps in Endoscopy Images
Eun Jeong Gong, Chang Seok Bang, Jae Jun Lee, Young Joo Yang, Gwang Ho Baik
Journal of Personalized Medicine.2022; 12(9): 1361. CrossRef - How to safely apply a 'Resect and Discard' policy for small colorectal polyps–real‐world data comparing endoscopic polyp evaluation and subsequent histological outcome
Georgios Marinopoulos, Maja Kopczynska, Robert Clark, Nadeem Sarwar, Yeng Ang, Arash Assadsangabi
GastroHep.2021; 3(1): 12. CrossRef - Characterization of Optical Coherence Tomography Images for Colon Lesion Differentiation under Deep Learning
Cristina L. Saratxaga, Jorge Bote, Juan F. Ortega-Morán, Artzai Picón, Elena Terradillos, Nagore Arbide del Río, Nagore Andraka, Estibaliz Garrote, Olga M. Conde
Applied Sciences.2021; 11(7): 3119. CrossRef - Clinical validation of the SIMPLE classification for optical diagnosis of colorectal polyps
Ahmed Amine Alaoui, Kussil Oumedjbeur, Roupen Djinbachian, Étienne Marchand, Paola N. Marques, Mickael Bouin, Simon Bouchard, Daniel von Renteln
Endoscopy International Open.2021; 09(05): E684. CrossRef - Colonoscopy and artificial intelligence: Bridging the gap or a gap needing to be bridged?
Tiing Leong Ang, James Weiquan Li
Artificial Intelligence in Gastrointestinal Endoscopy.2021; 2(2): 36. CrossRef - RNA-sequencing identification and validation of genes differentially expressed in high-risk adenoma, advanced colorectal cancer, and normal controls
Namjoo Kim, Jeong-An Gim, Beom Jae Lee, Byung il Choi, Seung Bin Park, Hee Sook Yoon, Sang Hee Kang, Seung Han Kim, Moon Kyung Joo, Jong-Jae Park, Chungyeul Kim, Han-Kyeom Kim
Functional & Integrative Genomics.2021; 21(3-4): 513. CrossRef - Computer-Aided Diagnosis of Diminutive Colorectal Polyps in Endoscopic Images: Systematic Review and Meta-analysis of Diagnostic Test Accuracy
Chang Seok Bang, Jae Jun Lee, Gwang Ho Baik
Journal of Medical Internet Research.2021; 23(8): e29682. CrossRef - Automatic image and text-based description for colorectal polyps using BASIC classification
Roger Fonollà, Quirine E.W. van der Zander, Ramon M. Schreuder, Sharmila Subramaniam, Pradeep Bhandari, Ad A.M. Masclee, Erik J. Schoon, Fons van der Sommen, Peter H.N. de With
Artificial Intelligence in Medicine.2021; 121: 102178. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Diagnosis and Treatment of Diminutive Polyps in the Colon
Iness Soltani, Daniel von Renteln
Current Treatment Options in Gastroenterology.2020; 18(2): 175. CrossRef - Colon capsule endoscopy in colorectal cancer screening: a randomised controlled trial
Lasse Kaalby, Ulrik Deding, Morten Kobaek-Larsen, Anne-Line Volden Havshoi, Erik Zimmermann-Nielsen, Marianne Kirstine Thygesen, Rasmus Kroijer, Thomas Bjørsum-Meyer, Gunnar Baatrup
BMJ Open Gastroenterology.2020; 7(1): e000411. CrossRef - A CNN CADx System for Multimodal Classification of Colorectal Polyps Combining WL, BLI, and LCI Modalities
Roger Fonollà, Quirine E. W. van der Zander, Ramon M. Schreuder, Ad A. M. Masclee, Erik J. Schoon, Fons van der Sommen, Peter H. N. de With
Applied Sciences.2020; 10(15): 5040. CrossRef - UEG Week 2019 Poster Presentations
United European Gastroenterology Journal.2019; 7(S8): 189. CrossRef
- The role of artificial intelligence in colonoscopy
- 9,462 View
- 281 Download
- 22 Web of Science
- 25 Crossref

Original Articles
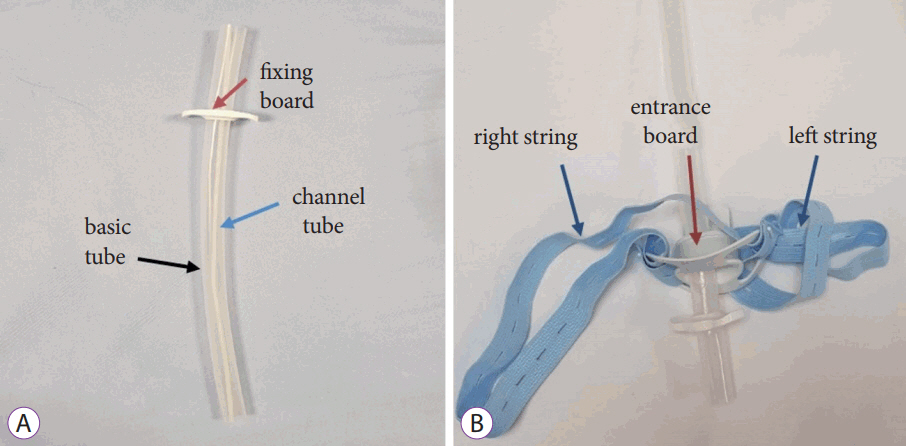
- Technical Feasibility of a Guidetube for Various Endoscopic Procedures in Human Gastrointestinal Simulators
- Dong Seok Lee, Byeong Gwan Kim, Kook Lae Lee, Yong Jin Jung, Ji Won Kim
- Clin Endosc 2019;52(3):247-251. Published online November 9, 2018
- DOI: https://doi.org/10.5946/ce.2018.147
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Many gastrointestinal (GI) endoscopic procedures are difficult and cumbersome owing to the limitation of currently available endoscopic devices. This study aimed to develop an endoscopic guidetube for multipurpose endoscopic procedures and assess its use in a realistic GI endoscopic simulator.
Methods
The guidetube used is a soft overtube composed of neoprene and is designed to assist various endoscopic procedures on demand. In total, 15 types of procedures were performed in GI simulators. Four procedures were performed in the stomach model and 11 in the colon model. The procedures include repeated endoscopic insertion and foreign body removal in various positions. The mean insertion and procedure time were assessed in each session. All procedures were performed by 5 expert endoscopists.
Results
Endoscopic procedures with the new guidetube were faster and more effective than the conventional endoscopic techniques. The mean insertion time of the endoscope with the guidetube was significantly shorter than that without the guidetube. The guidetube was safely inserted without scratch using low pushing force. Objects of various sizes larger than the endoscopic channel were easily removed by the guidetube-assisted endoscopic procedures.
Conclusions
This preliminary study shows that guidetube-assisted endoscopic procedures are faster, easier, safer and cheaper than conventional endoscopic procedures. -
Citations
Citations to this article as recorded by- Efficacy of an assistive guide tube for improved endoscopic access to gastrointestinal lesions: an in vivo study in a porcine model
Dong Seok Lee, Jeong-Sik Byeon, Sang Gyun Kim, Ji Won Kim, Kook Lae Lee, Ji Bong Jeong, Yong Jin Jung, Hyoun Woo Kang
Clinical Endoscopy.2024; 57(1): 82. CrossRef - Technical feasibility of a newly designed bendable forceps for difficult endoscopic tissue samplings (with video)
Dong Seok Lee, Ji Won Kim, Kook Lae Lee, Byeong Gwan Kim, Su Hwan Kim, Jeong-Sik Byeon
Surgical Endoscopy.2020; 34(10): 4692. CrossRef - Usefulness of an Overtube Device in Gastrointestinal Endoscopy
Seung Han Kim
Clinical Endoscopy.2019; 52(3): 203. CrossRef
- Efficacy of an assistive guide tube for improved endoscopic access to gastrointestinal lesions: an in vivo study in a porcine model
- 5,220 View
- 94 Download
- 3 Web of Science
- 3 Crossref

- Efficacy of Endoscopic Submucosal Dissection of Esophageal Neoplasms under General Anesthesia
- Koichi Hamada, Koichiro Kawano, Atsushi Yamauchi, Ryota Koyanagi, Yoshinori Horikawa, Shinya Nishida, Yoshiki Shiwa, Noriyuki Nishino, Michitaka Honda
- Clin Endosc 2019;52(3):252-257. Published online May 23, 2019
- DOI: https://doi.org/10.5946/ce.2018.151
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Evidence that general anesthesia (GA) reduces the operative time of esophageal endoscopic submucosal dissection (ESD) is currently insufficient. This study aims to evaluate the efficacy and safety of esophageal ESD under GA.
Methods
A total of 227 lesions from 198 consecutive patients with superficial esophageal neoplasms treated by ESD at 3 Japanese institutions between April 2011 and September 2017 were included in this retrospective study. For ESD, GA and deep sedation (DS) were used in 102 (51.5%, GA group) and 96 patients (48.5%, DS group), respectively.
Results
There were no statistically significant differences in age, sex, or comorbidities between the groups. In the GA group, the tumor size was larger (21 [3–77] mm vs. 14 [3–63] mm, p<0.001), luminal circumference was larger (≥2/3; 13.9% vs. 5.4%, p=0.042), procedure time was shorter (28 [5–202] min vs. 40 [8–249] min, p<0.001), and submucosal dissection speed was faster (25.2 [7.8–157.2] mm2 /min vs. 16.2 [2.4–41.3] mm2 /min, p<0.001). The rates of intraoperative perforation and aspiration pneumonia were lower in the GA group, but the difference did not achieve statistical significance (p=0.242 and p=0.242).
Conclusions
GA shortens the procedure time of esophageal ESD. -
Citations
Citations to this article as recorded by- General Anesthesia and Endoscopic Upper Gastrointestinal Tumor Resection
Seung Hyun Kim
Journal of Digestive Cancer Research.2023; 11(3): 125. CrossRef - Endoscopic resection of esophageal squamous cell carcinoma: Current indications and treatment outcomes
Seiichiro Abe, Yuichiro Hirai, Takeshi Uozumi, Mai Ego Makiguchi, Satoru Nonaka, Haruhisa Suzuki, Shigetaka Yoshinaga, Ichiro Oda, Yutaka Saito
DEN Open.2022;[Epub] CrossRef - Subtotal esophageal endoscopic submucosal dissection for long-segment Barrett’s esophagus and adenocarcinoma
Dai Kubota, Yoshiki Sakaguchi, Sayaka Nagao, Yosuke Tsuji, Mitsuhiro Fujishiro
Endoscopy.2022; 54(10): E583. CrossRef - Translation from manual to automatic endoscopic insufflation enhanced by a pressure limiter
Yuki Ushimaru, Tsuyoshi Takahashi, Kotaro Yamashita, Takuro Saito, Koji Tanaka, Kazuyoshi Yamamoto, Tomoki Makino, Yukinori Kurokawa, Hidetoshi Eguchi, Yuichiro Doki, Kiyokazu Nakajima
Surgical Endoscopy.2022; 36(9): 7038. CrossRef - Gel immersion endoscopic submucosal dissection: clinical experience with 13 cases of superficial esophageal cancer
Yuya Nakano, Tomoaki Tashima, Ryuhei Jinushi, Rie Terada, Yumi Mashimo, Tomonori Kawasaki, Toshio Uraoka, Shomei Ryozawa
Endoscopy International Open.2022; 10(09): E1302. CrossRef - Computer-aided diagnosis of esophageal cancer and neoplasms in endoscopic images: a systematic review and meta-analysis of diagnostic test accuracy
Chang Seok Bang, Jae Jun Lee, Gwang Ho Baik
Gastrointestinal Endoscopy.2021; 93(5): 1006. CrossRef - Efficacy of sedation with dexmedetomidine plus propofol during esophageal endoscopic submucosal dissection
Keiichi Ashikari, Takashi Nonaka, Takuma Higurashi, Tomohiro Takatsu, Tsutomu Yoshihara, Noboru Misawa, Jun Arimoto, Kenji Kanoshima, Tetsuya Matsuura, Akiko Fuyuki, Hidenori Ohkubo, Hideyuki Chiba, Atsushi Nakajima
Journal of Gastroenterology and Hepatology.2021; 36(7): 1920. CrossRef - Bilateral tension pneumothorax during endoscopic submucosal dissection under general anesthesia diagnosed by point-of-care ultrasound - A case report -
Seok Kyeong Oh, Seung Inn Cho, Young Ju Won, Jin Hee Yun
Anesthesia and Pain Medicine.2021; 16(2): 171. CrossRef - Perforation of a Gastric Tear during Esophageal Endoscopic Submucosal Dissection under General Anesthesia
Tomoaki Yamasaki, Yuhei Sakata, Takehisa Suekane, Hiroko Nebiki
Clinical Endoscopy.2021; 54(6): 916. CrossRef - Feasibility of the lidocaine injection method during esophageal endoscopic submucosal dissection
Tetsuya Yoshizaki, Daisuke Obata, Chise Ueda, Norio Katayama, Yasuhiro Aoki, Norihiro Okamoto, Hiroki Hashimura, Masanori Matsumoto, Megumi Takagi, Seitaro Ikeoka, Ryutaro Yoshida, Kenji Momose, Takaaki Eguchi, Hiroshi Yamashita, Akihiko Okada
JGH Open.2020; 4(2): 251. CrossRef - Commentary on “Efficacy of Endoscopic Submucosal Dissection of Esophageal Neoplasms under General Anesthesia”
Soo In Choi, Jun Chul Park
Clinical Endoscopy.2019; 52(3): 205. CrossRef
- General Anesthesia and Endoscopic Upper Gastrointestinal Tumor Resection
- 5,434 View
- 109 Download
- 11 Web of Science
- 11 Crossref

- Endoscopic Findings in Children with Isolated Lower Gastrointestinal Bleeding
- Ari Silbermintz, Manar Matar, Amit Assa, Noam Zevit, Yael Mozer Glassberg, Raanan Shamir
- Clin Endosc 2019;52(3):258-261. Published online May 14, 2019
- DOI: https://doi.org/10.5946/ce.2018.046
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Colorectal polyps are a common cause of lower gastrointestinal bleeding in children. Our aim was to study the causes of isolated lower gastrointestinal bleeding and to analyze the characteristics of the colorectal polyps found in our cohort.
Methods
We retrospectively reviewed colonoscopic procedures performed between 2007 and 2015. Children with isolated lower gastrointestinal bleeding were included in the study.
Results
A total of 185 colonoscopies were performed for isolated lower gastrointestinal bleeding. The median patient age was 8 years, and 77 patients (41.6%) were found to have colonic polyps. Normal colonoscopy findings were observed and acute colitis was detected in 77 (41.6%) and 14 (7.4%) patients, respectively. Single colonic polyps and 2–3 polyps were detected in 73 (94.8%) and 4 (5.2%) patients with polyps, respectively. Of the single polyps, 69 (94.5%) were juvenile polyps, among which 65 (94.2%) were located in the left colon.
Conclusions
Single left-sided juvenile polyps were the most common cause of isolated lower gastrointestinal bleeding in our study. It was rare to find multiple polyps and polyps proximal to the splenic flexure in our cohort. A full colonoscopy is still recommended in all patients in order to properly diagnose the small but significant group of patients with pathologies found proximal to the splenic flexure. -
Citations
Citations to this article as recorded by- Blutiger Stuhl beim Säugling und Kleinkind – Differenzialdiagnosen und Management
Burkhard Rodeck
Pädiatrie up2date.2024; 19(01): 29. CrossRef - Características clínicas e incidencia de pólipos colónicos en niños durante una década
Génesis Rojas, Dianora Navarro, Karolina López, Katiuska Belandria, Elennys Moya, Libia Alonso, Christian Núñez, Deivis Maury, Gleydis Villarroel
Revista GEN.2024; 77(4): 174. CrossRef - Fecal Calprotectin Levels Significantly Correlate with Polyp Size in Children and Adolescents with Juvenile Colorectal Polyps
Yu Bin Kim, Ju Young Kim, Sujin Choi, Yoo Min Lee, So Yoon Choi, Soon Chul Kim, Hyo-Jeong Jang, Yoon Lee, In Sook Jeong, Dae Yong Yi, Yunkoo Kang, Kyung Jae Lee, Byung-Ho Choe, Ben Kang
Pediatric Gastroenterology, Hepatology & Nutrition.2023; 26(1): 34. CrossRef - Practice Patterns of Colorectal Polypectomy in Pediatric Endoscopic Specialists in South Korea: A Nationwide Survey Study
Yoon Lee, Sujin Choi, Ben Kang
Pediatric Gastroenterology, Hepatology & Nutrition.2023; 26(1): 15. CrossRef - Associations of Polyp Characteristics in Children and Adolescents Presenting with Less Than Five Colorectal Polyps: A Full Colonoscopy Is Still Required
Ju Young Kim, Yu Bin Kim, Sujin Choi, Yoo Min Lee, Hyun Jin Kim, Soon Chul Kim, Hyo-Jeong Jang, So Yoon Choi, Dae Yong Yi, Yoon Lee, You Jin Choi, Yunkoo Kang, Kyung Jae Lee, Suk Jin Hong, Jun Hyun Hwang, Sanggyu Kwak, Byung-Ho Choe, Ben Kang
Gut and Liver.2023; 17(3): 441. CrossRef - Endoscopic Mucosal Resection in Children
David S. Vitale, Kelly Wang, Laith H. Jamil, Kenneth H. Park, Quin Y. Liu
Journal of Pediatric Gastroenterology and Nutrition.2022; 74(1): 20. CrossRef - Potential Utility of Fecal Calprotectin in Discriminating Colorectal Polyps From Other Major Etiologies in Children Presenting With Isolated Hematochezia
Yu Bin Kim, Ju Young Kim, Sujin Choi, Hyun Jin Kim, Yoo Min Lee, Yoon Lee, Hyo-Jeong Jang, Eun Hye Lee, Kyung Jae Lee, Soon Chul Kim, So Yoon Choi, Yunkoo Kang, Dae Yong Yi, You Jin Choi, Byung-Ho Choe, Ben Kang
Journal of Korean Medical Science.2022;[Epub] CrossRef - The management of colonic polyps in children: a 13-year retrospective study
Valeria Dipasquale, Claudio Romano, Mauro Iannelli, Andrea Tortora, Alessandro Princiotta, Marco Ventimiglia, Giuseppinella Melita, Socrate Pallio
European Journal of Pediatrics.2021; 180(7): 2281. CrossRef - Faecal calprotectin and ultrasonography as non-invasive screening tools for detecting colorectal polyps in children with sporadic rectal bleeding: a prospective study
Giovanni Di Nardo, Francesco Esposito, Chiara Ziparo, Caterina Strisciuglio, Francesca Vassallo, Marco Di Serafino, Maria Pia Villa, Pasquale Parisi, Melania Evangelisti, Claudia Pacchiarotti, Vito Domenico Corleto
Italian Journal of Pediatrics.2020;[Epub] CrossRef - The Most Common Cause of Lower Gastrointestinal Bleeding without Other Symptoms in Children is Colonic Polyp: Is Total Colonoscopy Needed?
Yeoun Joo Lee, Jae Hong Park
Clinical Endoscopy.2019; 52(3): 207. CrossRef
- Blutiger Stuhl beim Säugling und Kleinkind – Differenzialdiagnosen und Management
- 5,183 View
- 111 Download
- 9 Web of Science
- 10 Crossref

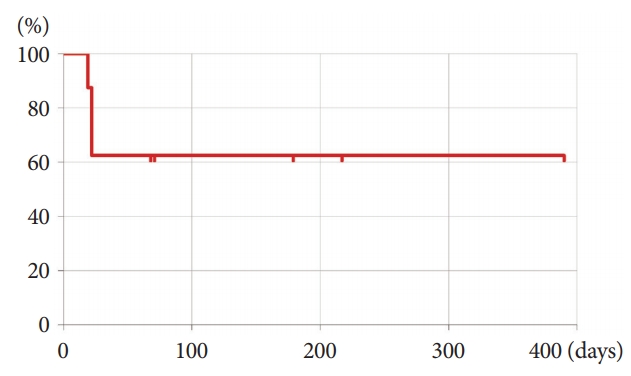
- Endoscopic Ultrasonography-Guided Gallbladder Drainage as a Treatment Option for Acute Cholecystitis after Metal Stent Placement in Malignant Biliary Strictures
- Fumisato Kozakai, Yoshihide Kanno, Kei Ito, Shinsuke Koshita, Takahisa Ogawa, Hiroaki Kusunose, Kaori Masu, Toshitaka Sakai, Toji Murabayashi, Keisuke Yonamine, Yujiro Kawakami, Yuki Fujii, Kazuaki Miyamoto, Yutaka Noda
- Clin Endosc 2019;52(3):262-268. Published online March 15, 2019
- DOI: https://doi.org/10.5946/ce.2018.183
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: It is often difficult to manage acute cholecystitis after metal stent (MS) placement in unresectable malignant biliary strictures. The aim of this study was to evaluate the feasibility of endoscopic ultrasonography-guided gallbladder drainage (EUS-GBD) for acute cholecystitis.
Methods
The clinical outcomes of 10 patients who underwent EUS-GBD for acute cholecystitis after MS placement between January 2011 and August 2018 were retrospectively evaluated. The procedural outcomes of percutaneous transhepatic gallbladder drainage (PTGBD) with tube placement (n=11 cases) and aspiration (PTGBA) (n=27 cases) during the study period were evaluated as a reference.
Results
The technical success and clinical effectiveness rates of EUS-GBD were 90% (9/10) and 89% (8/9), respectively. Severe bile leakage that required surgical treatment occurred in one case. Acute cholecystitis recurred after stent dislocation in 38% (3/8) of the cases. Both PTGBD and PTGBA were technically successful in all cases without severe adverse events and clinically effective in 91% and 63% of the cases, respectively.
Conclusions
EUS-GBD after MS placement was a feasible option for treating acute cholecystitis. However, it was a rescue technique following the established percutaneous intervention in the current setting because of the immature technical methodology, including dedicated devices, which need further development. -
Citations
Citations to this article as recorded by- Risk factors and treatment strategies for cholecystitis after metallic stent placement for malignant biliary obstruction: a multicenter retrospective study
Akihiro Matsumi, Hironari Kato, Taiji Ogawa, Toru Ueki, Masaki Wato, Masakuni Fujii, Tatsuya Toyokawa, Ryo Harada, Yuki Ishihara, Masahiro Takatani, Hirofumi Tsugeno, Naoko Yunoki, Takeshi Tomoda, Toshiharu Mitsuhashi, Motoyuki Otsuka
Gastrointestinal Endoscopy.2024; 100(1): 76. CrossRef - Endoscopic Transpapillary Gallbladder Drainage for Recurrent Cholecystitis after Covered Self-expandable Metal Stent Placement for Unresectable Malignant Biliary Obstruction
Akinori Maruta, Takuji Iwashita, Kaori Banno, Takuya Koizumi, Soichi Iritani, Kensaku Yoshida, Shogo Shimizu, Masahito Shimizu
Internal Medicine.2023; 62(2): 237. CrossRef - Treatment Strategy for Acute Cholecystitis Induced by a Metallic Stent Placed in Malignant Biliary Strictures: Role of Percutaneous Transhepatic Gallbladder Aspiration
Fumisato Kozakai, Yoshihide Kanno, Shinsuke Koshita, Takahisa Ogawa, Hiroaki Kusunose, Toshitaka Sakai, Keisuke Yonamine, Kazuaki Miyamoto, Hideyuki Anan, Haruka Okano, Kei Ito
Internal Medicine.2023; 62(5): 673. CrossRef - Prospective feasibility study on the efficacy and safety of a novel spiral dilator for endoscopic ultrasound‐guided drainage
Takahisa Ogawa, Yoshihide Kanno, Shinsuke Koshita, Hiroaki Kusunose, Toshitaka Sakai, Keisuke Yonamine, Kazuaki Miyamoto, Fumisato Kozakai, Haruka Okano, Hideyuki Anan, Kento Hosokawa, Kei Ito
DEN Open.2023;[Epub] CrossRef - Endoscopic ultrasound (EUS)-guided cholecystostomy versus percutaneous cholecystostomy (PTC) in the management of acute cholecystitis in patients unfit for surgery: a systematic review and meta-analysis
Matheus Candido Hemerly, Diogo Turiani Hourneaux de Moura, Epifanio Silvino do Monte Junior, Igor Mendonça Proença, Igor Braga Ribeiro, Erika Yuki Yvamoto, Pedro Henrique Boraschi Vieira Ribas, Sergio A. Sánchez-Luna, Wanderley Marques Bernardo, Eduardo G
Surgical Endoscopy.2023; 37(4): 2421. CrossRef - Trial sequential analysis of EUS-guided gallbladder drainage versus percutaneous cholecystostomy in patients with acute cholecystitis
Alessandro Cucchetti, Cecilia Binda, Elton Dajti, Monica Sbrancia, Giorgio Ercolani, Carlo Fabbri
Gastrointestinal Endoscopy.2022; 95(3): 399. CrossRef - Percutaneous Cholecystostomy: A Bridge to Less Morbidity
Anil Kumar Singh
The Arab Journal of Interventional Radiology.2022; 06(01): 003. CrossRef - Determinants of outcomes of transmural EUS-guided gallbladder drainage: systematic review with proportion meta-analysis and meta-regression
Carlo Fabbri, Cecilia Binda, Monica Sbrancia, Elton Dajti, Chiara Coluccio, Giorgio Ercolani, Andrea Anderloni, Alessandro Cucchetti
Surgical Endoscopy.2022; 36(11): 7974. CrossRef - Endoscopic ultrasound-guided cholecystogastrostomy as an alternative biliary drainage route in malignant obstructions
Marco A. D’Assuncao, Fernando P. Marson, Saverio T. N. Armellini, Fernando L. Mota, Fernando J. S. de Oliveira, Eduardo M. A. Pereira Junior
Endoscopy.2021; 53(07): E277. CrossRef - Efficacy and safety of conversion of percutaneous cholecystostomy to endoscopic transpapillary gallbladder stenting in high-risk surgical patients
Hyung Ku Chon, Chan Park, Dong Eun Park, Tae Hyeon Kim
Hepatobiliary & Pancreatic Diseases International.2021; 20(5): 478. CrossRef - Endoscopic Transpapillary Gallbladder Drainage for Acute Cholecystitis After Biliary Self-Expandable Metal Stent Placement
Kazunari Nakahara, Ryo Morita, Yosuke Michikawa, Keigo Suetani, Nozomi Morita, Akashi Fujita, Junya Sato, Yosuke Igarashi, Hiroki Ikeda, Kotaro Matsunaga, Tsunamasa Watanabe, Shinjiro Kobayashi, Takehito Otsubo, Fumio Itoh
Surgical Laparoscopy, Endoscopy & Percutaneous Techniques.2020; 30(5): 416. CrossRef - Endoscopic Management of Acute Cholecystitis Following Metal Stent Placement for Malignant Biliary Strictures: A View from the Inside Looking in
Sean Bhalla, Ryan Law
Clinical Endoscopy.2019; 52(3): 209. CrossRef
- Risk factors and treatment strategies for cholecystitis after metallic stent placement for malignant biliary obstruction: a multicenter retrospective study
- 5,877 View
- 127 Download
- 12 Web of Science
- 12 Crossref

Case Reports
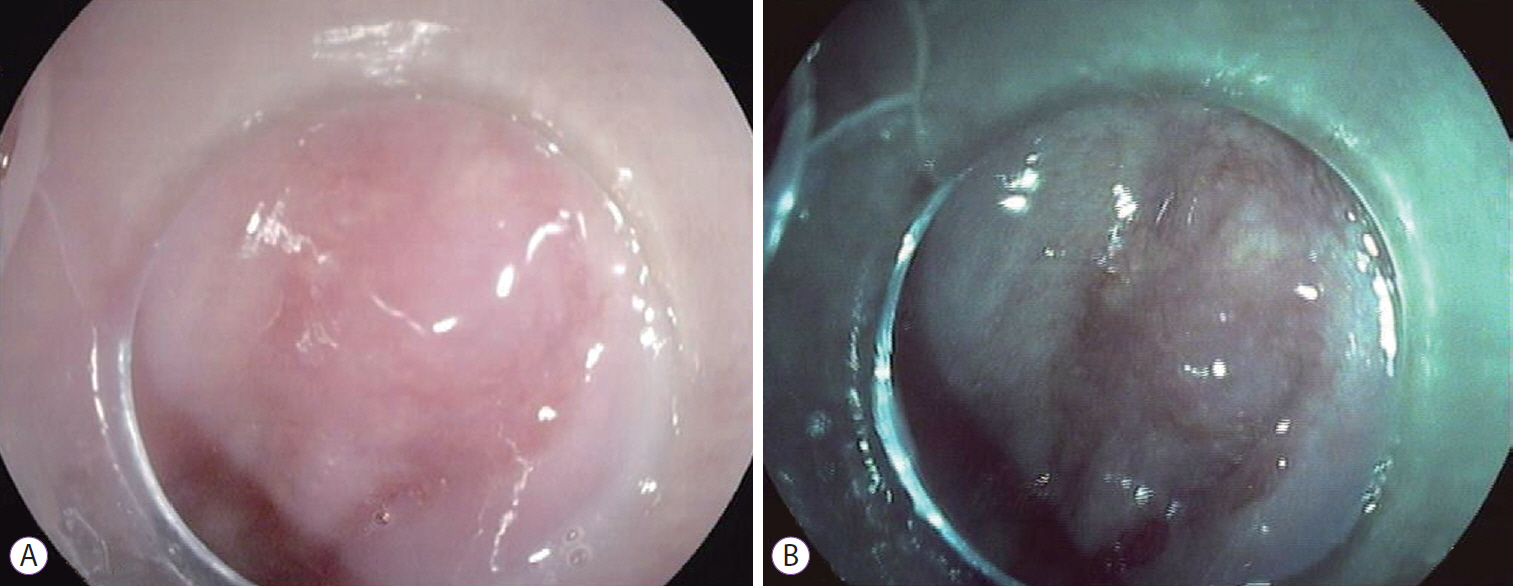
- Buried Barrett’s Esophagus with High-Grade Dysplasia after Radiofrequency Ablation
- Joana Castela, Miguel Serrano, Susana Mão de Ferro, Daniela Vinha Pereira, Paula Chaves, António Dias Pereira
- Clin Endosc 2019;52(3):269-272. Published online October 5, 2018
- DOI: https://doi.org/10.5946/ce.2018.124
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Radiofrequency ablation therapy is an effective endoscopic option for the eradication of Barrett’s esophagus that appears to reduce the risk of esophageal cancer. A concern associated with this technique is the development of subsquamous/buried intestinal metaplasia, whose clinical relevance and malignant potential have not yet been fully elucidated. Fewer than 20 cases of subsquamous neoplasia after the successful radiofrequency ablation of Barrett’s esophagus have been reported to date. Here, we describe a new case of subsquamous neoplasia (high-grade dysplasia) following radiofrequency ablation that was managed with endoscopic resection. Our experience suggests that a meticulous endoscopic inspection prior to and after radiofrequency ablation is fundamental to reduce the risk of buried neoplasia development.
-
Citations
Citations to this article as recorded by- Allaying uncertainty in diagnosing buried Barrett's esophagus
Ryan Demkowicz, Prashanthi N. Thota, Tanmayee Benjamin, Rocio Lopez, Haiyan Lu, Deepa T. Patil, Erinn Downs-Kelly, Jennifer A. Jeung, Keith K. Lai, James Lapinski, Erica C. Savage, John R. Goldblum, Ilyssa O. Gordon
Annals of Diagnostic Pathology.2021; 51: 151672. CrossRef - Endoscopic features of buried Barrett’s mucosa
Linda S. Yang, Bronte A. Holt, Richard Williams, Richard Norris, Edward Tsoi, Georgina Cameron, Paul Desmond, Andrew C.F. Taylor
Gastrointestinal Endoscopy.2021; 94(1): 14. CrossRef - Post-ablation buried neoplasia in Barrett’s esophagus
Prabhat Kumar, Ilyssa O. Gordon, Prashanthi N. Thota
Scandinavian Journal of Gastroenterology.2021; 56(5): 624. CrossRef - Role of optical coherence tomography in Barrett’s esophagus
Nikhil Gupta, Raghav Yelamanchi, Himanshu Agrawal, Nitin Agarwal
Artificial Intelligence in Gastrointestinal Endoscopy.2021; 2(4): 149. CrossRef - Indications, contraindications and limitations of endoscopic therapy for Barrett’s esophagus and early esophageal adenocarcinoma
Carol Rouphael, Mythri Anil Kumar, Madhusudhan R. Sanaka, Prashanthi N. Thota
Therapeutic Advances in Gastroenterology.2020; 13: 175628482092420. CrossRef - Risk Factors for Self-Expandable Metal Stent Complications in the Treatment of Esophageal Cancer: A Scoping Review
Connor K. Wilson, Sara R. Frankowski, Susan C. Steelman, Issam Makhoul
SN Comprehensive Clinical Medicine.2020; 2(8): 1163. CrossRef - Multifocal Cryoballoon Ablation for Eradication of Barrett's Esophagus-Related Neoplasia: A Prospective Multicenter Clinical Trial
Marcia Irene Canto, Arvind J. Trindade, Julian Abrams, Michael Rosenblum, John Dumot, Amitabh Chak, Prasad Iyer, David Diehl, Harshit S. Khara, F. Scott Corbett, Matthew McKinley, Eun Ji Shin, Irving Waxman, Anthony Infantolino, Christina Tofani, Jason Sa
American Journal of Gastroenterology.2020; 115(11): 1879. CrossRef - Inflammatory bowel disease- and Barrett’s esophagus-associated neoplasia: the old, the new, and the persistent struggles
Dipti M Karamchandani, Qin Zhang, Xiao-Yan Liao, Jing-Hong Xu, Xiu-Li Liu
Gastroenterology Report.2019; 7(6): 379. CrossRef
- Allaying uncertainty in diagnosing buried Barrett's esophagus
- 6,690 View
- 161 Download
- 6 Web of Science
- 8 Crossref

- Blue Laser Imaging with a Small-Caliber Endoscope Facilitates Detection of Early Gastric Cancer
- Haruo Takahashi, Yoshimasa Miura, Hiroyuki Osawa, Takahito Takezawa, Yuji Ino, Masahiro Okada, Alan Kawarai Lefor, Hironori Yamamoto
- Clin Endosc 2019;52(3):273-277. Published online August 14, 2018
- DOI: https://doi.org/10.5946/ce.2018.100

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Conventional endoscopy often misses early gastric cancers with minimal red discoloration because they cannot be distinguished from inflamed mucosa. We treated a patient with a small early gastric cancer that was difficult to diagnose using conventional endoscopy. Conventional endoscopy using a small-caliber endoscope showed only subtle red discoloration of the gastric mucosa. However, blue laser imaging showed a clearly discolored area measuring 10 mm in diameter around the red lesion, which was distinct from the surrounding inflamed mucosa. Irregular vessels on the tumor surface (suspicious for early gastric cancer) were observed even with small-caliber endoscopy. Biopsy revealed a well-moderately differentiated tubular adenocarcinoma, and endoscopic submucosal dissection was performed. Histopathological examination of the specimen confirmed well-moderately differentiated adenocarcinoma localized to the mucosa with slight depression compared to the surrounding mucosa, consistent with the endoscopic findings. This small early gastric cancer became clearly visible with blue laser imaging using small-caliber endoscopy.
-
Citations
Citations to this article as recorded by- Diagnostic performance of blue laser imaging for early detection of gastric cancer: A systematic review and meta-analysis
Mohammed Rifat Shaik, Andrew Canakis, Nishat Anjum Shaik, Shivanand Bomman, Dushyant Singh Dahiya, Emily Gorman, Mohammad Bilal, Saurabh Chandan
Indian Journal of Gastroenterology.2024;[Epub] CrossRef - Improved detection of early gastric cancer with linked color imaging using an ultrathin endoscope: a video-based analysis
Tsevelnorov Khurelbaatar, Yoshimasa Miura, Hiroyuki Osawa, Yuji Ino, Takahito Takezawa, Chihiro Iwashita, Yoshie Nomoto, Masato Tsunoda, Takashi Ueno, Haruo Takahashi, Manabu Nagayama, Hisashi Fukuda, Alan Kawarai Lefor, Hironori Yamamoto
Endoscopy International Open.2022; 10(05): E644. CrossRef - Current status of the gastric cancer screening program in Korea
Young-Il Kim, Il Ju Choi
Journal of the Korean Medical Association.2022; 65(5): 250. CrossRef - Current Evidence for a Paradigm Shift in Gastric Cancer Prevention From Endoscopic Screening toHelicobacter pyloriEradication in Korea
Young-Il Kim, Il Ju Choi
Journal of Gastric Cancer.2022; 22(3): 169. CrossRef - Clinical Features of False-Negative Early Gastric Cancers: A Retrospective Study of Endoscopic Submucosal Dissection Cases
Kohei Oka, Naoto Iwai, Takashi Okuda, Tasuku Hara, Yutaka Inada, Toshifumi Tsuji, Toshiyuki Komaki, Junichi Sakagami, Yuji Naito, Keizo Kagawa, Yoshito Itoh, Fabiana Zingone
Gastroenterology Research and Practice.2021; 2021: 1. CrossRef - Appropriate Color Enhancement Settings for Blue Laser Imaging Facilitates the Diagnosis of Early Gastric Cancer with High Color Contrast
Yuji Hiraoka, Yoshimasa Miura, Hiroyuki Osawa, Yoshie Nomoto, Haruo Takahashi, Masato Tsunoda, Manabu Nagayama, Takashi Ueno, Alan Kawarai Lefor, Hironori Yamamoto
Journal of Gastric Cancer.2021; 21(2): 142. CrossRef - Linked Color Imaging and Blue Laser Imaging for Upper Gastrointestinal Screening
Hiroyuki Osawa, Yoshimasa Miura, Takahito Takezawa, Yuji Ino, Tsevelnorov Khurelbaatar, Yuichi Sagara, Alan Kawarai Lefor, Hironori Yamamoto
Clinical Endoscopy.2018; 51(6): 513. CrossRef
- Diagnostic performance of blue laser imaging for early detection of gastric cancer: A systematic review and meta-analysis
- 5,559 View
- 164 Download
- 9 Web of Science
- 7 Crossref

- Primary Gastric Small Cell Carcinoma (Presenting as Linitis Plastica) Diagnosed Using Endoscopic Ultrasound-Guided Biopsy: A Case Report
- Ra Ri Cha, Jin Kyu Cho, Wan Soo Kim, Jin Joo Kim, Jae Min Lee, Sang Soo Lee, Hyun Jin Kim
- Clin Endosc 2019;52(3):278-282. Published online October 5, 2018
- DOI: https://doi.org/10.5946/ce.2018.114

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Small cell carcinomas are the most aggressive, highly malignant neuroendocrine tumors; among these, gastric small cell carcinoma (GSCC) is extremely rare. Here we report a case of a patient with primary GSCC, presenting as linitis plastic, who was diagnosed using endoscopic ultrasound (EUS)-guided biopsy. With undiagnosed linitis plastica, an 80-year-old woman was referred to our institution. Abdominal computed tomography revealed irregular wall thickening extending from the gastric body to the antrum. Endoscopy suspected to have Borrmann type IV advanced gastric cancer. EUS of the stomach showed diffuse submucosal thickening of the gastric wall, mainly the antrum. EUS-guided bite-on-bite biopsy confirmed the diagnosis of GSCC. In general, GSCC is difficult to diagnose and careful examination is necessary to determine the therapeutic strategy; however, EUS is particularly helpful in the differential diagnosis of a lesion presenting as linitis plastica.
-
Citations
Citations to this article as recorded by- An online tool for survival prediction of extrapulmonary small cell carcinoma with random forest
Xin Zhang
Frontiers in Oncology.2023;[Epub] CrossRef - Gastric linitis plastica due to signet-ring cell carcinoma with Krukenberg tumors diagnosed by endoscopic ultrasound-guided fine-needle aspiration
Takeshi Okamoto, Hidekazu Suzuki, Katsuyuki Fukuda
Clinical Journal of Gastroenterology.2021; 14(4): 994. CrossRef - A case report of advanced gastric small cell carcinoma with neoadjuvant chemotherapy followed by radical total gastrectomy
Bing Wang, Nanlin Jiao, Lianghui Shi
Asian Journal of Surgery.2020; 43(12): 1205. CrossRef
- An online tool for survival prediction of extrapulmonary small cell carcinoma with random forest
- 7,142 View
- 153 Download
- 3 Web of Science
- 3 Crossref

- Omental Patching and Purse-String Endosuture Closure after Endoscopic Full-Thickness Resection in Patients with Gastric Gastrointestinal Stromal Tumors
- Faisal Inayat, Aysha Aslam, Mathew D. Grunwald, Qulsoom Hussain, Abu Hurairah, Shahzad Iqbal
- Clin Endosc 2019;52(3):283-287. Published online October 5, 2018
- DOI: https://doi.org/10.5946/ce.2018.116
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract, primarily arising from the stomach. With the widespread utilization of and technical advancements in endoscopy, gastric GISTs are being increasingly detected at an early stage, enabling complete endoscopic resection. Endoscopic full-thickness resection (EFTR) is an advanced technique that has been recognized as a treatment tool for neoplasms in the digestive tract in selected patients. Although a number of methods are available, closing large iatrogenic defects after EFTR can be a concern in clinical practice. If this potential problem is appropriately solved, patients with gastric GISTs would be suitable candidates for resection utilizing this technique. To our knowledge, this is the first study to propose omental patching and purse-string endosuture closure following EFTR as a feasible endoscopic option in patients with gastric GISTs.
-
Citations
Citations to this article as recorded by- Endoscopic Full Thickness Resection: A Systematic Review
Partha Pal, Mohan Ramchandani, Pradev Inavolu, Duvvuru Nageshwar Reddy, Manu Tandan
Journal of Digestive Endoscopy.2022; 13(03): 152. CrossRef - Colon Sparing Endoscopic Full-Thickness Resection for Advanced Colorectal Lesions: Is It Time for Global Adoption?
Zhong-Wei Wu, Chao-Hui Ding, Yao-Dong Song, Zong-Chao Cui, Xiu-Qian Bi, Bo Cheng
Frontiers in Oncology.2022;[Epub] CrossRef - Endoscopic Therapy in the Management of Patients With Severe Rectal Bleeding Following Transrectal Ultrasound-Guided Prostate Biopsy: A Case-Based Systematic Review
Adnan Malik, Rizwan Ishtiaq, Muhammad Hassan Naeem Goraya, Faisal Inayat, Vinaya V. Gaduputi
Journal of Investigative Medicine High Impact Case Reports.2021; 9: 232470962110132. CrossRef - Closure techniques in exposed endoscopic full-thickness resection: Overview and future perspectives in the endoscopic suturing era
Antonino Granata, Alberto Martino, Dario Ligresti, Francesco Paolo Zito, Michele Amata, Giovanni Lombardi, Mario Traina
World Journal of Gastrointestinal Surgery.2021; 13(7): 645. CrossRef - Use of omental patch and endoscopic closure technique as an alternative to surgery after endoscopic full thickness resection of gastric intestinal stromal tumors: A series of cases
Amit H Sachdev, Shahzad Iqbal, Igor Braga Ribeiro, Diogo Turiani Hourneaux de Moura
World Journal of Clinical Cases.2020; 8(1): 120. CrossRef - Endoscopic full-thickness resection
David Friedel, Xiaocen Zhang, Rani Modayil, Stavros N. Stavropoulos
Techniques in Gastrointestinal Endoscopy.2019; 21(1): 19. CrossRef
- Endoscopic Full Thickness Resection: A Systematic Review
- 5,526 View
- 120 Download
- 5 Web of Science
- 6 Crossref

- Pneumoperitoneum after Endoscopic Duodenal Stent Insertion in a Patient with Percutaneous Transhepatic Biliary Drainage and Biliary Stent: A Case Report
- Jinwoo Choi, Min Ji Lee, Hyodeok Lee, Yook Kim, Joung-Ho Han, Seon Mee Park
- Clin Endosc 2019;52(3):288-292. Published online August 29, 2018
- DOI: https://doi.org/10.5946/ce.2018.128

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Early removal of a percutaneous transhepatic biliary drainage (PTBD) tube commonly causes pneumoperitoneum. However, we encountered a patient who developed pneumoperitoneum even with an indwelling PTBD tube. An 84-year-old man was admitted with type III combined duodenal and biliary obstruction secondary to metastatic bladder cancer. A biliary stent was placed using a percutaneous approach, and a duodenal stent was placed endoscopically. A large amount of subphrenic free air was detected after the procedures. Laboratory tests indicated intestinal perforation; however, peritoneal signs were absent. The patient was treated conservatively using an indwelling Levin tube. Seven days later, the massive amount of subphrenic free air disappeared. Follow-up tubography revealed unrestricted bile flow into the small intestine, and the PTBD tube was removed. Prolonged endoscopic procedures in patients with a PTBD tract communicating with the gastrointestinal tract can precipitate pneumoperitoneum. Clinicians should be careful to avoid misdiagnosing this condition as intestinal perforation.
-
Citations
Citations to this article as recorded by- The characteristics of residual pneumoperitoneum after laparoscopic colorectal surgery
Sotaro Fukuhara, Hiroyuki Egi, Masatoshi Kochi, Wataru Shimizu, Yuji Takakura, Kazuhiro Taguchi, Ikki Nakashima, Yusuke Sumi, Shintaro Akabane, Koki Sato, Hisaaki Yoshinaka, Yoshifumi Teraoka, Minoru Hattori, Hideki Ohdan
Asian Journal of Endoscopic Surgery.2022; 15(2): 320. CrossRef
- The characteristics of residual pneumoperitoneum after laparoscopic colorectal surgery
- 5,457 View
- 122 Download
- 1 Web of Science
- 1 Crossref

Brief Report
- Microforceps-Assisted Diagnosis of Cystic Pancreatic Neuroendocrine Tumor
- Guru Trikudanathan, Dale Snover, Shawn J Mallery
- Clin Endosc 2019;52(3):293-294. Published online May 24, 2019
- DOI: https://doi.org/10.5946/ce.2018.166
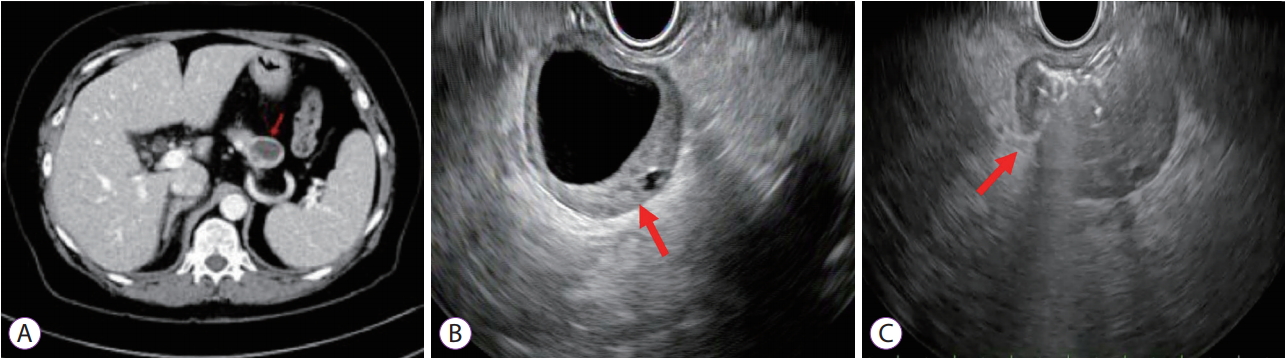
-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- Role of Endoscopic Ultrasound in the Diagnosis of Pancreatic Neuroendocrine Neoplasms
Tatsuya Ishii, Akio Katanuma, Haruka Toyonaga, Koki Chikugo, Hiroshi Nasuno, Toshifumi Kin, Tsuyoshi Hayashi, Kuniyuki Takahashi
Diagnostics.2021; 11(2): 316. CrossRef
- Role of Endoscopic Ultrasound in the Diagnosis of Pancreatic Neuroendocrine Neoplasms
- 3,336 View
- 61 Download
- 1 Web of Science
- 1 Crossref


 KSGE
KSGE




 First
First Prev
Prev



