Previous issues
- Page Path
- HOME > Browse Articles > Previous issues
Commentarys
- Quality Indicator for Gastric Cancer Detection Based on Helicobacter pylori Status
- Jae Myung Park
- Clin Endosc 2020;53(6):629-630. Published online November 30, 2020
- DOI: https://doi.org/10.5946/ce.2020.270
-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- Cancer Patient Perception Survey to Develop Korean Medicine Clinical Practice Guidelines for Gastric Cancer
Dong Hyeon Kim, Jong Hee Kim, Ji Hye Park, Hwa Seung Yoo, Hyeong Joon Jeon, So Jung Park
Journal of Physiology & Pathology in Korean Medicine.2023; 37(4): 81. CrossRef - Endoscopic diagnosis of early gastric cancer
Dong Chan Joo, Gwang Ha Kim
Journal of the Korean Medical Association.2022; 65(5): 267. CrossRef - Construction of Stomach Cancer Lesion Detection Combined with Drug Therapy Based on Artificial Intelligence
Shengyong Zhai, Lihong Yu, Jing Li, Sandip K. Mishra
Contrast Media & Molecular Imaging.2022; 2022: 1. CrossRef - Tailored eradication strategy vs concomitant therapy for Helicobacter pylori eradication treatment in Korean patients
Youn I Choi, Jun-Won Chung, Kyoung Oh Kim, Kwang An Kwon, Yoon Jae Kim, Jung Ho Kim, Ja Young Seo, Dong Kyun Park
World Journal of Gastroenterology.2021; 27(31): 5247. CrossRef
- Cancer Patient Perception Survey to Develop Korean Medicine Clinical Practice Guidelines for Gastric Cancer
- 3,365 View
- 97 Download
- 3 Web of Science
- 4 Crossref

- Ideal Method for Small Bowel Preparation before Video Capsule Endoscopy
- Jun Lee, Shai Friedland
- Clin Endosc 2020;53(6):631-632. Published online November 6, 2020
- DOI: https://doi.org/10.5946/ce.2020.264
- 3,126 View
- 81 Download

- The Need for a Better-Designed Study of the Outcomes of Endoscopic Management of Bile Leak
- Hyung Ku Chon, Eun Ji Shin, Seong-Hun Kim
- Clin Endosc 2020;53(6):633-635. Published online November 13, 2020
- DOI: https://doi.org/10.5946/ce.2020.263
- 2,865 View
- 76 Download

- Changing Trends in Biliary Stenting for Unresectable Malignant Perihilar Obstructions
- Lubna Kamani, Muhammad Arshad
- Clin Endosc 2020;53(6):636-637. Published online August 31, 2020
- DOI: https://doi.org/10.5946/ce.2020.165
- 3,831 View
- 74 Download

Focused Review Series: Cutting Edges of Advanced Therapeutic Endoscopy
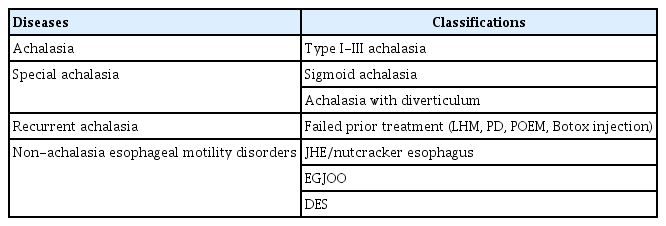
- Peroral Endoscopic Myotomy for Esophageal Motility Disorders
- Jun Young Kim, Yang Won Min
- Clin Endosc 2020;53(6):638-645. Published online November 20, 2020
- DOI: https://doi.org/10.5946/ce.2020.223
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Peroral endoscopic myotomy (POEM) is one of the most clinically successful tunnel-based minimally invasive endoscopic treatments. The classic indications of POEM include achalasia of all types, including failed prior treatments, and expanded indications include the non-achalasia esophageal motility disorders, such as esophagogastric junction outflow obstruction, diffuse esophageal spasm, and jackhammer esophagus. For achalasia treatment, POEM has achieved a comparable surgical efficacy and a safety outcome and, therefore, has emerged as a first-line treatment. For non-achalasia esophageal motility disorders, POEM has also shown high clinical response rates. The complication rate of POEM for esophageal motility disorders is low and most complications are managed with conservative treatment. Currently, POEM is a representative procedure of natural orifice transluminal endoscopic surgery, which has shown a good clinical efficacy with low complication rates for esophageal motility disorders including achalasia. However, further studies are needed to treat non-achalasia motility disorder via POEM.
-
Citations
Citations to this article as recorded by- Diffuse Esophageal Spasm: An Alternative Treatment Approach
McKenzie K Allen , Wayne Frei
Cureus.2024;[Epub] CrossRef - Clinical and financial outcomes of per-oral endoscopic myotomy compared to laparoscopic heller myotomy for treatment of achalasia
Lena Shally, Kashif Saeed, Derek Berglund, Mark Dudash, Katie Frank, Vladan N. Obradovic, Anthony T. Petrick, David L. Diehl, Jon D. Gabrielsen, David M. Parker
Surgical Endoscopy.2023; 37(7): 5526. CrossRef - Benefit of extending the protocol for high resolution manometry according to the version 4.0 of the Chicago criteria. A multicenter study
Luis G. Alcalá‐González, Alberto Ezquerra‐Duran, Ariadna Aguilar, Claudia Barber, Elizabeth Barba, Isis K. Araujo, Ingrid Marin, Juan Naves, Jordi Serra
Neurogastroenterology & Motility.2023;[Epub] CrossRef - Role of Endoscopy in Motility Disorders of Upper Gastrointestinal Tract
Jin Hee Noh, Hwoon-Yong Jung
Journal of Neurogastroenterology and Motility.2023; 29(1): 7. CrossRef - Surveillance Endoscopy After Foregut Surgery: Is It Necessary?
Yahya Alwatari, Daniel Scheese, Graham Gardner, Vignesh Vudatha, Walker Julliard, Carlos Puig Gilbert, Rachit D. Shah
Foregut: The Journal of the American Foregut Society.2023; 3(1): 89. CrossRef - Per oral endoscopic myotomy for achalasia: A Taiwanese single‐center experience
Yu‐Chi Lee, Wei‐Chen Tai, Keng‐Liang Wu, Chih‐Chien Yao, Seng‐Kee Chuah
Advances in Digestive Medicine.2022; 9(4): 241. CrossRef - Pediatric anesthesia and achalasia: 10 years’ experience in peroral endoscopy myotomy management
Fabio Sbaraglia, Pietro Familiari, Federica Maiellare, Marco Mecarello, Annamaria Scarano, Demetrio Del Prete, Rosa Lamacchia, Federica Antonicelli, Marco Rossi
Journal of Anesthesia, Analgesia and Critical Care.2022;[Epub] CrossRef - A rare complication: Tension pneumothorax after peroral endoscopic myotomy
Seokin Kang, Yuri Kim, Do Hoon Kim
International Journal of Gastrointestinal Intervention.2022; 11(3): 139. CrossRef - Endoscopic Management of Dysphagia
Min Ji Kim, Yang Won Min
The Korean Journal of Gastroenterology.2021; 77(2): 77. CrossRef - An update on endoscopic treatment for achalasia: From per oral endoscopic myotomy to endolumenal functional lumen imaging probe
Wei‐Chen Tai, Keng‐Liang Wu, Seng‐Kee Chuah
Advances in Digestive Medicine.2021; 8(1): 8. CrossRef
- Diffuse Esophageal Spasm: An Alternative Treatment Approach
- 4,444 View
- 133 Download
- 10 Web of Science
- 10 Crossref

-
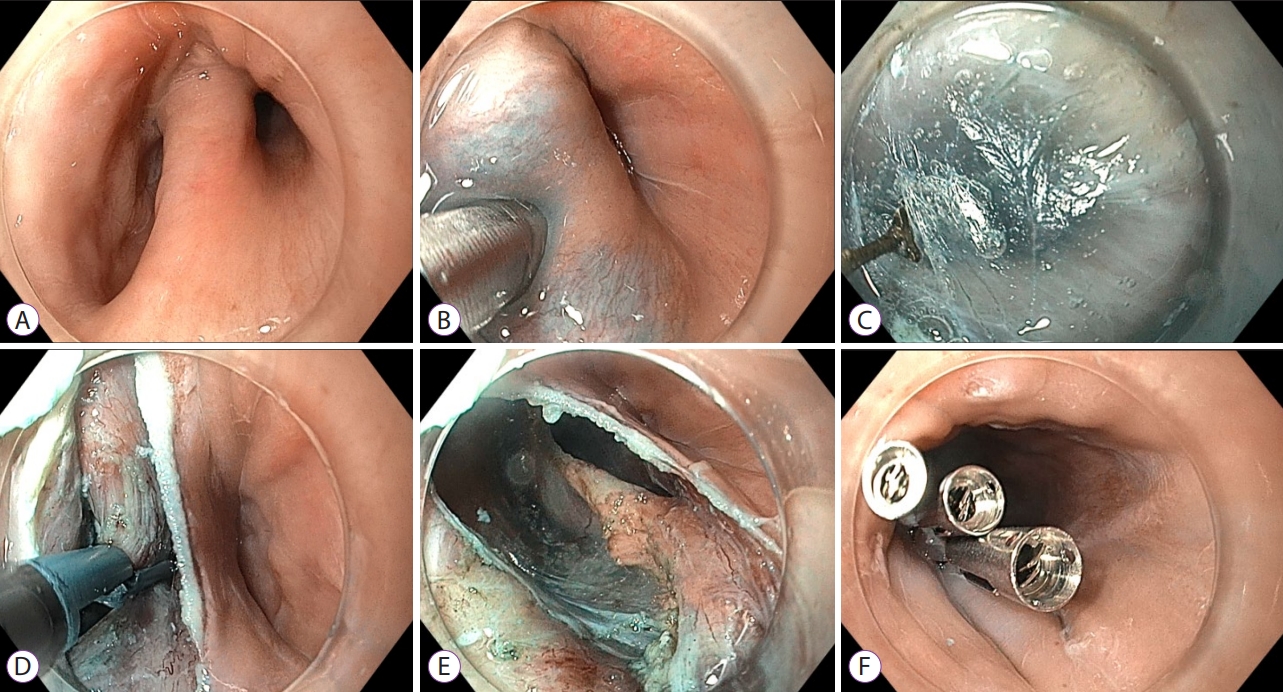
Role of Peroral Endoscopic Myotomy (POEM) in the Management of Esophageal Diverticula

- Bogdan P. Miutescu, Sarah Khan, Shruti Mony, Mouen A. Khashab
- Clin Endosc 2020;53(6):646-651. Published online November 26, 2020
- DOI: https://doi.org/10.5946/ce.2020.262
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Esophageal diverticula are uncommon; however, when present, they can cause symptoms of dysphagia, regurgitation, and chest pain. Based on location and pathophysiological characteristics, they are classified as pulsion- and traction-type diverticula. In the past, the open surgical approach was the only treatment available; however, in the past few decades, transoral incisionless approaches in the form of rigid and flexible endoscopy have gained popularity. Diverticular peroral endoscopic myotomy has emerged as an alternative treatment option. In this paper, we reviewed the role of peroral endoscopic myotomy as a treatment option for different types of esophageal diverticula. Although a safe and effective procedure, this novel submucosal tunneling technique for the treatment of esophageal diverticula requires further validation, and head-to-head comparisons between the different approaches for the treatment of esophageal diverticula are warranted.
-
Citations
Citations to this article as recorded by- Efficacy and safety of peroral endoscopic myotomy for esophageal diverticula
Elise M. Wessels, Jeroen M. Schuitenmaker, Barbara A.J. Bastiaansen, Paul Fockens, Gwen M.C. Masclee, Albert J. Bredenoord
Endoscopy International Open.2023; 11(05): E546. CrossRef - Peroral endoscopic myotomy (POEM) for esophageal diverticula
Jayanta SAMANTA, Zaheer NABI, Jahnvi DHAR, Harshal S. MANDAVDHARE
Minerva Gastroenterology.2023;[Epub] CrossRef - Multimodal Endoscopic Management of Esophageal Perforations as a Complication of Peroral Endoscopic Myotomy for a Zenker's Diverticulum
Erlison Mauricio Daza Castro, Carlos Fernando Fuentes, Andrea Carolina Córdoba Guzmán, Diego Aponte, José Nicolás Rocha, Carlos González, Luis Carlos Sabbagh
ACG Case Reports Journal.2023; 10(6): e01059. CrossRef - A rare case of bilateral Killian-Jamieson diverticula treated endoscopically
Catarina Félix, Pedro Barreiro, José Rodrigues, Rui Mendo, Catarina O’Neill, Cristina Chagas
Endoscopy.2022; 54(06): E283. CrossRef - Peroral endoscopic myotomy, septotomy, and restoration of esophageal lumen with over-the-scope clips: closing the circle of esophageal diverticula management
Eduardo Albéniz, Fermín Estremera-Arévalo, Marta Gómez Alonso, Pedro J. Rosón, Francisco J. Gallego Rojo, Juan Vila, Sheyla Montori
Endoscopy.2022; 54(11): E666. CrossRef - Successful D-POEM after failed surgical myotomy and diverticulectomy
Andrew Ross Leopold, Raymond E. Kim
VideoGIE.2022; 7(6): 211. CrossRef - Peroral Endoscopic Myotomy for the Treatment of Esophageal Diverticula
Antonio Facciorusso, Daryl Ramai, Yervant Ichkhanian, Rena Yadlapati, Vito Annese, Sachin Wani, Mouen A. Khashab
Journal of Clinical Gastroenterology.2022; 56(10): 853. CrossRef
- Efficacy and safety of peroral endoscopic myotomy for esophageal diverticula
- 6,115 View
- 197 Download
- 8 Web of Science
- 7 Crossref

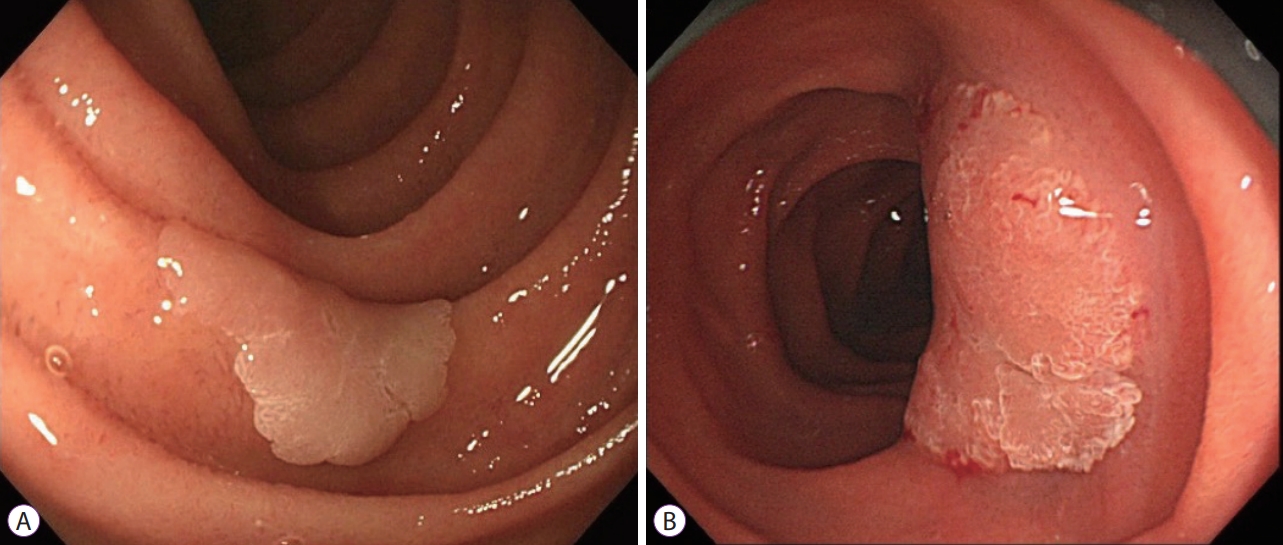
- Present Status of Endoscopic Submucosal Dissection for Non-Ampullary Duodenal Epithelial Tumors
- Naomi Kakushima, Masao Yoshida, Yohei Yabuuchi, Noboru Kawata, Kohei Takizawa, Yoshihiro Kishida, Sayo Ito, Kenichiro Imai, Kinichi Hotta, Hirotoshi Ishiwatari, Hiroyuki Matsubayashi, Hiroyuki Ono
- Clin Endosc 2020;53(6):652-658. Published online January 15, 2020
- DOI: https://doi.org/10.5946/ce.2019.184
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Prediction of histology by endoscopic examination is important in the clinical management of non-ampullary duodenal epithelial tumors (NADETs), including adenoma and adenocarcinoma. The use of a simple scoring system based on the findings of white-light endoscopy or magnified endoscopy with narrow-band imaging is useful to differentiate between Vienna category 3 (C3) and C4/5 lesions. Less invasive endoscopic resection procedures, such as cold snare polypectomy, are quick to perform and convenient for small (<10 mm) C3 lesions. Neoplasms with higher grade histology, such as C4/5 lesions, should be treated by endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), or surgery. Although EMR often requires piecemeal resection, the complication rate is acceptable. Excellent complete resection rates could be achieved by ESD; however, it remains a challenging method considering the high risk of complications. Shielding or closure of the ulcer after ESD is effective at decreasing the risk of delayed bleeding and perforation. Laparoscopic endoscopic cooperative surgery is an ideal treatment with a high rate of en bloc resection and a low rate of complications, although it is limited to high-volume centers. Patients with NADETs could benefit from a multidisciplinary approach to stratify the optimal treatment based on endoscopic diagnoses.
-
Citations
Citations to this article as recorded by- The Characteristics and Treatment Outcomes of 71 Duodenal Brunner’s Gland Adenomas with Endoscopic Submucosal Dissection
Ying Xiang, Jinyan Liu, Nan ya Wang, Dehua Tang, Lei Wang, Ping xiao Zou, Guifang Xu, Qin Huang
Digestive Diseases.2023; 41(6): 852. CrossRef - Endoscopic diagnosis and treatment of superficial non-ampullary duodenal epithelial tumors: A review
Zheng Zhao, Yue Jiao, Shuyue Yang, Anni Zhou, Guiping Zhao, Shuilong Guo, Peng Li, Shutian Zhang
Journal of Translational Internal Medicine.2023; 11(3): 206. CrossRef - Current Treatment Strategy for Superficial Nonampullary Duodenal Epithelial Tumors
Tetsuya Suwa, Kohei Takizawa, Noboru Kawata, Masao Yoshida, Yohei Yabuuchi, Yoichi Yamamoto, Hiroyuki Ono
Clinical Endoscopy.2022; 55(1): 15. CrossRef - Rapid and chronological expression of angiogenetic genes is a major mechanism involved in cell sheet transplantation in a rat gastric ulcer model
Shun Yamaguchi, Miki Higashi, Kengo Kanetaka, Yasuhiro Maruya, Shinichiro Kobayashi, Keiichi Hashiguchi, Masaaki Hidaka, Kazuhiko Nakao, Susumu Eguchi
Regenerative Therapy.2022; 21: 372. CrossRef - Survival comparison between endoscopic and surgical resection for non-ampullary duodenal neuroendocrine tumor (1–2 cm)
Jiebin Xie, Yuan Zhang, Ming He, Xu Liu, Pin Xie, Yueshan Pang
Scientific Reports.2022;[Epub] CrossRef - Efficacy, feasibility, and safety of endoscopic double closure in the GI tract
Ahmad M. Al-Taee, Kohtaro Ooka, Gregory B. Haber, Jonathan Cohen
iGIE.2022; 1(1): 19. CrossRef - Current endoscopic diagnosis treatment strategy for superficial nonampullary duodenal tumours
Aichun Li, Jianwei Shen
European Journal of Medical Research.2022;[Epub] CrossRef - The incidence of non‐ampullary duodenal cancer in Japan: The first analysis of a national cancer registry
Masao Yoshida, Yohei Yabuuchi, Naomi Kakushima, Motohiko Kato, Mikitaka Iguchi, Yorimasa Yamamoto, Kengo Kanetaka, Toshio Uraoka, Mitsuhiro Fujishiro, Masayuki Sho
Journal of Gastroenterology and Hepatology.2021; 36(5): 1216. CrossRef - White light and/or magnifying endoscopy with narrow band imaging for superficial nonampullary duodenal epithelial tumors
Naomi Kakushima, Masao Yoshida, Kohei Takizawa, Yohei Yabuuchi, Noboru Kawata, Yoshihiro Kishida, Sayo Ito, Kenichiro Imai, Kinichi Hotta, Hirotoshi Ishiwatari, Hiroyuki Matsubayashi, Hiroyuki Ono
Scandinavian Journal of Gastroenterology.2021; 56(2): 211. CrossRef - Endoscopic management of superficial nonampullary duodenal tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline
Geoffroy Vanbiervliet, Alan Moss, Marianna Arvanitakis, Urban Arnelo, Torsten Beyna, Olivier Busch, Pierre H. Deprez, Lumir Kunovsky, Alberto Larghi, Gianpiero Manes, Bertrand Napoleon, Kumanan Nalankilli, Manu Nayar, Enrique Pérez-Cuadrado-Robles, Stefan
Endoscopy.2021; 53(05): 522. CrossRef - Endoscopic Treatment for Superficial Nonampullary Duodenal Tumors
Hyo-Joon Yang
The Korean Journal of Gastroenterology.2021; 77(4): 164. CrossRef - Duodenal endoscopic submucosal dissection: Is it ready for primetime? (with video)
Sergey V. Kantsevoy
Gastrointestinal Endoscopy.2020; 91(5): 1138. CrossRef - Life on a knife edge: the optimal approach to the management of perforations during endoscopic submucosal dissection (ESD)
Shria Kumar, Young Hoon Youn, Jeffrey H. Lee
Expert Review of Gastroenterology & Hepatology.2020; 14(10): 965. CrossRef - Tapering body stiffness shortens upper gastrointestinal examination via transoral insertion with ultrathin endoscope
Satoshi Ono, Shun Ito, Kyohei Maejima, Shosuke Hosaka, Kiyotaka Umeki, Shin-ichiro Sato
Endoscopy International Open.2020; 08(12): E1748. CrossRef
- The Characteristics and Treatment Outcomes of 71 Duodenal Brunner’s Gland Adenomas with Endoscopic Submucosal Dissection
- 7,157 View
- 239 Download
- 14 Web of Science
- 14 Crossref

- Management of Remnant or Recurrent Lesions after Endoscopic Papillectomy
- Ichiro Yasuda, Saito Kobayashi, Kosuke Takahashi, Sohachi Nanjo, Hiroshi Mihara, Shinya Kajiura, Takayuki Ando, Kazuto Tajiri, Haruka Fujinami
- Clin Endosc 2020;53(6):659-662. Published online December 3, 2019
- DOI: https://doi.org/10.5946/ce.2019.171
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
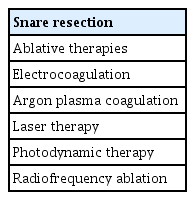
ePub - Endoscopic papillectomy (EP) for ampullary adenomas achieves cure rates ranging from 76% to 90%, and recurrence rates are as high as 33%. If remnant or recurrent lesions after prior EP are endoscopically visible and are not suspected of intraductal extension into the biliary or pancreatic duct, repeated snaring and cutting can be performed until all visible lesions are completely resected. However, endoscopic ablative therapies, particularly argon plasma coagulation, can be attempted for tiny or uncertain remnant and recurrent lesions. In addition, intraductal radiofrequency ablation has recently been attempted for residual intraductal lesions after EP at several institutions. Although still under investigation, it has shown some promise. It might be offered as an alternative to surgery, particularly in patients who are unfit for surgery or those who refuse to undergo surgery.
-
Citations
Citations to this article as recorded by- The timing of recurrence after endoscopic papillectomy
Samuel Han, Joshua A. Turkeltaub, Daniel Jonas, Augustin R. Attwell, Anna M. Duloy, Steven A. Edmundowicz, Hazem T. Hammad, Mihir S. Wagh, Sachin Wani, Raj J. Shah
Surgical Endoscopy.2024; 38(2): 688. CrossRef - Outcomes of rescue procedures in the management of locally recurrent ampullary tumors: A Pancreas 2000/EPC study
Elias Karam, Marcus Hollenbach, Einas Abou Ali, Francesco Auriemma, Aiste Gulla, Christian Heise, Sara Regner, Sébastien Gaujoux, Jean M. Regimbeau, Georg Kähler, Steffen Seyfried, Jean C. Vaillant, Charles De Ponthaud, Alain Sauvanet, David Birnbaum, Nic
Surgery.2023; 173(5): 1254. CrossRef - The first Russian experience of radiofrequency ablation in the treatment of adenoma of the major duodenal papilla with intraductal growth in the common bile duct
L.R. Tigiyev, Yu.S. Teterin, P.A. Yartsev, S.S. Petrikov
Khirurgiya. Zhurnal im. N.I. Pirogova.2023; (8): 70. CrossRef - The Significance of Histopathological Findings on Clinical Outcomes in Endoscopic Papillectomy with Endocut
Sayaka Miyamoto, Masahiro Serikawa, Yasutaka Ishii, Yumiko Tatsukawa, Shinya Nakamura, Juri Ikemoto, Yosuke Tamura, Kazuki Nakamura, Masaru Furukawa, Yumiko Yamashita, Noriaki Iijima, Koji Arihiro, Shiro Oka
Journal of Clinical Medicine.2023; 12(21): 6853. CrossRef - Clinical outcomes of endoscopic papillectomy of ampullary adenoma: A multi-center study
Seong Ji Choi, Hong Sik Lee, Jiyeong Kim, Jung Wan Choe, Jae Min Lee, Jong Jin Hyun, Jai Hoon Yoon, Hyo Jung Kim, Jae Seon Kim, Ho Soon Choi
World Journal of Gastroenterology.2022; 28(17): 1845. CrossRef - Endoscopic papillectomy for ampullary adenoma and early adenocarcinoma: Analysis of factors related to treatment outcome and long‐term prognosis
Hiroki Kawashima, Eizaburo Ohno, Takuya Ishikawa, Tadashi Iida, Hiroyuki Tanaka, Kazuhiro Furukawa, Masanao Nakamura, Takashi Honda, Senju Hashimoto, Akihiro Itoh, Masatoshi Ishigami, Yoshiki Hirooka, Mitsuhiro Fujishiro
Digestive Endoscopy.2021; 33(5): 858. CrossRef - Predictive factor of recurrence after endoscopic papillectomy for ampullary neoplasms
Kosuke Takahashi, Eisuke Ozawa, Ichiro Yasuda, Naohiro Komatsu, Hisamitsu Miyaaki, Ken Ohnita, Takuji Yamao, Kazuo Oba, Tatsuki Ichikawa, Kazuhiko Nakao
Journal of Hepato-Biliary-Pancreatic Sciences.2021; 28(7): 625. CrossRef - Management of obstructive jaundice in patients with neoplasms of the major duodenal papilla
Yu.S. Teterin, L.R. Tigiev, P.A. Yartsev, E.V. Stepan, M.L. Rogal, Yu.D. Kulikov
Khirurgiya. Zhurnal im. N.I. Pirogova.2021; (7): 49. CrossRef - Underwater endoscopic papillectomy for residual tumor after endoscopic papillectomy: First report
Yuki Mori, Akira Kurita, Shujiro Yazumi
Digestive Endoscopy.2020;[Epub] CrossRef - Diagnosis and treatment of benign neoplasms of the major duodenal papilla
Yu.S. Teterin, P.A. Yartsev, M.L. Rogal, L.R. Tigiev, N.V. Shavrina, K.A. Nugumanova, E.V. Stepan
Khirurgiya. Zhurnal im. N.I. Pirogova.2020; (11): 32. CrossRef
- The timing of recurrence after endoscopic papillectomy
- 5,488 View
- 215 Download
- 7 Web of Science
- 10 Crossref

Reviews
- Clinical Practice Guideline for the Management of Antithrombotic Agents in Patients Undergoing Gastrointestinal Endoscopy
- Hyun Lim, Eun Jeong Gong, Byung-Hoon Min, Seung Joo Kang, Cheol Min Shin, Jeong-Sik Byeon, Miyoung Choi, Chan Guk Park, Joo Young Cho, Soo Teik Lee, Ho Gak Kim, Hoon Jai Chun
- Clin Endosc 2020;53(6):663-677. Published online November 26, 2020
- DOI: https://doi.org/10.5946/ce.2020.192
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
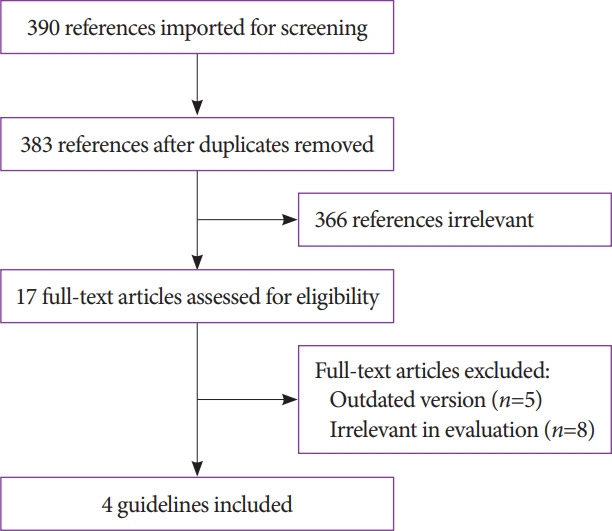
ePub - Antithrombotic agents, including antiplatelet agents and anticoagulants, are increasingly used in South Korea. The management of patients using antithrombotic agents and requiring gastrointestinal endoscopy is an important clinical challenge. Although clinical practice guidelines (CPGs) for the management of patients receiving antithrombotic agents and undergoing gastrointestinal endoscopy have been developed in the Unites States, Europe, and Asia Pacific region, it is uncertain whether these guidelines can be adopted in South Korea. After reviewing current CPGs, we identified unmet needs and recognized significant discrepancies in the clinical practice among regions. This is the first CPG in Korea providing information that may assist endoscopists in the management of patients on antithrombotic agents who require diagnostic or elective therapeutic endoscopy. This guideline was developed through the adaptation process as an evidence-based method, with four guidelines retrieved by systematic review. Eligible guidelines were evaluated according to the Appraisal of Guidelines for Research and Evaluation II process, and 13 statements were established using a grading system. This guideline was reviewed by external experts before an official. It will be revised as necessary to cover changes in technology, evidence, or other aspects of clinical practice.
-
Citations
Citations to this article as recorded by- Clinical Practice Guideline for Percutaneous Endoscopic Gastrostomy
Chung Hyun Tae, Ju Yup Lee, Moon Kyung Joo, Chan Hyuk Park, Eun Jeong Gong, Cheol Min Shin, Hyun Lim, Hyuk Soon Choi, Miyoung Choi, Sang Hoon Kim, Chul-Hyun Lim, Jeong-Sik Byeon, Ki-Nam Shim, Geun Am Song, Moon Sung Lee, Jong-Jae Park, Oh Young Lee
Gut and Liver.2024; 18(1): 10. CrossRef - A systematic critical appraisal of clinical practice guidelines of antithrombotic agents in gastrointestinal endoscopy using the AGREE II tool
Denisse Camille Dayto, Wojciech Blonski, Tea Reljic, Farina Klocksieben, Jeffrey Gill, Rene D. Gomez‐Esquivel, Brijesh Patel, Pushpak Taunk, Andrew Sephien, Camille Thelin, Ambuj Kumar
Journal of Gastroenterology and Hepatology.2024; 39(5): 818. CrossRef - The Impact of Sedation on Cardio-Cerebrovascular Adverse Events after Surveillance Esophagogastroduodenoscopy in Patients with Gastric Cancer: A Nationwide Population-Based Cohort Study
Sang Yoon Kim, Jun Kyu Lee, Kwang Hyuck Lee, Jae-Young Jang, Byung-Wook Kim
Gut and Liver.2024; 18(2): 245. CrossRef - International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
Seung Joo Kang, Chung Hyun Tae, Chang Seok Bang, Cheol Min Shin, Young-Hoon Jeong, Miyoung Choi, Joo Ha Hwang, Yutaka Saito, Philip Wai Yan Chiu, Rungsun Rerknimitr, Christopher Khor, Vu Van Khien, Kee Don Choi, Ki-Nam Shim, Geun Am Song, Oh Young Lee
Clinical Endoscopy.2024; 57(2): 141. CrossRef - Top tips on the management of antithrombotic agents in the periendoscopic period
Alberto Tringali
Gastrointestinal Endoscopy.2024; 99(6): 1021. CrossRef - Assessment of delayed bleeding after endoscopic submucosal dissection of early-stage gastrointestinal tumors in patients receiving direct oral anticoagulants
Mitsushige Sugimoto, Masaki Murata, Takashi Kawai
World Journal of Gastroenterology.2023; 29(19): 2916. CrossRef - Clinical practice guidelines for percutaneous endoscopic gastrostomy
Chung Hyun Tae, Ju Yup Lee, Moon Kyung Joo, Chan Hyuk Park, Eun Jeong Gong, Cheol Min Shin, Hyun Lim, Hyuk Soon Choi, Miyoung Choi, Sang Hoon Kim, Chul-Hyun Lim, Jeong-Sik Byeon, Ki-Nam Shim, Geun Am Song, Moon Sung Lee, Jong-Jae Park, Oh Young Lee
Clinical Endoscopy.2023; 56(4): 391. CrossRef - Clinical Practice Guideline for Percutaneous Endoscopic Gastrostomy
Chung Hyun Tae, Ju Yup Lee, Moon Kyung Joo, Chan Hyuk Park, Eun Jeong Gong, Cheol Min Shin, Hyun Lim, Hyuk Soon Choi, Miyoung Choi, Sang Hoon Kim, Chul-Hyun Lim, Jeong-Sik Byeon, Ki-Nam Shim, Geun Am Song, Moon Sung Lee, Jong-Jae Park, Oh Young Lee
The Korean Journal of Gastroenterology.2023; 82(3): 107. CrossRef - Predicting the Bleeding Risk for Patients on Anticoagulant Therapy Prior to Gastric Endoscopic Submucosal Dissection
Jie-Hyun Kim
Journal of Gastric Cancer.2022; 22(1): 1. CrossRef - Utility of a deep learning model and a clinical model for predicting bleeding after endoscopic submucosal dissection in patients with early gastric cancer
Ji Eun Na, Yeong Chan Lee, Tae Jun Kim, Hyuk Lee, Hong-Hee Won, Yang Won Min, Byung-Hoon Min, Jun Haeng Lee, Poong-Lyul Rhee, Jae J Kim
World Journal of Gastroenterology.2022; 28(24): 2721. CrossRef
- Clinical Practice Guideline for Percutaneous Endoscopic Gastrostomy
- 12,890 View
- 945 Download
- 9 Web of Science
- 10 Crossref

- Practical Approaches for High-Risk Surgical Patients with Acute Cholecystitis: The Percutaneous Approach versus Endoscopic Alternatives
- Rungsun Rerknimitr, Khanh Cong Pham
- Clin Endosc 2020;53(6):678-685. Published online January 9, 2020
- DOI: https://doi.org/10.5946/ce.2019.186

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - In high-risk surgical patients with acute cholecystitis who are not candidates for early laparoscopic cholecystectomy, gallbladder (GB) drainage is an alternative treatment option. Percutaneous transhepatic gallbladder drainage (PTGBD) is a recommended first line intervention because of its high efficacy and feasibility in most centers. However, with the advent of endoscopic accessories and technology, endoscopic GB drainage has been chosen as a more favorable choice by endoscopists. Endoscopic transpapillary gallbladder drainage (ETGBD) can be performed under either fluoroscopic or peroral cholangioscopic guidance via endoscopic retrograde cholangiopancreatography by the transpapillary placement of a long double-pigtail stent. In a patient with common bile duct stones, this procedure is accompanied with stone removal. ETGBD is especially useful for acute cholecystitis patients who are contraindicated for PTGBD or those with severe coagulopathy, thrombocytopenia, and abnormal anatomy. Moreover, the advantage of ETGBD is its preservation of the external GB structure. Thereby it would not disturb the future cholecystectomy. Recently, endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) using plastic, fully covered metallic, or lumen-apposing metal stents transmurally has emerged as a modality for GB drainage with higher technical and clinical success rates. EUS-GBD can provide a more permanent GB drainage than PTGBD and ETGBD.
-
Citations
Citations to this article as recorded by- Acute cholecystitis management in high-risk, critically ill, and unfit-for-surgery patients: the Italian Society of Emergency Surgery and Trauma (SICUT) guidelines
Federico Coccolini, Eugenio Cucinotta, Andrea Mingoli, Mauro Zago, Gaia Altieri, Alan Biloslavo, Roberto Caronna, Ismail Cengeli, Enrico Cicuttin, Roberto Cirocchi, Luigi Cobuccio, Gianluca Costa, Valerio Cozza, Camilla Cremonini, Giovanni Del Vecchio, Gi
Updates in Surgery.2024; 76(2): 331. CrossRef - Bile as a liquid biopsy matrix: potential applications and limitations
Maria Arechederra, Maria Rullán, Daniel Oyón, Matias A. Ávila, Jesús M. Urman, Carmen Berasain
Exploration of Digestive Diseases.2024; : 5. CrossRef - Endosonografische Drainage der Gallenblase wegen akuter Cholezystitis bei Patienten mit hohem Operationsrisiko
Markus Zachäus, Michael Bartels, Andreas Flade, Andreas Schubert-Hartmann, Regina Lamberts, Alireza Sepehri-Shamloo, Ulrich Paul Halm
Zentralblatt für Chirurgie - Zeitschrift für Allgemeine, Viszeral-, Thorax- und Gefäßchirurgie.2023; 148(02): 140. CrossRef - Retrospective comparison of clinical outcomes of ultrasound-guided percutaneous cholecystostomy in patients with and without coagulopathy: a single center’s experience
Hayato Yamahata, Minoru Yabuta, Mahbubur Rahman
Japanese Journal of Radiology.2023; 41(9): 1015. CrossRef - Acute cholecystitis: “There’s more than one way to skin a cat”!
Guido Costamagna
Digestive Endoscopy.2022; 34(1): 73. CrossRef - Ultimate outcomes of three modalities for non-surgical gallbladder drainage in acute cholecystitis with or without concomitant common bile duct stones
Wiriyaporn Ridtitid, Thanawat Luangsukrerk, Panida Piyachaturawat, Nicha Teeratorn, Phonthep Angsuwatcharakon, Pradermchai Kongkam, Rungsun Rerknimitr
Annals of Hepato-Biliary-Pancreatic Surgery.2022; 26(1): 104. CrossRef - Endosonography-Guided Versus Percutaneous Gallbladder Drainage Versus Cholecystectomy in Fragile Patients with Acute Cholecystitis—A High-Volume Center Study
Hayato Kurihara, Francesca M. Bunino, Alessandro Fugazza, Enrico Marrano, Giulia Mauri, Martina Ceolin, Ezio Lanza, Matteo Colombo, Antonio Facciorusso, Alessandro Repici, Andrea Anderloni
Medicina.2022; 58(11): 1647. CrossRef - A Case of Xanthogranulomatous Cholecystitis that was Difficult to Differentiate from Gallbladder Cancer after Long-term Placement of an Endoscopic Transpapillary Gallbladder Drainage Tube
Moeko KATO, Toshiro MASUDA, Takihiro KAMIO, Hiroshi TAKAMORI
Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association).2022; 83(10): 1800. CrossRef - Endoscopic Ultrasound-Guided Gallbladder Drainage Versus Percutaneous Transhepatic Gallbladder Drainage for Acute Cholecystitis with High Surgical Risk: An Up-to-Date Meta-Analysis and Systematic Review
Yunxiao Lyu, Ting Li, Bin Wang, Yunxiao Cheng, Liang Chen, Sicong Zhao
Journal of Laparoendoscopic & Advanced Surgical Techniques.2021; 31(11): 1232. CrossRef - Efficacy and safety of conversion of percutaneous cholecystostomy to endoscopic transpapillary gallbladder stenting in high-risk surgical patients
Hyung Ku Chon, Chan Park, Dong Eun Park, Tae Hyeon Kim
Hepatobiliary & Pancreatic Diseases International.2021; 20(5): 478. CrossRef - A Case of Iatrogenic Pseudoaneurysm Caused by EGBS and Penetrating the Cystic Duct
Takumi Habu, Akihiro Sako, Kotaro Nishida, Koichi Komatsu, Keiichi Arakawa, Takehito Maruyama, Shigeo Aoki, Hideyuki Mishima, Yuichi Matsui
The Japanese Journal of Gastroenterological Surgery.2021; 54(12): 869. CrossRef - Intraductal Ultrasonography Can Enhance the Success of Endoscopic Transpapillary Gallbladder Drainage in Patients with Acute Cholecystitis
Clement Chun Ho Wu, Christopher Jen Lock Khor
Clinical Endoscopy.2020; 53(2): 114. CrossRef - Clinical Evaluation of a Newly Developed Guidewire for Pancreatobiliary Endoscopy
Shigeto Ishii, Toshio Fujisawa, Hiroyuki Isayama, Shingo Asahara, Shingo Ogiwara, Hironao Okubo, Hisafumi Yamagata, Mako Ushio, Sho Takahashi, Hiroki Okawa, Wataru Yamagata, Yoshihiro Okawa, Akinori Suzuki, Yusuke Takasaki, Kazushige Ochiai, Ko Tomishima,
Journal of Clinical Medicine.2020; 9(12): 4059. CrossRef
- Acute cholecystitis management in high-risk, critically ill, and unfit-for-surgery patients: the Italian Society of Emergency Surgery and Trauma (SICUT) guidelines
- 6,321 View
- 368 Download
- 11 Web of Science
- 13 Crossref

Original Articles
- Effect of Aspiration Therapy on Obesity-Related Comorbidities: Systematic Review and Meta-Analysis
- Pichamol Jirapinyo, Diogo T. H. de Moura, Laura C. Horton, Christopher C. Thompson
- Clin Endosc 2020;53(6):686-697. Published online February 28, 2020
- DOI: https://doi.org/10.5946/ce.2019.181
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
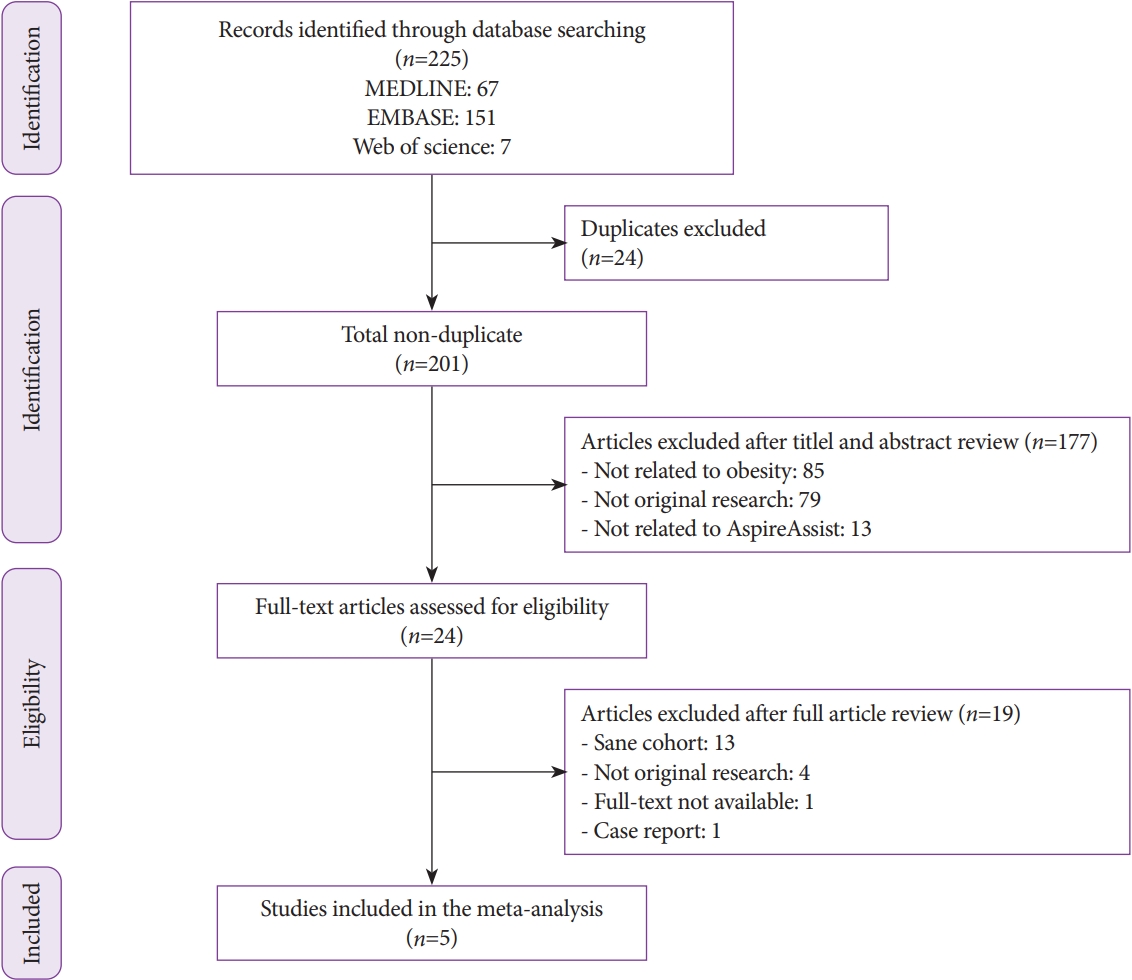
ePub - Background
/Aims: Aspiration therapy (AT) involves endoscopic placement of a gastrostomy tube with an external device that allows patients to drain 30% of ingested calories after meals. Its efficacy for inducing weight loss has been shown. This study aimed to assess the effect of AT on obesity-related comorbidities.
Methods
A meta-analysis of studies that assessed AT outcomes was conducted through December 2018. Primary outcomes were changes in comorbidities at 1 year following AT. Secondary outcomes were the amount of weight loss at up to 4 years and pooled serious adverse events (SAEs).
Results
Five studies with 590 patients were included. At 1 year, there were improvements in metabolic conditions: mean difference (MD) in systolic blood pressure: -7.8 (-10.7 – -4.9) mm Hg; MD in diastolic blood pressure: -5.1 (-7.0 – 3.2) mm Hg; MD in triglycerides: -15.8 (-24.0 – -7.6) mg/dL; MD in high-density lipoprotein: 3.6 (0.7–6.6) mg/dL; MD in hemoglobin A1c (HbA1c): -1.3 (-1.8 – -0.8) %; MD in aspartate transaminase: -2.7 (-4.1 – -1.3) U/L; MD in alanine transaminase: -7.5 (-9.8 – -5.2) U/L. At 1 (n=218), 2 (n=125), 3 (n=46), and 4 (n=27) years, the patients experienced 17.8%, 18.3%, 19.1%, and 18.6% total weight loss (TWL), corresponding to 46.3%, 46.2%, 48.0%, and 48.7% excess weight loss (EWL) (p<0.0001 for all). Subgroup analysis of 2 randomized controlled trials (n=225) showed that AT patients lost more weight than did controls by 11.6 (6.5–16.7) %TWL and 25.6 (16.0–35.3) %EWL and experienced greater improvement in HbA1c and alanine transaminase by 1.3 (0.8–1.8) % and 9.0 (3.9–14.0) U/L. The pooled SAE rate was 4.1%.
Conclusions
Obesity-related comorbidities significantly improved at 1 year following AT. Additionally, a subgroup of patients who continued to use AT appeared to experience significant weight loss that persisted up to at least 4 years. -
Citations
Citations to this article as recorded by- Update on Endoscopic Treatments for Obesity
Fernanda Pessorrusso, Sagar V. Mehta, Shelby Sullivan
Current Obesity Reports.2024; 13(2): 364. CrossRef - Role of endoscopic duodenojejunal bypass liner in obesity management and glycemic control
Willian Ferreira Igi, Victor Lira de Oliveira, Ayah Matar, Diogo Turiani Hourneaux de Moura
Clinical Endoscopy.2024; 57(3): 309. CrossRef - Updates in Endoscopic Bariatric and Metabolic Therapies
Hammad Qureshi, Naba Saeed, Manol Jovani
Journal of Clinical Medicine.2023; 12(3): 1126. CrossRef - The Role Bariatric Surgery and Endobariatric Therapies in Nonalcoholic Steatohepatitis
Aaron Yeoh, Robert Wong, Ashwani K. Singal
Clinics in Liver Disease.2023; 27(2): 413. CrossRef - Obesity management for cardiovascular disease prevention
Rama Hritani, Mahmoud Al Rifai, Anurag Mehta, Charles German
Obesity Pillars.2023; 7: 100069. CrossRef - Overview on the endoscopic treatment for obesity: A review
Maheeba Abdulla, Nafeesa Mohammed, Jehad AlQamish
World Journal of Gastroenterology.2023; 29(40): 5526. CrossRef - Endoscopic removal of a weight-loss device with stoma closure using a tack-and-suture device
Areebah Waseem, Joseph Wawrzynski, Daniel B. Maselli, Ashley Kucera, Chase Wooley, Christopher McGowan
VideoGIE.2023; 8(11): 441. CrossRef - Effect of Endoscopic Bariatric and Metabolic Therapies on Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis
Pichamol Jirapinyo, Thomas R. McCarty, Russell D. Dolan, Raj Shah, Christopher C. Thompson
Clinical Gastroenterology and Hepatology.2022; 20(3): 511. CrossRef - Endobariatrics: well past infancy and maturing rapidly
Shreesh Shrestha, Esha Shrestha, Tilak Shah
Current Opinion in Gastroenterology.2022; 38(6): 592. CrossRef - Advances in endobariatrics: past, present, and future
Abhishek Shenoy, Allison R Schulman
Gastroenterology Report.2022;[Epub] CrossRef - Various Novel and Emerging Technologies in Endoscopic Bariatric and Metabolic Treatments
Hee Kyong Na, Diogo Turiani Hourneaux De Moura
Clinical Endoscopy.2021; 54(1): 25. CrossRef - Endoscopic Sleeve Gastroplasty (ESG) for High-Risk Patients, High Body Mass Index (> 50 kg/m2) Patients, and Contraindication to Abdominal Surgery
Renjie Li, Wilfried Veltzke-Schlieker, Andreas Adler, Maximilian Specht, Wael Eskander, Mahmoud Ismail, Harun Badakhshi, Manoel Passos Galvao, Ricardo Zorron
Obesity Surgery.2021; 31(8): 3400. CrossRef - Obesity Primer for the Practicing Gastroenterologist
Pichamol Jirapinyo, Christopher C. Thompson
American Journal of Gastroenterology.2021; 116(5): 918. CrossRef - Bariatric and metabolic endoscopy: impact on obesity and related comorbidities
Amit Mehta, Reem Z. Sharaiha
Therapeutic Advances in Gastrointestinal Endoscopy.2021; 14: 263177452110191. CrossRef - Advanced endoscopic gastrointestinal techniques for the bariatric patient: implications for the anesthesia provider
Andrew Kim, Joshua A. Spiro, Thomas J. Hatzidais, Norman D. Randolph, Rosie Q. Li, Diana Ayubcha, Mark S. Weiss
Current Opinion in Anaesthesiology.2021; 34(4): 490. CrossRef - Preparing for the NASH Epidemic: A Call to Action
Fasiha Kanwal, Jay H. Shubrook, Zobair Younossi, Yamini Natarajan, Elisabetta Bugianesi, Mary E. Rinella, Stephen A. Harrison, Christos Mantzoros, Kim Pfotenhauer, Samuel Klein, Robert H. Eckel, Davida Kruger, Hashem El-Serag, Kenneth Cusi
Gastroenterology.2021; 161(3): 1030. CrossRef - Preparing for the NASH epidemic: A call to action
Fasiha Kanwal, Jay H. Shubrook, Zobair Younossi, Yamini Natarajan, Elisabetta Bugianesi, Mary E. Rinella, Stephen A. Harrison, Christos Mantzoros, Kim Pfotenhauer, Samuel Klein, Robert H. Eckel, Davida Kruger, Hashem El-Serag, Kenneth Cusi
Metabolism.2021; 122: 154822. CrossRef - Preparing for the NASH epidemic: A call to action
Fasiha Kanwal, Jay H. Shubrook, Zobair Younossi, Yamini Natarajan, Elisabetta Bugianesi, Mary E. Rinella, Stephen A. Harrison, Christos Mantzoros, Kim Pfotenhauer, Samuel Klein, Robert H. Eckel, Davida Kruger, Hashem El‐Serag, Kenneth Cusi
Obesity.2021; 29(9): 1401. CrossRef - Preparing for the NASH Epidemic: A Call to Action
Fasiha Kanwal, Jay H. Shubrook, Zobair Younossi, Yamini Natarajan, Elisabetta Bugianesi, Mary E. Rinella, Stephen A. Harrison, Christos Mantzoros, Kim Pfotenhauer, Samuel Klein, Robert H. Eckel, Davida Kruger, Hashem El-Serag, Kenneth Cusi
Diabetes Care.2021; 44(9): 2162. CrossRef - Endobariatrics and Metabolic Endoscopy: Can We Solve the Obesity Epidemic with Our Scope?
Jad Farha, Shahem Abbarh, Zadid Haq, Mohamad I. Itani, Andreas Oberbach, Vivek Kumbhari, Dilhana Badurdeen
Current Gastroenterology Reports.2020;[Epub] CrossRef
- Update on Endoscopic Treatments for Obesity
- 5,664 View
- 133 Download
- 19 Web of Science
- 20 Crossref

- Quality Indicators for the Detection of Helicobacter pylori-Negative Early Gastric Cancer: A Retrospective Observational Study
- Fumiaki Ishibashi, Konomi Kobayashi, Keita Fukushima, Ryu Tanaka, Tomohiro Kawakami, Junko Kato, Kazuaki Sugihara
- Clin Endosc 2020;53(6):698-704. Published online March 13, 2020
- DOI: https://doi.org/10.5946/ce.2019.203
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: While Helicobacter pylori (HP)-negative gastric cancer is frequently reported, little is known about the predictors for detecting HP-negative early gastric cancer (EGC). We aimed to evaluate the predictors for the detection of HP-negative EGC.
Methods
We retrospectively reviewed 13,477 consecutive asymptomatic cases where upper endoscopy was performed by nine physicians from April 2017 to March 2019 and analyzed the detection rate of high-risk lesions (HRLs), including EGC, tubular adenoma, and lymphoma, according to the status of HP infection. The observation time was corrected for multiple regression analyses.
Results
For all physicians, the average observation time for screening HP-eradicated and -naïve patients was shorter than that for screening HP-positive patients (p<0.05). Multiple regression analyses revealed that the observation time in the three groups was an independent predictor for detecting HRLs in HP-eradicated patients (p=0.03106, 0.01263, and 0.02485, respectively), while experience of endoscopy was an independent predictor for detecting HRLs in HP-naïve patients (p=0.02638).
Conclusions
While observation time during screening endoscopy was a quality indicator for detecting HRLs in HP-eradicated patients, experience of endoscopy was a quality indicator for detecting HRLs in HP-naïve patients. -
Citations
Citations to this article as recorded by- Exploring quality indicators for the detection of Helicobacter pylori-naïve gastric cancer: a cross-sectional nationwide survey
Fumiaki Ishibashi, Toshiaki Hirasawa, Hiroya Ueyama, Yohei Minato, Sho Suzuki
Clinical Endoscopy.2023; 56(4): 460. CrossRef - A Close Follow-Up Strategy in the Short Period of Time after Helicobacter pylori Eradication Contributes to Earlier Detection of Gastric Cancer
Fumiaki Ishibashi, Sho Suzuki, Mizuki Nagai, Kentaro Mochida, Konomi Kobayashi, Tetsuo Morishita
Digestion.2023; 104(3): 165. CrossRef - Endoscopic diagnosis of early gastric cancer
Dong Chan Joo, Gwang Ha Kim
Journal of the Korean Medical Association.2022; 65(5): 267. CrossRef - Risk factors for early gastric cancer: focus on Helicobacter pylori gastritis
Hee Seok Moon
Journal of the Korean Medical Association.2022; 65(5): 259. CrossRef - Tailored eradication strategy vs concomitant therapy for Helicobacter pylori eradication treatment in Korean patients
Youn I Choi, Jun-Won Chung, Kyoung Oh Kim, Kwang An Kwon, Yoon Jae Kim, Jung Ho Kim, Ja Young Seo, Dong Kyun Park
World Journal of Gastroenterology.2021; 27(31): 5247. CrossRef - Quality management system for screening esophagogastroduodenoscopy improves detection of Helicobacter pylori-negative interval gastric cancer
Fumiaki Ishibashi, Konomi Kobayashi, Tomohiro Kawakami, Ryu Tanaka, Kazuaki Sugihara, Satoshi Baba
Endoscopy International Open.2021; 09(12): E1900. CrossRef - Quality Indicator for Gastric Cancer Detection Based on Helicobacter pylori Status
Jae Myung Park
Clinical Endoscopy.2020; 53(6): 629. CrossRef
- Exploring quality indicators for the detection of Helicobacter pylori-naïve gastric cancer: a cross-sectional nationwide survey
- 4,936 View
- 180 Download
- 7 Web of Science
- 7 Crossref

- Clinical Outcomes of Percutaneous Endoscopic Gastrostomy in the Surgical Intensive Care Unit
- Gyu Young Pih, Hee Kyong Na, Suk-Kyung Hong, Ji Yong Ahn, Jeong Hoon Lee, Kee Wook Jung, Do Hoon Kim, Kee Don Choi, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung
- Clin Endosc 2020;53(6):705-716. Published online March 31, 2020
- DOI: https://doi.org/10.5946/ce.2019.196

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Percutaneous endoscopic gastrostomy (PEG) is usually performed on patients with chronic underlying diseases in the general ward (GW). This study evaluated the clinical outcomes of PEG performed on patients in the surgical intensive care unit (SICU) compared with those of PEG performed in the GW.
Methods
The medical records of 27 patients in the SICU and 263 in the GW, who underwent PEG between January 2013 and July 2017, were retrospectively reviewed.
Results
The median age of the 27 SICU patients was 66 years, and their median body mass index was 21.1 kg/m2. In the SICU group, the median baseline Sequential Organ Failure Assessment (SOFA) score was 4, and the median Acute Physiology and Chronic Health Evaluation II (APACHE II) score was 16. The median interval between surgery and PEG in SICU patients was 30 days, with a PEG failure rate of 3.7%. Acute complications in SICU patients included bleeding (7.4%) and ileus (11.1%), while chronic complications included aspiration pneumonia (7.4%) and tube obstruction (3.7%). The rates of acute and chronic complications did not differ significantly between the SICU and GW groups. The 30-day mortality rate was 14.8% in SICU patients and 5.3% in GW patients (p=0.073).
Conclusions
PEG is a safe and feasible method of enteral feeding for critically ill patients who require ICU care after surgery. -
Citations
Citations to this article as recorded by- Clinical Practice Guideline for Percutaneous Endoscopic Gastrostomy
Chung Hyun Tae, Ju Yup Lee, Moon Kyung Joo, Chan Hyuk Park, Eun Jeong Gong, Cheol Min Shin, Hyun Lim, Hyuk Soon Choi, Miyoung Choi, Sang Hoon Kim, Chul-Hyun Lim, Jeong-Sik Byeon, Ki-Nam Shim, Geun Am Song, Moon Sung Lee, Jong-Jae Park, Oh Young Lee
Gut and Liver.2024; 18(1): 10. CrossRef - A Multicenter Survey of Percutaneous Endoscopic Gastrostomy in 2019 at Korean Medical Institutions
Jun Woo Park, Tae Gyun Kim, Kwang Bum Cho, Jeong Seok Kim, Jin Woong Cho, Jung Won Jeon, Sun Gyo Lim, Chan Gyoo Kim, Hong Jun Park, Tae Jun Kim, Eun Sun Kim, Su Jin Jeong, Yong Hwan Kwon
Gut and Liver.2024; 18(1): 77. CrossRef - Clinical practice guidelines for percutaneous endoscopic gastrostomy
Chung Hyun Tae, Ju Yup Lee, Moon Kyung Joo, Chan Hyuk Park, Eun Jeong Gong, Cheol Min Shin, Hyun Lim, Hyuk Soon Choi, Miyoung Choi, Sang Hoon Kim, Chul-Hyun Lim, Jeong-Sik Byeon, Ki-Nam Shim, Geun Am Song, Moon Sung Lee, Jong-Jae Park, Oh Young Lee
Clinical Endoscopy.2023; 56(4): 391. CrossRef - Risk factors and natural history of bedside percutaneous endoscopic versus fluoroscopy-guided gastrostomy tubes in intensive care unit patients
Lucy Ching Chau, Ryan Soheim, Michael Dix, Sarah Chung, Nadia Obeid, Arielle Hodari-Gupta, Cletus Stanton
Surgical Endoscopy.2023; 37(11): 8742. CrossRef - Clinical Practice Guideline for Percutaneous Endoscopic Gastrostomy
Chung Hyun Tae, Ju Yup Lee, Moon Kyung Joo, Chan Hyuk Park, Eun Jeong Gong, Cheol Min Shin, Hyun Lim, Hyuk Soon Choi, Miyoung Choi, Sang Hoon Kim, Chul-Hyun Lim, Jeong-Sik Byeon, Ki-Nam Shim, Geun Am Song, Moon Sung Lee, Jong-Jae Park, Oh Young Lee
The Korean Journal of Gastroenterology.2023; 82(3): 107. CrossRef - Relative contraindications to percutaneous endoscopic gastrostomy (review of literature)
Yu. O. Zharikov, M. Kh. Gurtsiev, S. Zh. Antonyan, S. F. Askerova, E. I. Chairkina, P. A. Yartsev
Grekov's Bulletin of Surgery.2022; 180(6): 105. CrossRef
- Clinical Practice Guideline for Percutaneous Endoscopic Gastrostomy
- 4,947 View
- 128 Download
- 6 Web of Science
- 6 Crossref

- The Efficacy of 4 Liters of Clear Liquids for Small Bowel Preparation Prior to Video Capsule Endoscopy
- Nicholas Placone, Runalia Bahar, Surinder Mann
- Clin Endosc 2020;53(6):713-718. Published online March 31, 2020
- DOI: https://doi.org/10.5946/ce.2019.213
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Optimal small bowel (SB) preparation for video capsule endoscopy (VCE) is controversial. Our study aimed to support the use of a specified volume of 4 liters of clear liquids for bowel preparation for VCE.
Methods
A retrospective review of 284 patients who underwent SB preparation with 2 liters of polyethylene glycol (PEG) and 284 patients who had 4 liters of clear liquid preparation. We analyzed image quality, endoscopic findings, completion rate, and transit times.
Results
The 4-liter clear liquid group had significantly higher mean image quality scores when compared to the PEG group (2.908±0.77 to 2.669±0.64, p<0.0001), as well as more studies with adequate preparation (72% to 64%, p=0.0214). Although the PEG group had more endoscopic findings on VCE (40% to 23%, p<0.0001), there was a significant difference in the indications for the procedure between the groups. There was no difference in the capsule completion rate or SB transit time.
Conclusions
Our data demonstrate significantly higher mean image quality scores when using a specified volume of 4 liters of clear liquid compared to 2 liters of PEG. This study supports the growing evidence of the effectiveness of a 4-liter clear liquid SB preparation as opposed to PEG for VCE. -
Citations
Citations to this article as recorded by- Small bowel cleansing for capsule endoscopy, systematic review and meta- analysis: Timing is the real issue
Clelia Marmo, Maria Elena Riccioni, Marco Pennazio, Giulio Antonelli, Cristiano Spada, Guido Costamagna
Digestive and Liver Disease.2023; 55(4): 454. CrossRef - Disease surveillance evaluation of primary small-bowel follicular lymphoma using capsule endoscopy images based on a deep convolutional neural network (with video)
Akihiko Sumioka, Akiyoshi Tsuboi, Shiro Oka, Yusuke Kato, Yuka Matsubara, Issei Hirata, Hidehiko Takigawa, Ryo Yuge, Fumio Shimamoto, Tomohiro Tada, Shinji Tanaka
Gastrointestinal Endoscopy.2023; 98(6): 968. CrossRef - A colorectal cancer missed by colon capsule endoscopy: a case report
C. MacLeod, R. Oliphant, J. G. Docherty, A. J. M. Watson
BMC Gastroenterology.2022;[Epub] CrossRef - Value of the diving method for capsule endoscopy in the examination of small-intestinal disease: a prospective randomized controlled trial
Xianhui Zeng, Liansong Ye, Jianrong Liu, Xianglei Yuan, Shan Jiang, Minghui Huang, Xiujiang Huang, Chengwei Tang, Bing Hu
Gastrointestinal Endoscopy.2021; 94(4): 795. CrossRef - Bowel Preparation for Small Bowel Capsule Endoscopy: Is There Still a Role for Polyethylene Glycol?
Paul Collins, Neil Haslam, Anthony Morris, Thomas Skouras, Ashley Bond
Journal of Digestive Endoscopy.2020; 11(03): 215. CrossRef - Capsule endoscopy – Recent developments and future directions.
Stefania Zammit Chetcuti, Reena Sidhu
Expert Review of Gastroenterology & Hepatology.2020;[Epub] CrossRef - Ideal Method for Small Bowel Preparation before Video Capsule Endoscopy
Jun Lee, Shai Friedland
Clinical Endoscopy.2020; 53(6): 631. CrossRef
- Small bowel cleansing for capsule endoscopy, systematic review and meta- analysis: Timing is the real issue
- 3,865 View
- 94 Download
- 6 Web of Science
- 7 Crossref

- Positive Fecal Occult Blood Test is a Predictive Factor for Gastrointestinal Bleeding after Capsule Endoscopy in Patients with Unexplained Iron Deficiency Anemia: A Korean Multicenter CAPENTRY Study
- Ji Young Chang, Chang Mo Moon, Ki-Nam Shim, Dae Young Cheung, Hyun Seok Lee, Yun Jeong Lim, Seong Ran Jeon, Soo Jung Park, Kyeong Ok Kim, Hyun Joo Song, Hyun Joo Jang, Ji Hyun Kim
- Clin Endosc 2020;53(6):719-726. Published online November 6, 2020
- DOI: https://doi.org/10.5946/ce.2019.149
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Capsule endoscopy (CE) is recommended as the primary method for the evaluation of unexplained anemia. This study aimed to assess the diagnostic yield of CE in patients with unexplained iron deficiency anemia (IDA) without overt bleeding, and to evaluate their long-term outcomes and related clinical factors.
Methods
Data of patients who underwent CE for the evaluation of IDA were reviewed from a CE registry in Korea. Additional clinical data were collected by the involved investigators of each hospital through a review of medical records.
Results
Among a total of 144 patients, the diagnostic yield of CE was 34%. Gastrointestinal (GI) bleeding was found in 6.3% (n=9) of the patients (occult bleeding in four patients and overt bleeding in five patients) during a mean follow-up of 17.8 months. Patients with a positive fecal occult blood test (FOBT) result at the initial diagnosis had a higher rate of GI bleeding after CE (p=0.004). In addition, a positive FOBT result was the only independent predictive factor for GI bleeding (hazard ratio, 5.30; 95% confidence interval, 1.41–19.85; p=0.013).
Conclusions
Positive FOBT is a predictive factor for GI bleeding during follow-up after CE in patients with unexplained IDA without overt bleeding. Thus, patients with positive FOBT need to be more closely followed up. -
Citations
Citations to this article as recorded by- Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Guideline – Update 2022
Marco Pennazio, Emanuele Rondonotti, Edward J. Despott, Xavier Dray, Martin Keuchel, Tom Moreels, David S. Sanders, Cristiano Spada, Cristina Carretero, Pablo Cortegoso Valdivia, Luca Elli, Lorenzo Fuccio, Begona Gonzalez Suarez, Anastasios Koulaouzidis,
Endoscopy.2023; 55(01): 58. CrossRef - Predictive Model for Positive Video Capsule Endoscopy in Iron Deficiency Anemia
Shadi Hamdeh, Jihan Fathallah, Hui Zhang, Amber Charoen, Barakat Aburajab Altamimi, Florence-Damilola Odufalu, Devashree Dave, Amer El Sayed, Laura R. Glick, Scott Grisolano, Christine Hachem, Muhammad Bader Hammami, Khaldoun Haj Mahmoud, Alexander N. Lev
Digestive Diseases and Sciences.2023; 68(7): 3083. CrossRef - Predictors of Positive Video Capsule Endoscopy Findings for Chronic Unexplained Abdominal Pain: Single-Center Retrospective Study and Meta-Analysis
Wonshik Kim, Beomjae Lee, Ahyoung Yoo, Seunghan Kim, Moonkyung Joo, Jong-Jae Park
Diagnostics.2021; 11(11): 2123. CrossRef
- Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Guideline – Update 2022
- 5,585 View
- 115 Download
- 4 Web of Science
- 3 Crossref

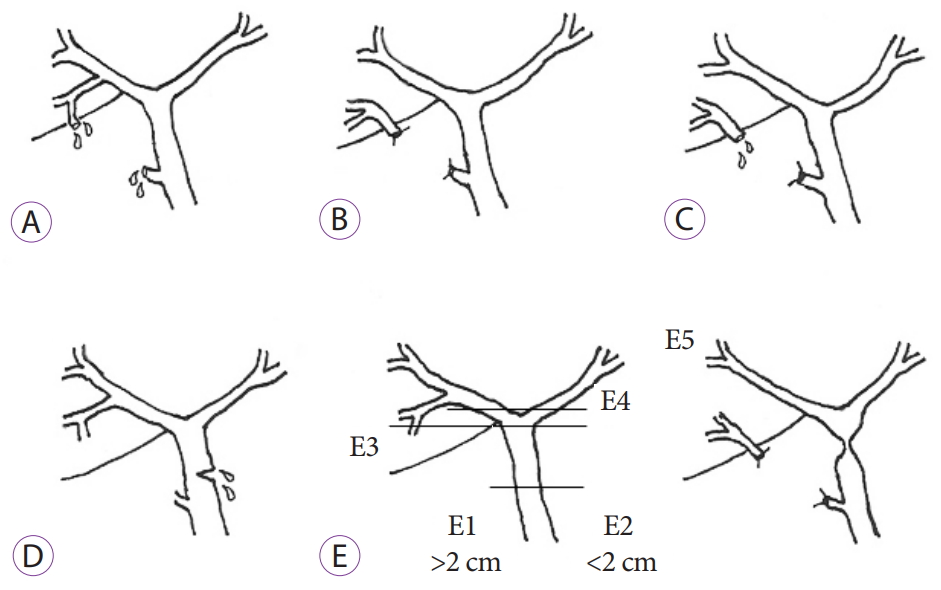
- Outcomes of Endoscopic Management among Patients with Bile Leak of Various Etiologies at a Tertiary Care Center
- Suprabhat Giri, Sridhar Sundaram, Harish Darak, Sanjay Kumar, Shobna Bhatia
- Clin Endosc 2020;53(6):727-734. Published online August 21, 2020
- DOI: https://doi.org/10.5946/ce.2020.017
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Bile leak is a common complication of cholecystectomy, and it is also observed in other conditions such as ruptured liver abscess, hydatid cyst, and trauma. Endoscopic retrograde cholangiopancreatography (ERCP) is the first-line management for such conditions. However, studies on the outcomes of endoscopic management for bile leaks with etiologies other than post-cholecystectomy injury are extremely limited.
Methods
We conducted a retrospective review of patients with symptomatic bile leak who were referred to a tertiary care center and who underwent ERCP between April 2016 and April 2019. The primary outcome was complete symptomatic resolution without extravasation of the contrast medium during the second ERCP conducted after 6 weeks.
Results
In total, 71 patients presented with symptomatic bile leak. The etiologies of bile leak were post-cholecystectomy injury in 34 (47.8%), liver abscess in 20 (28.1%), and post-hydatid cyst surgery in 11 (15.4%) patients. All patients were managed with ERCP, sphincterotomy, and stent placement for 6 weeks, except for one who underwent surgery. The primary outcome was achieved in 65 (91.5%) of 71 patients. There was no significant difference in terms of outcome in relation to the interval between the diagnosis of bile leak and ERCP.
Conclusions
Most patients with bile leak can be successfully managed with ERCP even when performed on an elective basis. -
Citations
Citations to this article as recorded by- A critical appraisal of the ISGLS definition of biliary leakage after liver resection
Svenja Sliwinski, Jan Heil, Josephine Franz, Hanan El Youzouri, Michael Heise, Wolf O. Bechstein, Andreas A. Schnitzbauer
Langenbeck's Archives of Surgery.2023;[Epub] CrossRef - Progress in ERCP Treatment of Biliary Complications in Patients with Hepatic Echinococcosis
燕泽 林
Advances in Clinical Medicine.2023; 13(03): 4013. CrossRef - Diagnosis and management of bile leaks after severe liver injury: A Trauma Association of Canada multicenter study
Morgan Schellenberg, Chad G. Ball, Natthida Owattanapanich, Brent Emigh, Patrick B. Murphy, Bradley Moffat, Brett Mador, Andrew Beckett, Jennie Lee, Emilie Joos, Samuel Minor, Matt Strickland, Kenji Inaba
Journal of Trauma and Acute Care Surgery.2022; 93(6): 813. CrossRef - Postoperative bile leakage caused by intrahepatic duct injury during right hemicolectomy
Jaram Lee, Ook Song, Hyeong-Min Park, Soo Young Lee, Chang Hyun Kim, Hyeong Rok Kim
Medicine.2021; 100(46): e27877. CrossRef - The Need for a Better-Designed Study of the Outcomes of Endoscopic Management of Bile Leak
Hyung Ku Chon, Eun Ji Shin, Seong-Hun Kim
Clinical Endoscopy.2020; 53(6): 633. CrossRef
- A critical appraisal of the ISGLS definition of biliary leakage after liver resection
- 4,503 View
- 116 Download
- 4 Web of Science
- 5 Crossref

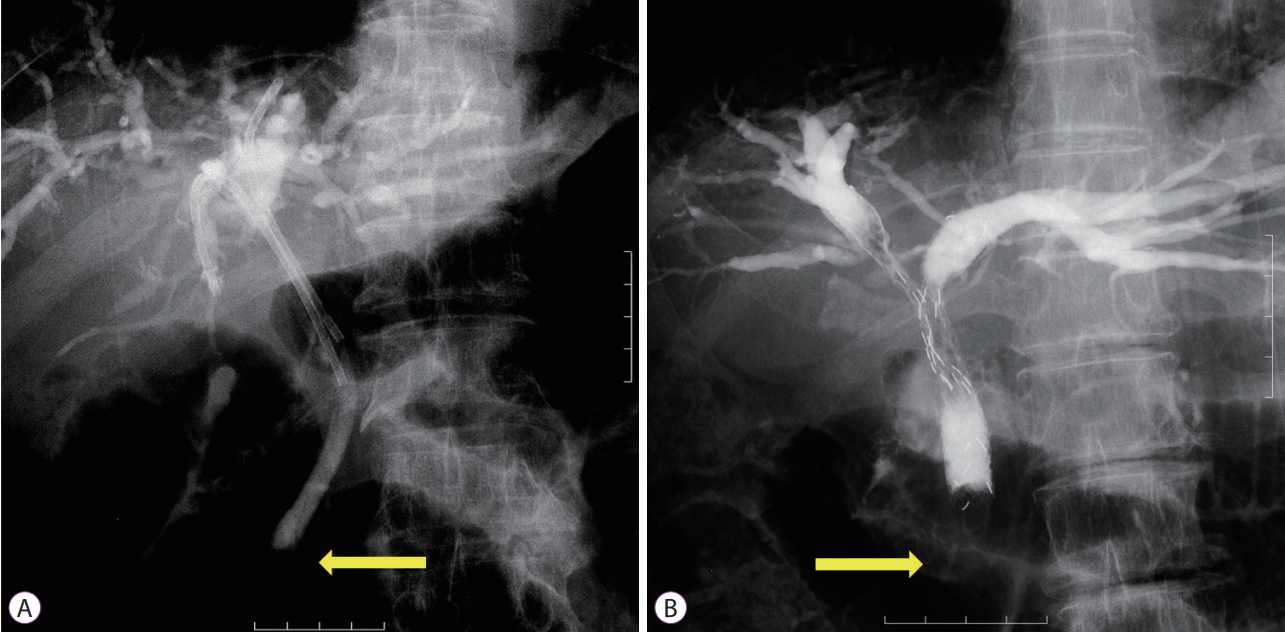
- Inside Plastic Stents versus Metal Stents for Treating Unresectable Malignant Perihilar Biliary Obstructions: A Retrospective Comparative Study
- Yoshihide Kanno, Shinsuke Koshita, Takahisa Ogawa, Hiroaki Kusunose, Kaori Masu, Toshitaka Sakai, Keisuke Yonamine, Kazuaki Miyamoto, Toji Murabayashi, Fumisato Kozakai, Jun Horaguchi, Yutaka Noda, Kei Ito
- Clin Endosc 2020;53(6):735-742. Published online March 4, 2020
- DOI: https://doi.org/10.5946/ce.2020.003
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The aim of this study was to evaluate outcomes of inside plastic stents (iPSs) versus those of metal stents (MSs) for treating unresectable perihilar malignant obstructions.
Methods
For all patients who underwent endoscopic suprapapillary placement of iPS(s) or MS(s) as the first permanent biliary drainage for unresectable malignant perihilar obstructions between January 2014 and August 2019, clinical outcomes using iPSs (n=20) and MSs (n=85), including clinical efficacy, adverse events, and time to recurrence of biliary obstruction (RBO), were retrospectively evaluated.
Results
There were no differences in clinical effectiveness (95% for the iPS group vs. 92% for the MS group, p=1.00). Procedure-related adverse events, including pancreatitis, acute cholangitis, acute cholecystitis, and death, were observed for 8% of the MS group, although no patient in the iPS group developed such adverse events. The median time to RBO was 561 days (95% confidence interval, 0–1,186 days) for iPSs and 209 days (127–291 days) for MSs, showing a significant difference (p=0.008).
Conclusions
Time to RBO after iPS placement was significantly longer than that after MS placement. IPSs, which are removable, unlike MSs, were an acceptable option. -
Citations
Citations to this article as recorded by- Comparison of unilateral and bilateral intraductal plastic stent placement for unresectable malignant hilar biliary obstruction: A propensity score‐matched cohort analysis
Mitsuru Okuno, Keisuke Iwata, Tsuyoshi Mukai, Yuhei Iwasa, Shinya Uemura, Kensaku Yoshida, Akinori Maruta, Takuji Iwashita, Ichiro Yasuda, Masahito Shimizu
Journal of Hepato-Biliary-Pancreatic Sciences.2024; 31(4): 284. CrossRef - A Novel Method of Calculating the Drained Liver Volume Using a 3D Volume Analyzer for Biliary Drainage of Unresectable Malignant Hilar Biliary Obstruction
Naoto Imagawa, Mitsuharu Fukasawa, Shinichi Takano, Satoshi Kawakami, Yoshimitsu Fukasawa, Hiroyuki Hasegawa, Natsuhiko Kuratomi, Shota Harai, Naruki Shimamura, Dai Yoshimura, Shoji Kobayashi, Takashi Yoshida, Mitsuaki Sato, Yuichiro Suzuki, Nobuyuki Enom
Digestive Diseases and Sciences.2024; 69(3): 969. CrossRef - Partial Stent-in-Stent Method with an Uncovered Self-Expandable Metallic Stent for Unresectable Malignant Hilar Bile Duct Obstruction
Takuya Shimosaka, Yohei Takeda, Taro Yamashita, Yuta Seki, Shiho Kawahara, Takayuki Hirai, Noriyuki Suto, Yuri Sakamoto, Wataru Hamamoto, Hiroki Koda, Takumi Onoyama, Kazuya Matsumoto, Kazuo Yashima, Hajime Isomoto, Naoyuki Yamaguchi
Journal of Clinical Medicine.2024; 13(3): 820. CrossRef - Current status of preoperative endoscopic biliary drainage for distal and hilar biliary obstruction
Hirotoshi Ishiwatari, Junya Sato, Hiroki Sakamoto, Takuya Doi, Hiroyuki Ono
Digestive Endoscopy.2024;[Epub] CrossRef - Biliary drainage in hilar and perihilar cholangiocarcinoma: 25-year experience at a tertiary cancer center
Ahmad Al Nakshabandi, Faisal S. Ali, Iyad Albustami, Hyunsoo Hwang, Wei Qiao, Nicole C. Johnston, Abdullah S. Shaikh, Emmanuel Coronel, Phillip S. Ge, William Ross, Brian Weston, Jeffrey H. Lee
Gastrointestinal Endoscopy.2024; 99(6): 938. CrossRef - Clinical Outcomes of Inside Stents and Conventional Plastic Stents as Bridge-to-Surgery Options for Malignant Hilar Biliary Obstruction
Hirotoshi Ishiwatari, Takanori Kawabata, Hiroki Kawashima, Yousuke Nakai, Shin Miura, Hironari Kato, Hideyuki Shiomi, Nao Fujimori, Takeshi Ogura, Osamu Inatomi, Kensuke Kubota, Toshio Fujisawa, Mamoru Takenaka, Hiroshi Mori, Kensaku Noguchi, Yuki Fujii,
Digestive Diseases and Sciences.2023; 68(4): 1139. CrossRef - Development of novel biliary metal stent with coil-spring structure and its application in vivo swine biliary stricture model
In Rae Cho, Sang Hyub Lee, Jin Ho Choi, Namyoung Park, Min Woo Lee, Joo Seong Kim, Seok Jeong, Don Haeng Lee, Tae-Won Jeong, Byoung-Yun Ki, Woo Hyun Paik, Ji Kon Ryu, Yong-Tae Kim
Frontiers in Oncology.2023;[Epub] CrossRef - Endoscopic retrograde stent drainage therapies for malignant biliary obstruction: the distal opening of stent location above or across the duodenal papilla? A systematic review and meta-analysis
Dong Fang, Yi Han, Chenglin Zhu, Zhenwang Shi, Deming Bao, Liming Wang, Qin Xu
Scandinavian Journal of Gastroenterology.2023; 58(9): 1071. CrossRef - Suprapapillary placement of plastic versus metal stents for malignant biliary hilar obstructions: a multicenter, randomized trial
Yoshihide Kanno, Kei Ito, Kazunari Nakahara, Shinya Kawaguchi, Yoshiharu Masaki, Toru Okuzono, Hironari Kato, Masaki Kuwatani, Shotaro Ishii, Toji Murabayashi, Sho Hasegawa, Masatsugu Nagahama, Yuji Iwashita, Yosuke Michikawa, Shuzo Terada, Yujiro Kawakam
Gastrointestinal Endoscopy.2023; 98(2): 211. CrossRef - Déjà vu but with a different conclusion
Richard Kozarek
Gastrointestinal Endoscopy.2023; 98(5): 787. CrossRef - Utility of bilateral intraductal plastic stent for malignant hilar biliary obstruction compared with bilateral self-expandable metal stent: a propensity score–matched cohort analysis
Mitsuru Okuno, Keisuke Iwata, Takuji Iwashita, Tsuyoshi Mukai, Kota Shimojo, Yosuke Ohashi, Yuhei Iwasa, Akihiko Senju, Shota Iwata, Ryuichi Tezuka, Hironao Ichikawa, Naoki Mita, Shinya Uemura, Kensaku Yoshida, Akinori Maruta, Eiichi Tomita, Ichiro Yasuda
Gastrointestinal Endoscopy.2023; 98(5): 776. CrossRef - Endoscopic nasobiliary drainage versus endoscopic biliary stenting for preoperative biliary drainage in patients with malignant hilar biliary obstruction: Propensity score‐matched multicenter comparative study
Hirotoshi Ishiwatari, Takanori Kawabata, Hiroki Kawashima, Yousuke Nakai, Shin Miura, Hironari Kato, Hideyuki Shiomi, Nao Fujimori, Takeshi Ogura, Osamu Inatomi, Kensuke Kubota, Toshio Fujisawa, Mamoru Takenaka, Hiroshi Mori, Kensaku Noguchi, Yuki Fujii,
Digestive Endoscopy.2023;[Epub] CrossRef - Cross‐wired metal stents for endoscopic bilateral stent‐in‐stent deployment in malignant hilar biliary obstruction: A multicenter, single‐arm, prospective study
Kentaro Yamao, Takeshi Ogura, Hideyuki Shiomi, Takaaki Eguchi, Hisakazu Matsumoto, Zhao Liang Li, Hiroaki Hashimoto, Yasutaka Chiba, Mamoru Takenaka, Tomohiro Watanabe, Masatoshi Kudo, Tsuyoshi Sanuki
DEN Open.2022;[Epub] CrossRef - Recent advances regarding endoscopic biliary drainage for unresectable malignant hilar biliary obstruction
Hironari Kato, Kazuyuki Matsumoto, Hiroyuki Okada
DEN Open.2022;[Epub] CrossRef - Double‐scope method is helpful to rescue a retrieval thread attached to a stent caught on the duodenoscope forceps elevator
Kengo Matsumoto, Dai Nakamatsu, Tsutomu Nishida
Digestive Endoscopy.2022;[Epub] CrossRef - The feasibility of percutaneous transhepatic gallbladder aspiration for acute cholecystitis after self-expandable metallic stent placement for malignant biliary obstruction: a 10-year retrospective analysis in a single center
Akihisa Ohno, Nao Fujimori, Toyoma Kaku, Masayuki Hijioka, Ken Kawabe, Naohiko Harada, Makoto Nakamuta, Takamasa Oono, Yoshihiro Ogawa
Clinical Endoscopy.2022; 55(6): 784. CrossRef - Self‐expandable metal stents have longer patency and less cholangitis than inside stents in malignant perihilar biliary obstruction
Akinobu Koiwai, Morihisa Hirota, Tomofumi Katayama, Ryo Kin, Keita Kawamura, Katsuya Endo, Takayuki Kogure, Atsuko Takasu, Takayoshi Meguro, Kennichi Satoh
JGH Open.2022; 6(5): 317. CrossRef - Two-devices-in-one-channel method for preventing the preceding stent migration in case of multiple indwelling biliary inside plastic stents
Tesshin Ban, Yoshimasa Kubota, Takuya Takahama, Shun Sasoh, Tomoaki Ando, Makoto Nakamura, Takashi Joh
Endoscopy.2022; 54(S 02): E948. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Endoscopic or percutaneous biliary drainage in hilar cholangiocarcinoma: When and how?
Tudor Mocan, Adelina Horhat, Emil Mois, Florin Graur, Cristian Tefas, Rares Craciun, Iuliana Nenu, Mihaela Spârchez, Zeno Sparchez
World Journal of Gastrointestinal Oncology.2021; 13(12): 2050. CrossRef - Changing Trends in Biliary Stenting for Unresectable Malignant Perihilar Obstructions
Lubna Kamani, Muhammad Arshad
Clinical Endoscopy.2020; 53(6): 636. CrossRef
- Comparison of unilateral and bilateral intraductal plastic stent placement for unresectable malignant hilar biliary obstruction: A propensity score‐matched cohort analysis
- 5,456 View
- 243 Download
- 20 Web of Science
- 21 Crossref

Case Reports
- A Case of Suspicious Allergic Reaction to Peracetic Acid Following Endoscopy
- Naohiko Harada, Manami Hirowatari, Eikichi Ihara, Etsuko Ishihara, Mitsuko Inoue, Tomoya Miyamura, Makoto Nakamuta
- Clin Endosc 2020;53(6):743-745. Published online March 16, 2020
- DOI: https://doi.org/10.5946/ce.2019.129
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - A 43-year-old man with rheumatic arthritis was admitted to our hospital for symptoms of cough, left chest pain, and left elbow pain, and further examination revealed an elevated level of C-reactive protein. On day 2 after admission, he underwent esophagogastroduodenoscopy. On the morning of day 7, he developed a high fever of 39.7°C, several hours after bronchoscopy. On day 13, he underwent colonoscopy. Five minutes after the colonoscopy, he developed a high fever of 39.9°C, accompanied by stridor, indicating a decrease in arterial oxygen saturation level. An intradermal test for peracetic acid which was used for cleaning flexible endoscopy was positive. We suspect that he suffered from an allergic reaction to peracetic acid following the flexible endoscopy. This is the first case reported on suspicious allergic reaction to peracetic acid following a flexible endoscopy procedure.
-
Citations
Citations to this article as recorded by- The Economics of Cystoscopy: A Microcost Analysis
Alexander P. Kenigsberg, Samuel Gold, Lorie Grant, Yair Lotan
Urology.2021; 157: 29. CrossRef
- The Economics of Cystoscopy: A Microcost Analysis
- 4,060 View
- 127 Download
- 1 Web of Science
- 1 Crossref

- Rare and Fatal Gastrointestinal Mucormycosis (Zygomycosis) in a COVID-19 Patient: A Case Report
- Epifanio Silvino do Monte Junior, Marcos Eduardo Lera dos Santos, Igor Braga Ribeiro, Gustavo de Oliveira Luz, Elisa Ryoka Baba, Bruno Salomão Hirsch, Mateus Pereira Funari, Eduardo Guimarães Hourneaux de Moura
- Clin Endosc 2020;53(6):746-749. Published online November 19, 2020
- DOI: https://doi.org/10.5946/ce.2020.180

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - The novel coronavirus disease (COVID-19) quickly spread to all continents. However, data regarding all the signs and symptoms of COVID-19 are insufficient. Patients with COVID-19 might present higher susceptibility to fungal coinfections. Mucormycosis is a rare and often life-threatening fungal disease characterized by vascular invasion by hyphae, resulting in thrombosis and necrosis. This is the first case report of mucormycosis in a COVID-19 patient. An 86-year-old male patient was admitted to the emergency room with acute diarrhea, cough, dyspnea, and fever from 5 days prior. Blood tests revealed a hemoglobin level of 14.3 mg/dL. Five days following the admission, the patient presented with melena and a hemoglobin level of 5.6 mg/dL. A transfusion of three units of red blood cells was required. Esophagogastroduodenoscopy revealed two giant gastric ulcers with necrotic debris and a deep hemorrhagic base without active bleeding. Furthermore, biopsies confirmed mucormycosis. Despite intensive care, the patient died 36 hours after the esophagogastroduodenoscopy.
-
Citations
Citations to this article as recorded by- The potential for rapid antigen testing for mucormycosis in the context of COVID-19
Christopher R. Thornton
Expert Review of Molecular Diagnostics.2024; 24(3): 161. CrossRef - A New Proposed Combined CT and MRI Staging System for Covid-19-Associated Rhino-Orbito-Cerebral Fungal Infection: A Multi-center Study with Pathological Correlation
Noha Yahia Ebaid, Haitham Foda, Doaa Khedr Mohamed Khedr, Ahmed Ebeed, Mahmoud Ahmed Ebada, Rabab Mohamed Abdelhay, Ali Awad, Amany Abd Al Badea, Basma Hamed Ibrahim, Emad Hassan Emara
Academic Radiology.2024; 31(3): 1055. CrossRef - Development of a Machine Learning Model to Predict Risk of Development of COVID-19-Associated Mucormycosis
Rajashri Patil, Sahjid Mukhida, Jyoti Ajagunde, Uzair Khan, Sameena Khan, Nageswari Gandham, Chanda Vyawhare, Nikunja K Das, Shahzad Mirza
Future Microbiology.2024; 19(4): 297. CrossRef - COVID-19 Second Wave with Mucormycosis, a Deadly Combination: A Systemic Review
Neetu Jain, Seema Bhadauria
Biomedical and Biotechnology Research Journal.2024; 8(1): 13. CrossRef - The cross-talk between mucormycosis, steroids and diabetes mellitus amidst the global contagion of COVID-19
Shrey Dwivedi, Princy Choudhary, Ayushi Gupta, Sangeeta Singh
Critical Reviews in Microbiology.2023; 49(3): 318. CrossRef - Magnetic resonance imaging spectrum of COVID-associated rhino-orbital-cerebral mucormycosis and assessment of anatomical severity
Ishan Kumar, Ashish Verma, Jyoti Dangwal, Pramod Kumar Singh, Ram Chandra Shukla, Jaya Chakravarty
The Neuroradiology Journal.2023; 36(4): 404. CrossRef - Mucormycosis and Its Upsurge During COVID-19 Epidemic: An Updated Review
Bharti Sharma, Skarma Nonzom
Current Microbiology.2023;[Epub] CrossRef - Mucormycosis: A hidden mystery of fungal infection, possible diagnosis, treatment and development of new therapeutic agents
Mohd Kamil Hussain, Shaista Ahmed, Andleeb Khan, Arif Jamal Siddiqui, Shahnaaz Khatoon, Sadaf Jahan
European Journal of Medicinal Chemistry.2023; 246: 115010. CrossRef -
cotH
Genes Are Necessary for Normal Spore Formation and Virulence in
Mucor lusitanicus
Csilla Szebenyi, Yiyou Gu, Teclegiorgis Gebremariam, Sándor Kocsubé, Sándor Kiss-Vetráb, Olivér Jáger, Roland Patai, Krisztina Spisák, Rita Sinka, Ulrike Binder, Mónika Homa, Csaba Vágvölgyi, Ashraf S. Ibrahim, Gábor Nagy, Tamás Papp, Anuradha Chowdhary
mBio.2023;[Epub] CrossRef - Post COVID-19: Risk Factors, Prevention, and Management of Black
Fungus
Aimen Salman, Suneela Dhaneshwar, Shaik Shafiulla
Anti-Infective Agents.2023; 21(1): 39. CrossRef - Surge of mucormycosis during the COVID-19 pandemic
Paulami Dam, Marlon H. Cardoso, Sukhendu Mandal, Octávio L. Franco, Pınar Sağıroğlu, Osman Ahmet Polat, Kerem Kokoglu, Rittick Mondal, Amit Kumar Mandal, Ismail Ocsoy
Travel Medicine and Infectious Disease.2023; 52: 102557. CrossRef - COVID-19 and Mucormycosis of Orofacial Region: A Scoping Review
Abhishek Banerjee, Moumalini Das, Pooja Verma, Abhishek Chatterjee, Karthikeyan Ramalingam, Kumar Chandan Srivastava
Cureus.2023;[Epub] CrossRef - Case Reports on Black Fungus of the Gastrointestinal Tract: A New Complication in COVID-19 Patients
Sachin Arora, Ashish Singh, Pallavi Prasad, Rahul, Rajneesh Singh
The Korean Journal of Gastroenterology.2023; 81(5): 221. CrossRef - Galangin for COVID-19 and Mucormycosis co-infection: a potential therapeutic strategy of targeting critical host signal pathways triggered by SARS-CoV-2 and Mucormycosis
Md. Imran Hasan, Md. Arju Hossain, Md Habibur Rahman, Md Sohel, Asif Ahsan, Md. Sadat Hossain Soikot, Md. Nazrul Islam, Mohammad Ruhul Amin, Deepak Kumar Jain
Network Modeling Analysis in Health Informatics and Bioinformatics.2023;[Epub] CrossRef - Opportunistic Fungal Invasion in COVID-19 Pandemic: A Critical Review in Diagnosis and Management
Abhishek Sharma, Gulnaz Bano, Abdul Malik, Yuman Rasool, Samrina Manzar, Tarun Singh, Manish Maity
Avicenna Journal of Medicine.2023; 13(03): 131. CrossRef - Effect of antifungal drugs against mucormycosis and impact on human health
Shivangi Giri, Sujata Sharma, Kumud Kant Awasthi, Lata Shahani
Materials Today: Proceedings.2023; 95: 43. CrossRef - Epidemiology, Risk Factors, Diagnosis and Treatment of Mucormycosis
(Black Fungus): A Review
Pragati Upadhayay, Keshav Bansal, Ahsas Goyal
Current Pharmaceutical Biotechnology.2023; 24(13): 1645. CrossRef - View of mucormycosis during the era of COVID-19 infection: A cross-sectional study
Ossama M. Zakaria, Dana W. Alkuwaity
Journal of Family Medicine and Primary Care.2023; 12(11): 2608. CrossRef - An Update on COVID‐19 Associated Mucormycosis Characteristics, Risk Factors, and Outcomes: a Systematic Review and Meta-Analysis
Kazem Khiabani, Mohammad Hosein Amirzade-Iranaq, Hanie Ahmadi
Current Fungal Infection Reports.2023; 17(4): 282. CrossRef - CT Findings of COVID-19–associated Pulmonary Mucormycosis: A Case Series and Literature Review
Mandeep Garg, Nidhi Prabhakar, Valliappan Muthu, Shameema Farookh, Harsimran Kaur, Vikas Suri, Ritesh Agarwal
Radiology.2022; 302(1): 214. CrossRef - Mucormycosis (black fungus) ensuing COVID-19 and comorbidity meets - Magnifying global pandemic grieve and catastrophe begins
Karthika Pushparaj, Haripriya Kuchi Bhotla, Vijaya Anand Arumugam, Manikantan Pappusamy, Murugesh Easwaran, Wen-Chao Liu, Utthapon Issara, Kannan R.R. Rengasamy, Arun Meyyazhagan, Balamuralikrishnan Balasubramanian
Science of The Total Environment.2022; 805: 150355. CrossRef - Mucormycosis: A Case Series of Patients Admitted in Non-COVID-19 Intensive Care Unit of a Tertiary Care Center during the Second Wave
Nikhil Kothari, Amit Goyal, Ankur Sharma, Shilpa Goyal, Pradeep K Bhatia, Sangam Yadav
Indian Journal of Critical Care Medicine.2022; 25(10): 1193. CrossRef - A systematic review on SARS‐CoV‐2‐associated fungal coinfections
Shringika Soni, Ramesh Namdeo Pudake, Utkarsh Jain, Nidhi Chauhan
Journal of Medical Virology.2022; 94(1): 99. CrossRef - Rhino-orbito-cerebral mucormycosis during the COVID-19 third wave in 2021: an Egyptian preliminary report from a single tertiary hospital
Taha K. Alloush, Osama Mansour, Adel T. Alloush, Tamer Roushdy, Eman Hamid, Mahmoud El-Shamy, Hossam M. Shokri
Neurological Sciences.2022; 43(2): 799. CrossRef - Cumulative Mortality and Factors Associated With Outcomes of Mucormycosis After COVID-19 at a Multispecialty Tertiary Care Center in India
Twinkle Choksi, Anamika Agrawal, Purva Date, Darshana Rathod, Anuja Gharat, Avinash Ingole, Bhushan Chaudhari, Nitin Pawar
JAMA Ophthalmology.2022; 140(1): 66. CrossRef - Using artificial intelligence-based models to predict the risk of mucormycosis among COVID-19 survivors: An experience from a public hospital in India
Shabbir Syed-Abdul, A. Shoban Babu, Raja Shekhar Bellamkonda, Ramaiah Itumalla, GVRK Acharyulu, Surya Krishnamurthy, Y. Venkat Santosh Ramana, Naresh Mogilicharla, Shwetambara Malwade, Yu-Chuan Li
Journal of Infection.2022; 84(3): 351. CrossRef - Bilateral Renal Mucor Mycosis Presenting as Anuria in a Covid 19 Recovered Patient: A Case Report and Review of Literature
Surya Prakash Vaddi, Seshu Mohan Khetavath, Dilip M. Babu, Nagarjuna Maturu, Bhulaxmi, Swathi, Krithika Mohan, Datta Prasad M, Jawahar B, Rajesh Reddy KRV
Urology.2022; 161: 12. CrossRef - Fungal Infections Other Than Invasive Aspergillosis in COVID-19 Patients
Kerri Basile, Catriona Halliday, Jen Kok, Sharon C-A. Chen
Journal of Fungi.2022; 8(1): 58. CrossRef - Gastrointestinal mucormycosis: A periodic systematic review of case reports from 2015 to 2021
Mojtaba Didehdar, Zahra chegini, Alireza Moradabadi, Ali Arash Anoushirvani, Seidamir Pasha Tabaeian, Milad Yousefimashouf, Aref Shariati
Microbial Pathogenesis.2022; 163: 105388. CrossRef - Gastrointestinal Mucormycosis: A Challenge during COVID-19 Pandemic
Jagdish Chander
Journal of Gastrointestinal Infections.2022; 11(1): 30. CrossRef - COVID-19 associated mucormycosis – An emerging threat
Chien-Ming Chao, Chih-Cheng Lai, Wen-Liang Yu
Journal of Microbiology, Immunology and Infection.2022; 55(2): 183. CrossRef - The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries
Martin Hoenigl, Danila Seidel, Agostinho Carvalho, Shivaprakash M Rudramurthy, Amir Arastehfar, Jean-Pierre Gangneux, Nosheen Nasir, Alexandro Bonifaz, Javier Araiza, Nikolai Klimko, Alexandra Serris, Katrien Lagrou, Jacques F Meis, Oliver A Cornely, John
The Lancet Microbe.2022; 3(7): e543. CrossRef - Fatal allograft mucormycosis complicating severe COVID‐19 infection and bacterial pyelonephritis
Abhilash Chandra, Namrata Rao S., Kiran Preet Malhotra
Transplant Infectious Disease.2022;[Epub] CrossRef - Mucormycosis in COVID-19 pandemic: study at tertiary hospital in India
Reshma P. Chavan, Shivraj M. Ingole, Hamna Abdul Nazir, Wilson V. Desai, Gajanan S. Kanchewad
European Archives of Oto-Rhino-Laryngology.2022; 279(6): 3201. CrossRef - Mucormycosis and COVID-19 pandemic: Clinical and diagnostic approach
Asim Azhar, Wajihul Hasan Khan, Parvez Anwar Khan, Khaled Alhosaini, Mohammad Owais, Aijaz Ahmad
Journal of Infection and Public Health.2022; 15(4): 466. CrossRef - COVID19 associated mucormycosis: A review
PriyadharsiniR Palanisamy, Dhivya Elango
Journal of Family Medicine and Primary Care.2022; 11(2): 418. CrossRef - Mucormycosis: risk factors, diagnosis, treatments, and challenges during COVID-19 pandemic
Ayushi Sharma, Anjana Goel
Folia Microbiologica.2022; 67(3): 363. CrossRef - Retracted: Mucormycosis infection in patients with COVID‐19: A systematic review
SeyedAhmad SeyedAlinaghi, Amirali Karimi, Alireza Barzegary, Zahra Pashaei, Amir Masoud Afsahi, Sanam Alilou, Nazanin Janfaza, Alireza Shojaei, Fatemeh Afroughi, Parsa Mohammadi, Yasna Soleimani, Newsha Nazarian, Ava Amiri, Marcarious M. Tantuoyir, Shahra
Health Science Reports.2022;[Epub] CrossRef - Epidemiology, clinical presentation and management of COVID‐19 associated mucormycosis: A single centre experience from Pune, Western India
Ameet Dravid, Reema Kashiva, Zafer Khan, Balasaheb Bande, Danish Memon, Aparna Kodre, Milind Mane, Vishal Pawar, Dattatraya Patil, Suraj Kalyani, Prathamesh Raut, Madhura Bapte, Charlotte Saldanha, Dinesh Chandak, Teerthagouda Patil, Sateesh Reddy, Krushn
Mycoses.2022; 65(5): 526. CrossRef - Rhino-Orbital-Cerebral Mucormycosis in a Post-COVID-19 Patient from Peru
Linda Ponce-Rosas, Jose Gonzales-Zamora, Nelson Diaz-Reyes, Oliver Alarco-Cadillo, Jorge Alave-Rosas, Mohd Adnan
Case Reports in Infectious Diseases.2022; 2022: 1. CrossRef - Mucormycosis: A Lethal Disease
Pawan N. Karwa, Jyoti K. Soundarmal, Pallavi S. Shinde, Swapna R. Jalde
Asian Journal of Pharmacy and Technology.2022; : 41. CrossRef - An overview of COVID-19 related to fungal infections: what do we know after the first year of pandemic?
R. G. Vitale, J. Afeltra, S. Seyedmousavi, S. L. Giudicessi, S. M. Romero
Brazilian Journal of Microbiology.2022; 53(2): 759. CrossRef - Mucormycosis: A new threat to Coronavirus disease 2019 with special emphasis on India
Deganta Ghosh, Sagardeep Dey, Himanko Chakraborty, Sneha Mukherjee, Ankita Halder, Akash Sarkar, Pallab Chakraborty, Rajdeep Ghosh, Joy Sarkar
Clinical Epidemiology and Global Health.2022; 15: 101013. CrossRef - First Reported Cases of COVID-19-Associated Mucormycosis in Tunisia
Rim Khemakhem, Ichrak Bougharriou, Nesrine Kallel, Anis Bafoun, Feten Mahmoudi, Samy Kammoun
Electronic Journal of Medical and Dental Studies.2022; 12(1): em0097. CrossRef - Iranian patients co-infected with COVID-19 and mucormycosis: the most common predisposing factor, clinical outcomes, laboratory markers and diagnosis, and drug therapies
Hamideh Molaei, Ehsan Shojaeefar, Eghlim Nemati, Leila Khedmat, Sayed Yousef Mojtahedi, Nematollah Jonaidi Jafari, Morteza Izadi, Behzad Einollahi
Infectious Diseases.2022; 54(8): 600. CrossRef - COVID-19 and Plethora of Fungal Infections
Reetu Kundu, Nidhi Singla
Current Fungal Infection Reports.2022; 16(2): 47. CrossRef - A Comprehensive Review on the Management of COVID-19-Associated Mucormycosis (CAM): The New Basics
Divyam Girdhar, Ekta Manocha
BioMed.2022; 2(2): 181. CrossRef - Mucormycosis and COVID-19-Associated Mucormycosis: Insights of a Deadly but Neglected Mycosis
Laura C. García-Carnero, Héctor M. Mora-Montes
Journal of Fungi.2022; 8(5): 445. CrossRef - COVID-19-Associated Fungal Infections: An Urgent Need for Alternative Therapeutic Approach?
Marianna Domán, Krisztián Bányai
Frontiers in Microbiology.2022;[Epub] CrossRef - Current Treatment Options for COVID-19 Associated Mucormycosis: Present Status and Future Perspectives
Yasasve Madhavan, Kadambari Vijay Sai, Dilip Kumar Shanmugam, Aashabharathi Manimaran, Karthigadevi Guruviah, Yugal Kishore Mohanta, Divyambika Catakapatri Venugopal, Tapan Kumar Mohanta, Nanaocha Sharma, Saravanan Muthupandian
Journal of Clinical Medicine.2022; 11(13): 3620. CrossRef - Fungal Infection in Co-infected Patients With COVID-19: An Overview of Case Reports/Case Series and Systematic Review
Sima Sadat Seyedjavadi, Parmida Bagheri, Mohammad Javad Nasiri, Mehdi Razzaghi-Abyaneh, Mehdi Goudarzi
Frontiers in Microbiology.2022;[Epub] CrossRef - COVID-19-associated fungal infections in Iran: A systematic review
Tina Nazari, Fatemeh Sadeghi, Alireza Izadi, Setayesh Sameni, Shahram Mahmoudi, Felix Bongomin
PLOS ONE.2022; 17(7): e0271333. CrossRef - Effect of Indoor Bioaerosols (Fungal) Exposure on the Health of Post-COVID-19 Patients and Possible Mitigation Strategies
Yogesh Kumar Vishwakarma, Amrita Shahi, Ram Sharan Singh
COVID.2022; 2(7): 940. CrossRef - Mucormycosis in patients with COVID-19 in Russia: the results of a prospective multi-center study
S. N. Khostelidi, V. A. Zaytsev, S. A. Vartanyan, N. A. Nikitin, G. N. Evtukh, M. N. Gilalov, G. V. Portnov, A. A. Zubareva, I. B. Baranova, T. S. Bogomolova, Yu. L. Avdeenko, O. V. Shadrivova, E. A. Desyatik, E. V. Shagdileeva, Yu. V. Borzova, Yu. A. Kri
Journal Infectology.2022; 14(2): 116. CrossRef - Development of a Monoclonal Antibody and a Serodiagnostic Lateral-Flow Device Specific to Rhizopus arrhizus (Syn. R. oryzae), the Principal Global Agent of Mucormycosis in Humans
Genna E. Davies, Christopher R. Thornton
Journal of Fungi.2022; 8(7): 756. CrossRef - Mucormycosis co-infection in COVID-19 patients: An update
Abdullah S. Alkhamiss, Ahmed A. Ahmed, Zafar Rasheed, Ruqaih Alghsham, Ali Shariq, Thamir Alsaeed, Sami A. Althwab, Suliman Alsagaby, Abdullah S. M. Aljohani, Fahad A. Alhumaydhi, Sharifa K. Alduraibi, Alaa K. Alduraibi, Homaidan T. Alhomaidan, Khaled S.
Open Life Sciences.2022; 17(1): 917. CrossRef - COVID-19-Associated Mucormycosis: A Matter of Concern Amid the SARS-CoV-2 Pandemic
Pankaj Chandley, Priyanka Subba, Soma Rohatgi
Vaccines.2022; 10(8): 1266. CrossRef - Antifungal therapy in the management of fungal secondary infections in COVID-19 patients: A systematic review and meta-analysis
Sujit Kumar Sah, Atiqulla Shariff, Niharika Pathakamuri, Subramanian Ramaswamy, Madhan Ramesh, Krishna Undela, Malavalli Siddalingegowda Srikanth, Teggina Math Pramod Kumar, Joy Sturtevant
PLOS ONE.2022; 17(7): e0271795. CrossRef - The role of SARS-CoV-2 immunosuppression and the therapy used to manage COVID-19 disease in the emergence of opportunistic fungal infections: A review
Nahid Akhtar, Atif Khurshid Wani, Surya Kant Tripathi, Ajit Prakash, M. Amin-ul Mannan
Current Research in Biotechnology.2022; 4: 337. CrossRef - Mortality-Related Risk Factors for Coronavirus Disease (COVID-19)-Associated Mucormycosis: a systematic review and meta-analysis
Vahid Reza Ostovan, Reza Tabrizi, Hanieh Bazrafshan, Zahra Bahrami, Hajar Khazraei, Samaneh Khazraei, Afshin Borhani-Haghighi, Mohsen Moghadami, Matthew Grant
Current Fungal Infection Reports.2022; 16(4): 143. CrossRef - Comparative risk assessment of COVID‐19 associated mucormycosis and aspergillosis: A systematic review
Prodip Kumar Baral, Md. Abdul Aziz, Mohammad Safiqul Islam
Health Science Reports.2022;[Epub] CrossRef - COVID-19 and Fungal infections: a double debacle
Sara Mina, Hajar Yaakoub, Cédric Annweiler, Vincent Dubée, Nicolas Papon
Microbes and Infection.2022; 24(8): 105039. CrossRef - Wave of Invasive Fungal Disease on the Shores of COVID-19: A Case Series of COVID-19 Associated Rhino-Orbital Fungal Rhinosinusitis and Literature Review
Sandeep Trehan, Neena Chaudhary, Ashwin Bhasarkar
Indian Journal of Otolaryngology and Head & Neck Surgery.2022; 74(S2): 3359. CrossRef - A study of rhino-orbito-cerebral mucormycosis with COVID-19: A new challenge in North West of Rajasthan
Surendra Kumar, Harish Kumar, Manoj Mali, BabuLal Meena
Annals of African Medicine.2022; 21(4): 383. CrossRef - Is the production of reactive oxygen and nitrogen species by macrophages associated with better infectious control in female mice with experimentally disseminated and pulmonary mucormycosis?
Amanda Ribeiro dos Santos, Thais Fernanda Fraga-Silva, Débora de Fátima Almeida-Donanzam, Angela Carolina Finatto, Camila Marchetti, Maria Izilda Andrade, Olavo Speranza de Arruda, Maria Sueli Parreira de Arruda, James Venturini, Michal A Olszewski
PLOS ONE.2022; 17(12): e0270071. CrossRef - Mucormycosis, COVID-19 Pandemic and the Lessons Learnt: A Review
Anila Varghese, Anita Upadhyay, RoyA Daniel, Twinkle Sharma, MShyam Mohan, Balaji Susindran, Priyanka Singh, Chandrakant Lahariya
Journal of Medical Evidence.2022; 3(3): 256. CrossRef - Gastritis enfisematosa secundaria a mucormicosis gástrica en paciente con COVID-19. Reporte de un caso
Martín Islas Torres, Ana Laura Castillo Luna, José Juan Rodríguez Moreno, Valeria Priscilla Rendón Muñoz, José Gerardo Zamora Inzuna, Albert Antonio Ibarra Trejo
Cirujano General.2022; 44(2): 87. CrossRef - A Fatal Case of Rhizopus azygosporus Pneumonia Following COVID-19
Anubhav Kanwar, Alex Jordan, Scott Olewiler, Kurt Wehberg, Michael Cortes, Brendan R. Jackson
Journal of Fungi.2021; 7(3): 174. CrossRef - The double‐edged sword of systemic corticosteroid therapy in viral pneumonia: A case report and comparative review of influenza‐associated mucormycosis versus COVID‐19 associated mucormycosis
Kazem Ahmadikia, Seyed Jamal Hashemi, Sadegh Khodavaisy, Muhammad Ibrahim Getso, Neda Alijani, Hamid Badali, Hossein Mirhendi, Mohammadreza Salehi, Azin Tabari, Mojtaba Mohammadi Ardehali, Mohammad Kord, Emmanuel Roilides, Sassan Rezaie
Mycoses.2021; 64(8): 798. CrossRef - Antibacterials/hydrocortisone/oseltamivir
Reactions Weekly.2021; 1845(1): 43. CrossRef - Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient – Case report and review of literature
Akshay Khatri, Kai-Ming Chang, Ilan Berlinrut, Frances Wallach
Journal of Medical Mycology.2021; 31(2): 101125. CrossRef - Research and Management of Rare Diseases in the COVID-19 Pandemic Era: Challenges and Countermeasures
Sanjana Fatema Chowdhury, Syed Muktadir Al Sium, Saeed Anwar
Frontiers in Public Health.2021;[Epub] CrossRef - Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India
Awadhesh Kumar Singh, Ritu Singh, Shashank R. Joshi, Anoop Misra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2021; 15(4): 102146. CrossRef - Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: Case series from the north-western part of India
Hardeva Ram Nehara, Inder Puri, Vipin Singhal, Sunil IH, Bhagirath Ram Bishnoi, Pramendra Sirohi
Indian Journal of Medical Microbiology.2021; 39(3): 380. CrossRef - COVID 19 infection and mucormycosis—a dangerously increasing combination
Satvinder Singh Bakshi, Vinoth Kumar Kalidoss
The Egyptian Journal of Otolaryngology.2021;[Epub] CrossRef - Autoptic identification of disseminated mucormycosis in a young male presenting with cerebrovascular event, multi-organ dysfunction and COVID-19 infection
Vidya Krishna, Jaymin Morjaria, Rona Jalandari, Fatima Omar, Sundeep Kaul
IDCases.2021; 25: e01172. CrossRef - Does COVID 19 generate a milieu for propagation of mucormycosis?
Deepak Pandiar, N. Siva Kumar, Rahul Anand, Mala Kamboj, Anjali Narwal, P.M. Shameena
Medical Hypotheses.2021; 152: 110613. CrossRef - Mucormycosis associated with COVID‐19 in two kidney transplant patients
Carolt Arana, Rafael E. Cuevas Ramírez, Marc Xipell, Joaquim Casals, Asunción Moreno, Sabina Herrera, Marta Bodro, Frederic Cofan, Fritz Diekmann, Núria Esforzado
Transplant Infectious Disease.2021;[Epub] CrossRef - COVID-19 associated mucormycosis: the urgent need to reconsider the indiscriminate use of immunosuppressive drugs
Alfonso J. Rodriguez-Morales, Ranjit Sah, Jose Millan-Oñate, Angel Gonzalez, Juan J. Montenegro-Idrogo, Sias Scherger, Carlos Franco-Paredes, Andrés F. Henao-Martínez
Therapeutic Advances in Infectious Disease.2021; 8: 204993612110270. CrossRef - COVID‐19‐associated mucormycosis: An updated systematic review of literature
Rimesh Pal, Birgurman Singh, Sanjay Kumar Bhadada, Mainak Banerjee, Ranjitpal Singh Bhogal, Neemu Hage, Ashok Kumar
Mycoses.2021; 64(12): 1452. CrossRef - Rare case of gastrointestinal mucormycosis with colonic perforation in an immunocompetent patient with COVID-19
Ravinder Pal Singh, Nishkarsh Gupta, Tanudeep Kaur, Anju Gupta
BMJ Case Reports.2021; 14(7): e244096. CrossRef - The double trouble: COVID-19 associated mucormycosis a focused review and future perspectives
Arun Kumar Agnihotri, Monika Vij, Okezie I. Aruoma, Vipul D Yagnik, Theeshan Bahorun, Maria Elena Villamil, Godfred A. Menezes, Vineet Gupta
Global Journal of Medical, Pharmaceutical, and Biomedical Update.2021; 16: 4. CrossRef - Mucormycosis: An opportunistic pathogen during COVID-19
Iyer Mahalaxmi, Kaavya Jayaramayya, Dhivya Venkatesan, Mohana Devi Subramaniam, Kaviyarasi Renu, Padmavathi Vijayakumar, Arul Narayanasamy, Abilash Valsala Gopalakrishnan, Nachimuthu Senthil Kumar, Palanisamy Sivaprakash, Krothapalli R.S. Sambasiva Rao, B
Environmental Research.2021; 201: 111643. CrossRef - Epidemiology of Systemic Mycoses in the COVID-19 Pandemic
María Guadalupe Frías-De-León, Rodolfo Pinto-Almazán, Rigoberto Hernández-Castro, Eduardo García-Salazar, Patricia Meza-Meneses, Carmen Rodríguez-Cerdeira, Roberto Arenas, Esther Conde-Cuevas, Gustavo Acosta-Altamirano, Erick Martínez-Herrera
Journal of Fungi.2021; 7(7): 556. CrossRef - EFFICACY OF ENDOSCOPIC TOPICAL MITOMYCIN C APPLICATION IN CAUSTIC ESOPHAGEAL STRICTURES IN THE PEDIATRIC POPULATION: A SYSTEMATIC REVIEW AND META-ANALYSIS OF RANDOMIZED CONTROLLED TRIALS
Marcelo Mochate FLOR, Igor Braga RIBEIRO, Diogo Turiani Hourneaux DE MOURA, Sérgio Barbosa MARQUES, Wanderley Marques BERNARDO, Eduardo Guimarães Hourneaux DE MOURA
Arquivos de Gastroenterologia.2021; 58(2): 253. CrossRef - Coronavirus Disease-Associated Mucormycosis from a Tertiary Care Hospital in India: A Case Series
Yudhyavir Singh, Venkata Ganesh, Shailendra Kumar, Nishant Patel, Richa Aggarwala, Kapil Dev Soni, Anjan Trikha
Cureus.2021;[Epub] CrossRef - Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature
Deepak Garg, Valliappan Muthu, Inderpaul Singh Sehgal, Raja Ramachandran, Harsimran Kaur, Ashish Bhalla, Goverdhan D. Puri, Arunaloke Chakrabarti, Ritesh Agarwal
Mycopathologia.2021; 186(2): 289. CrossRef - Mucormycosis and COVID‐19: An epidemic within a pandemic in India
Lav Selarka, Suktara Sharma, Dinesh Saini, Sanjay Sharma, Amit Batra, Vishal T. Waghmare, Pratibha Dileep, Sanket Patel, Monarch Shah, Tejas Parikh, Prakash Darji, Amit Patel, Gaurav Goswami, Anand Shah, Sandeep Shah, Harsh Lathiya, Moksha Shah, Pranita S
Mycoses.2021; 64(10): 1253. CrossRef - COVID-19 and mucormycosis superinfection: the perfect storm
Jaffar A. Al-Tawfiq, Saad Alhumaid, Abeer N. Alshukairi, Mohamad-Hani Temsah, Mazin Barry, Abbas Al Mutair, Ali A. Rabaan, Awadh Al-Omari, Raghavendra Tirupathi, Manaf AlQahtani, Salma AlBahrani, Kuldeep Dhama
Infection.2021; 49(5): 833. CrossRef - COVID-19 Associated Rhino-Orbital Mucormycosis Complicated by Gangrenous and Bone Necrosis—A Case Report from Honduras
Elsa Yolanda Palou, María Auxiliadora Ramos, Emec Cherenfant, Adoni Duarte, Itzel Carolina Fuentes-Barahona, Lysien I. Zambrano, Fausto Muñoz-Lara, Sandra Aracely Montoya-Ramirez, Alex Francisco Cardona-Ortiz, Jorge Alberto Valle-Reconco, Juan J. Monteneg
Vaccines.2021; 9(8): 826. CrossRef - A rare case of knee joint mucormycosis with pathological fracture after COVID-19 infection
Sergiu Andrei Iordache, Adrian Cursaru, Bogdan Şerban, Mihnea Ioan Gabriel Popa
Romanian Journal of Orthopaedic Surgery and Traumatology.2021; 4(1): 9. CrossRef - Epidemiology and Pathophysiology of COVID-19-Associated Mucormycosis: India Versus the Rest of the World
Valliappan Muthu, Shivaprakash M. Rudramurthy, Arunaloke Chakrabarti, Ritesh Agarwal
Mycopathologia.2021; 186(6): 739. CrossRef - COVID-19-associated mucormycosis: Case report and systematic review
Ahmet Dilek, Resat Ozaras, Sevket Ozkaya, Mustafa Sunbul, Elif Itir Sen, Hakan Leblebicioglu
Travel Medicine and Infectious Disease.2021; 44: 102148. CrossRef - COVID-19 and mucormycosis in Latin America – An emerging concern
Alfonso J. Rodriguez-Morales, Carlos S. Mamani-García, Janeth N. Nuñez-Lupaca, Darwin A. León-Figueroa, Mely Olarte-Durand, Robinson A. Yrene-Cubas, Diana M. Ticona, Sebastian Abanto-Urbano
Travel Medicine and Infectious Disease.2021; 44: 102156. CrossRef - Overview on the Prevalence of Fungal Infections, Immune Response, and Microbiome Role in COVID-19 Patients
Maryam Roudbary, Sunil Kumar, Awanish Kumar, Lucia Černáková, Fatemeh Nikoomanesh, Célia F. Rodrigues
Journal of Fungi.2021; 7(9): 720. CrossRef - A case report of rhino-facial mucormycosis in a non-diabetic patient with COVID-19: a systematic review of literature and current update
Faezeh Mohammadi, Milad Badri, Shapoor Safari, Nima Hemmat
BMC Infectious Diseases.2021;[Epub] CrossRef - Mucormycosis: A manifestation in COVID-19 infection
Abhishek Sharma, Gulnaz Bano, Abdul Malik
Indian Journal of Pharmacy and Pharmacology.2021; 8(3): 189. CrossRef - A Review of Coronavirus Disease Covid-19
Swapnali Zore
International Journal of Advanced Research in Science, Communication and Technology.2021; : 104. CrossRef - COVID-19 Associated Mucormycosis: A Systematic Review from Diagnostic Challenges to Management
Farah Yasmin, Hala Najeeb, Aisha Naeem, Kartik Dapke, Rachana Phadke, Muhammad Sohaib Asghar, Syed Muhammad Ismail Shah, Domenico De Berardis, Irfan Ullah
Diseases.2021; 9(4): 65. CrossRef - COVID-19-Associated Mucormycosis (CAM): An Updated Evidence Mapping
Salman Hussain, Harveen Baxi, Abanoub Riad, Jitka Klugarová, Andrea Pokorná, Simona Slezáková, Radim Líčeník, Abul Kalam Najmi, Miloslav Klugar
International Journal of Environmental Research and Public Health.2021; 18(19): 10340. CrossRef - Rhino-orbital Mucormycosis as a complication of severe COVID-19 pneumonia
Mohammed A. Alamin, Mohammed Abdulgayoom, Sushil Niraula, Elabbass Abdelmahmuod, Ashraf O. Ahmed, Mohammed I. Danjuma
IDCases.2021; 26: e01293. CrossRef - COVID-19-Associated Mucormycosis (CAM): Case-Series and Global Analysis of Mortality Risk Factors
Abanoub Riad, Alshaimaa Ahmed Shabaan, Julien Issa, Sally Ibrahim, Hatem Amer, Yossef Mansy, Islam Kassem, Amira Bisher Kassem, Hans-Peter Howaldt, Miloslav Klugar, Sameh Attia
Journal of Fungi.2021; 7(10): 837. CrossRef - Sinoorbital Mucormycosis Associated with Corticosteroid Therapy in COVID-19 Infection
Zeinab Mehrabi, Maryam Salimi, Kianoush Niknam, Farzaneh Mohammadi, Hesan Jelodari Mamaghani, Mohammad Reza Sasani, Mohammad Javad Ashraf, Amirhossein Salimi, Mohammad Hassan Zahedroozegar, Zohreh Erfani, Huban Atilla
Case Reports in Ophthalmological Medicine.2021; 2021: 1. CrossRef - Mucormycosis infection in severe COVID‐19 patient with multiple underlying health conditions
Zahra Heydarifard, Moslem Safaei, Sevrin Zadheidar, Soroush Ehsan, Nazanin Zahra Shafiei‐Jandaghi
Clinical Case Reports.2021;[Epub] CrossRef - Invasive Fungal Infections Complicating COVID-19: A Narrative Review
Giacomo Casalini, Andrea Giacomelli, Annalisa Ridolfo, Cristina Gervasoni, Spinello Antinori
Journal of Fungi.2021; 7(11): 921. CrossRef - Coronavirus Disease 2019–Associated Invasive Fungal Infection
John W Baddley, George R Thompson, Sharon C -A Chen, P Lewis White, Melissa D Johnson, M Hong Nguyen, Ilan S Schwartz, Andrej Spec, Luis Ostrosky-Zeichner, Brendan R Jackson, Thomas F Patterson, Peter G Pappas
Open Forum Infectious Diseases.2021;[Epub] CrossRef - Manifestations and risk factors of COVID-19 and mucormycosis: A mini-review
Jugal Sutradhar, BapiRay Sarkar
Journal of Acute Disease.2021; 10(6): 221. CrossRef - Invasive Mucormycosis – An Enigma
Anil Prasad, Minakshi Mishra, Kaushik Saha
Cureus.2021;[Epub] CrossRef - Salix spp. Bark Hot Water Extracts Show Antiviral, Antibacterial, and Antioxidant Activities—The Bioactive Properties of 16 Clones
Jenni Tienaho, Dhanik Reshamwala, Tytti Sarjala, Petri Kilpeläinen, Jaana Liimatainen, Jinze Dou, Anneli Viherä-Aarnio, Riikka Linnakoski, Varpu Marjomäki, Tuula Jyske
Frontiers in Bioengineering and Biotechnology.2021;[Epub] CrossRef - Mucormycosis – resurgence of a deadly opportunist during COVID-19 pandemic: Four case reports
Shalini Upadhyay, Tanisha Bharara, Manisha Khandait, Ankit Chawdhry, Bharat Bhushan Sharma
World Journal of Clinical Cases.2021; 9(36): 11338. CrossRef - Mucormycosis following COVID19: clinical case and literature review
Sofya N. Khostelidi, V.A. Zaytsev, E.V. Pelikh, E.V. Yashina, O.N. Rodionova, T.S. Bogomolova, Yu.L. Avdeenko, Nikolay N. Klimko
Clinical Microbiology and Antimicrobial Chemotherapy.2021; 23(3): 255. CrossRef
- The potential for rapid antigen testing for mucormycosis in the context of COVID-19
- 7,750 View
- 256 Download
- 107 Web of Science
- 111 Crossref

- Endoscopic Ultrasound-Guided Vascular Therapy for Portoduodenal Fistula
- Tanyaporn Chantarojanasiri, Apichet Sirinawasatien, Chalermrat Bunchorntavakul, Aroon Siripun, Sa-ard Treepongkaruna, Thawee Ratanachu-ek
- Clin Endosc 2020;53(6):750-753. Published online February 13, 2020
- DOI: https://doi.org/10.5946/ce.2019.167
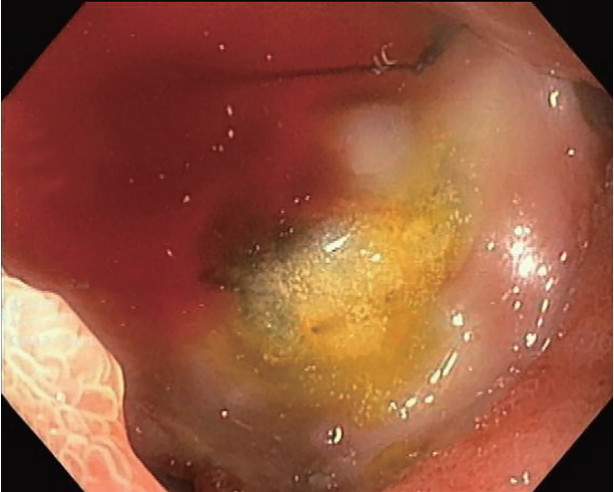
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Portoenteric fistula is a rare cause of massive upper gastrointestinal bleeding. Most cases can be treated with radiointervention or surgery, but portoenteric fistula is associated with a high mortality. We reported a case of intermittent massive upper gastrointestinal bleeding in a 33-year-old man with cholangiocarcinoma who underwent surgical resection followed by chemoradiation. A portoduodenal fistula due to chronic duodenal ulceration was identified. The bleeding was successfully controlled by endoscopic ultrasound-guided coil placement through the duodenal bulb using the anchoring technique. Follow-up endoscopy and computed tomography scan showed multiple coil placements between a part of the portal vein and the duodenal bulb without any evidence of portal vein thrombosis. There were no complications, and bleeding did not recur during the 8-month follow-up period.
-
Citations
Citations to this article as recorded by- Management of non-variceal upper gastrointestinal bleeding: role of endoscopic ultrasound-guided treatments
Chaoqun Han, Xin Ling, Jun Liu, Rong Lin, Zhen Ding
Therapeutic Advances in Gastroenterology.2022; 15: 175628482110561. CrossRef - A Case of an Internal Pancreatic Stent Penetrating the Portal Vein after Pancreaticoduodenectomy for Ampullary Carcinoma
Masanobu Taniguchi, Atsushi Mitsunaka, Yumi Zen, Takayuki Higashiguchi, Masaru Nagato, Yasuhisa Tango, Ichiro Nakamura, Tomoaki Nakamura, Hisanori Shiomi
The Japanese Journal of Gastroenterological Surgery.2022; 55(2): 99. CrossRef - Endoscopic ultrasound-guided portal vein coiling: troubleshooting interventional endoscopic ultrasonography
Shin Haba, Kazuo Hara, Nobumasa Mizuno, Takamichi Kuwahara, Nozomi Okuno, Akira Miyano, Daiki Fumihara, Moaz Elshair
Clinical Endoscopy.2022; 55(3): 458. CrossRef
- Management of non-variceal upper gastrointestinal bleeding: role of endoscopic ultrasound-guided treatments
- 4,101 View
- 85 Download
- 2 Web of Science
- 3 Crossref

Brief Report
- Multidisciplinary Approach to Diagnose and Treat Diffuse Esophageal Leiomyomatosis: A Case Report
- Luísa Martins Figueiredo, Maria Ana Rafael, Joana C. Branco, Catarina G. Rodrigues, António T. Alves, David Horta, Alexandra Martins
- Clin Endosc 2020;53(6):754-756. Published online November 24, 2020
- DOI: https://doi.org/10.5946/ce.2020.111
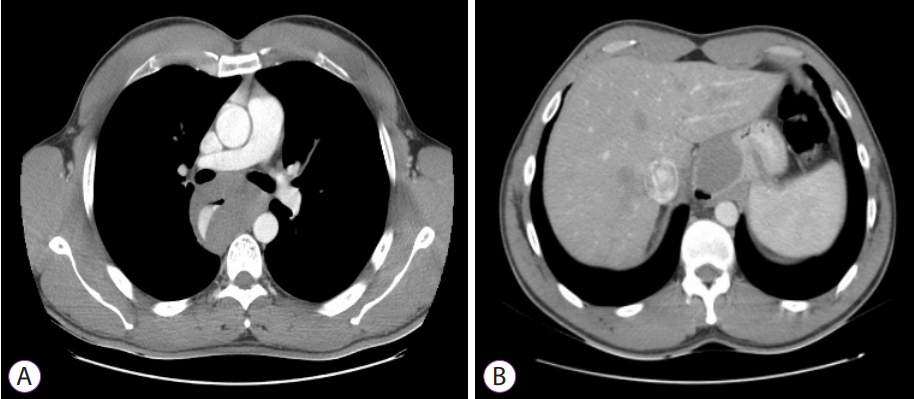
-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- Dysphagia Unmasked: A Case Report of Esophageal Leiomyomatosis
Lilian M Haji, Husain H Faraj, Bader N Abdulaziz, Hissa A Alaradi, Ahlam Alharbi
Cureus.2023;[Epub] CrossRef
- Dysphagia Unmasked: A Case Report of Esophageal Leiomyomatosis
- 2,990 View
- 61 Download
- 1 Web of Science
- 1 Crossref

Boost Your Learning with Quiz
- Esophageal Lesions: A High Index of Suspicion is Important for Diagnosis
- Jie-Hyun Kim
- Clin Endosc 2020;53(6):757-758. Published online November 27, 2020
- DOI: https://doi.org/10.5946/ce.2020.278
- 3,942 View
- 82 Download


 KSGE
KSGE




 First
First Prev
Prev



