Search
- Page Path
- HOME > Search
Original Articles
- Effectiveness of endoscopic ultrasound-guided tissue acquisition with stereomicroscopic on-site evaluation for preoperative diagnosis of resectable or borderline resectable pancreatic cancer: a prospective study
- Junro Ishizaki, Kosuke Okuwaki, Masafumi Watanabe, Hiroshi Imaizumi, Tomohisa Iwai, Rikiya Hasegawa, Takahiro Kurosu, Masayoshi Tadehara, Takaaki Matsumoto, Kai Adachi, Taro Hanaoka, Mitsuhiro Kida, Chika Kusano
- Received October 25, 2023 Accepted January 15, 2024 Published online May 24, 2024
- DOI: https://doi.org/10.5946/ce.2023.277 [Epub ahead of print]
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: To validate endoscopic ultrasound-guided tissue acquisition (EUS-TA) used in conjunction with stereomicroscopic on-site evaluation (SOSE) as a preoperative diagnostic tool for resectable pancreatic cancer (R-PC) and borderline resectable PC (BR-PC).
Methods
Seventy-eight consecutive patients who underwent EUS-TA for suspected R-PC or BR-PC were enrolled. The primary endpoint was the sensitivity of EUS-TA together with SOSE based on the stereomicroscopically visible white core (SVWC) cutoff value. One or two sites were punctured by using a 22-gauge biopsy needle for EUS-TA, based on the SOSE findings.
Results
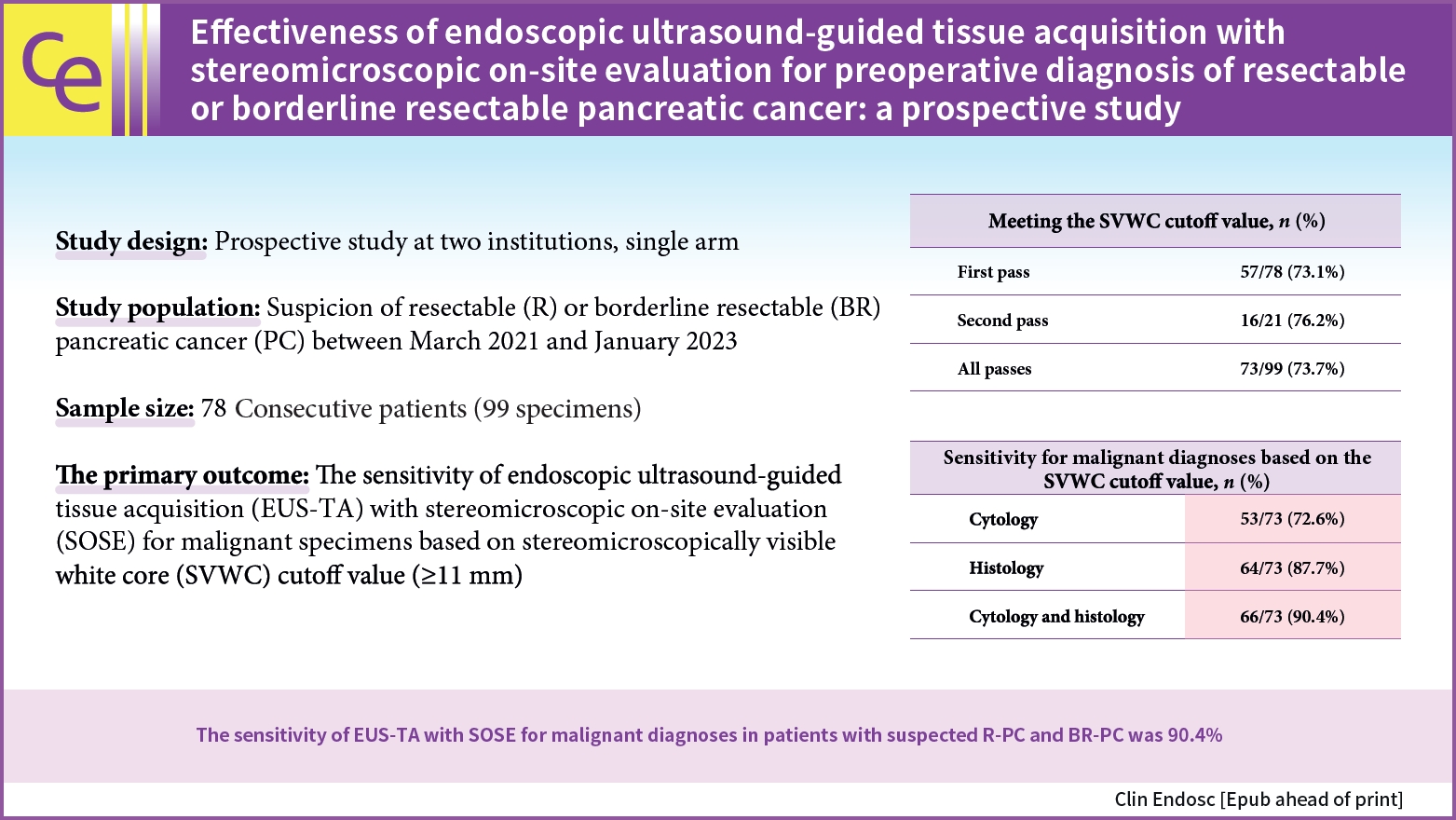
We collected 99 specimens from 56 and 22 patients with R-PC and BR-PC, respectively. Based on the SOSE results, we performed 57 procedures with one puncture. The SVWC cutoff values were met in 73.7% and 73.1% of all specimens and in those obtained during the first puncture, respectively. The final diagnoses were malignant and benign tumors in 76 and two patients, respectively. The overall sensitivity, specificity, and accuracy of EUS-TA for the 78 lesions were 90.8%, 100%, and 91.0%, respectively. The sensitivity for malignant diagnosis based on the SVWC cutoff value were 89.5% and 90.4% for the first puncture and all specimens, respectively.
Conclusions
The sensitivity of EUS-TA in conjunction with SOSE for malignancy diagnosis in patients with suspected R-PC or BR-PC was 90.4%.
- 1,303 View
- 26 Download

- Clinical utility of endoscopic ultrasound-guided tissue acquisition for comprehensive genomic profiling of patients with biliary tract cancer, especially with intrahepatic cholangiocarcinoma
- Takafumi Yanaidani, Kazuo Hara, Nozomi Okuno, Shin Haba, Takamichi Kuwahara, Yasuhiro Kuraishi, Nobumasa Mizuno, Sho Ishikawa, Masanori Yamada, Tsukasa Yasuda
- Clin Endosc 2024;57(3):384-392. Published online February 15, 2024
- DOI: https://doi.org/10.5946/ce.2023.139

-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: Endoscopic ultrasound-guided tissue acquisition (EUS-TA) is a standard diagnostic method for biliary tract cancer (BTC), and samples obtained in this manner may be used for comprehensive genomic profiling (CGP). This study evaluated the utility of EUS-TA for CGP in a clinical setting and determined the factors associated with the adequacy of CGP in patients with BTC.
Methods
CGP was attempted for 105 samples from 94 patients with BTC at the Aichi Cancer Center, Japan, from October 2019 to April 2022.
Results
Overall, 77.1% (81/105) of the samples were adequate for CGP. For 22-G or 19-G fine-needle biopsy (FNB), the sample adequacy was 85.7% (36/42), which was similar to that of surgical specimens (94%, p=0.45). Univariate analysis revealed that 22-G or larger FNB needle usage (86%, p=0.003), the target primary lesions (88%, p=0.015), a target size ≥30 mm (100%, p=0.0013), and number of punctures (90%, p=0.016) were significantly positively associated with CGP sample adequacy.
Conclusions
EUS-TA is useful for CGP tissue sampling in patients with BTC. In particular, the use of 22-G or larger FNB needles may allow for specimen adequacy comparable to that of surgical specimens. -
Citations
Citations to this article as recorded by- Is genomic analysis possible in a tissue acquired via endoscopic ultrasound-guided fine-needle biopsy in cholangiocarcinoma?
Jonghyun Lee, Sung Yong Han
Clinical Endoscopy.2024; 57(3): 332. CrossRef
- Is genomic analysis possible in a tissue acquired via endoscopic ultrasound-guided fine-needle biopsy in cholangiocarcinoma?
- 1,851 View
- 26 Download
- 1 Web of Science
- 1 Crossref

- 22-gauge Co-Cr versus stainless-steel Franseen needles for endoscopic ultrasound-guided tissue acquisition in patients with solid pancreatic lesions
- Yuki Tanisaka, Masafumi Mizuide, Akashi Fujita, Ryuhei Jinushi, Rie Shiomi, Takahiro Shin, Kei Sugimoto, Tomoaki Tashima, Yumi Mashimo, Shomei Ryozawa
- Clin Endosc 2024;57(2):237-245. Published online January 26, 2024
- DOI: https://doi.org/10.5946/ce.2023.011
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: Endoscopic ultrasound-guided tissue acquisition (EUS-TA) using Franseen needles is reportedly useful for its high diagnostic yield. This study compared the diagnostic yield and puncturing ability of EUS-TA using 22-gauge cobalt-chromium (CO-Cr) needles with those of stainless-steel Franseen needles in patients with solid pancreatic lesions.
Methods
Outcomes were compared between the 22-gauge Co-Cr Franseen needle (December 2019 to November 2020; group C) and stainless-steel needle (November 2020 to May 2022; group S).
Results
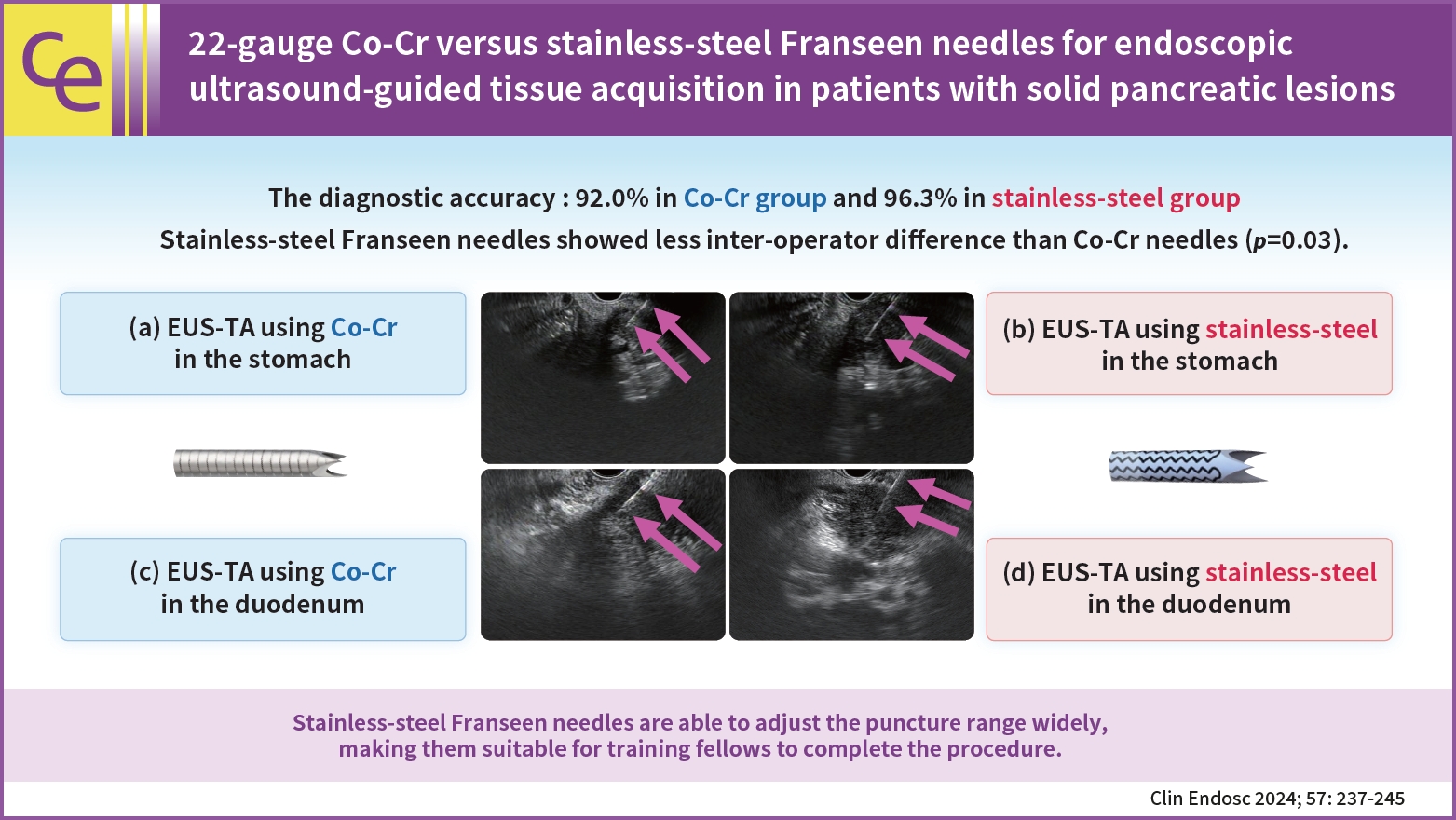
A total of 155 patients (group C, 75; group S, 80) were eligible. The diagnostic accuracy was 92.0% in group C and 96.3% in group S with no significant intergroup differences (p=0.32). The rate of change in the operator (from training fellows to experts) was 20.0% (15/75) in group C and 7.5% (6/80) in group S. Stainless-steel Franseen needles showed less inter-operator difference than Co-Cr needles (p=0.03).
Conclusions
Both Co-Cr and stainless-steel Franseen needles showed high diagnostic ability. Stainless-steel Franseen needles are soft and flexible; therefore, the range of puncture angles can be widely adjusted, making them suitable for training fellows to complete the procedure.
- 2,296 View
- 142 Download

- The role of needle-based confocal laser endomicroscopy in the diagnosis of pancreatic neuroendocrine tumors
- Masanori Yamada, Kazuo Hara, Nobumasa Mizuno, Shin Haba, Takamichi Kuwahara, Nozomi Okuno, Yasuhiro Kuraishi, Takafumi Yanaidani, Sho Ishikawa, Tsukasa Yasuda, Toshitaka Fukui
- Clin Endosc 2024;57(3):393-401. Published online September 12, 2023
- DOI: https://doi.org/10.5946/ce.2023.068
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is a highly accurate method for diagnosing pancreatic neuroendocrine tumors (PNETs); however, some PNETs are difficult to diagnose. Recently, the efficacy of needle-based confocal laser endomicroscopy (nCLE) in diagnosing solid pancreatic masses has been reported. However, the efficacy of nCLE in the diagnosis of PNETs remains unknown and only a small number of cases have been reported. Hence, this study aimed to evaluate the efficacy of nCLE in the diagnosis of PNETs.
Methods
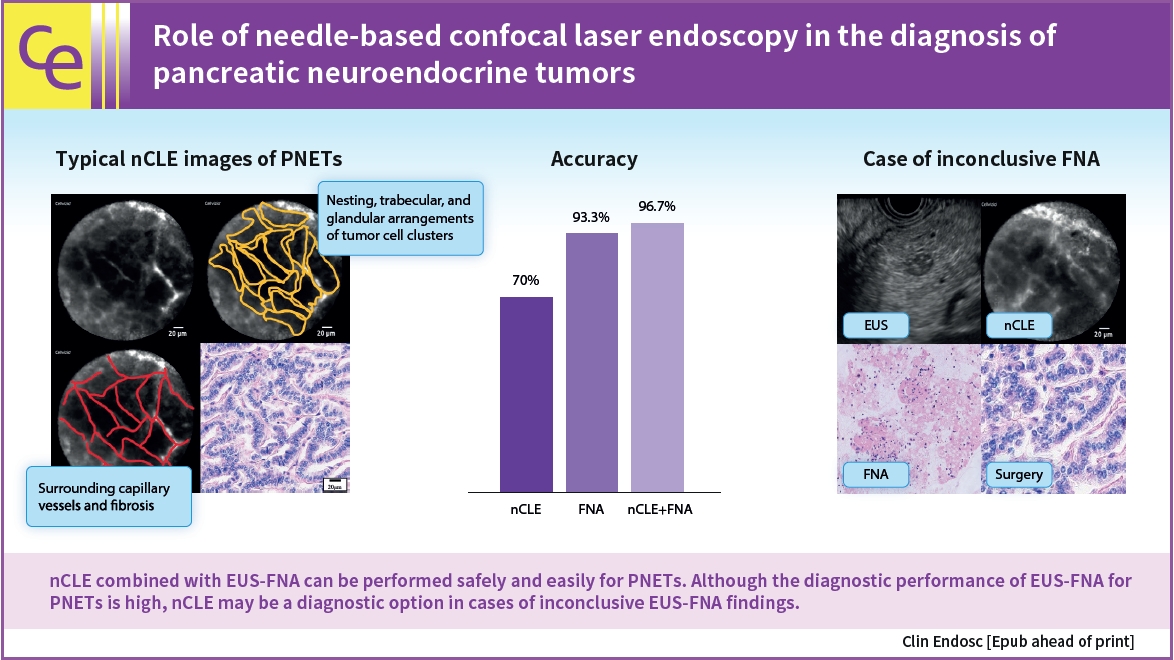
This single-center retrospective study evaluated 30 consecutive patients with suspected PNETs on contrast-enhanced computed tomography, who consented to nCLE combined with EUS-FNA and were diagnosed using EUS-FNA or surgical resection. The diagnostic criteria for PNETs using nCLE were based on the nesting and trabecular and glandular arrangement of tumor cell clusters surrounded by capillary vessels and fibrosis, as reported in previous studies.
Results
The diagnosis using nCLE was classified into three categories: misdiagnosis in three cases (10%), non-diagnostic in six cases (20%), and diagnostic in 21 cases (70%). nCLE was able to diagnose PNET in one of the two cases with inconclusive EUS-FNA.
Conclusions
Although further development of the resolution and optimization of the diagnostic criteria are required, nCLE may constitute a useful diagnostic option in cases of inconclusive EUS-FNA for PNETs. -
Citations
Citations to this article as recorded by- Recent developments in the diagnosis of pancreatic neuroendocrine neoplasms
Anna Battistella, Matteo Tacelli, Paola Mapelli, Marco Schiavo Lena, Valentina Andreasi, Luana Genova, Francesca Muffatti, Francesco De Cobelli, Stefano Partelli, Massimo Falconi
Expert Review of Gastroenterology & Hepatology.2024; 18(4-5): 155. CrossRef
- Recent developments in the diagnosis of pancreatic neuroendocrine neoplasms
- 2,167 View
- 110 Download
- 1 Web of Science
- 1 Crossref

- Comparison of 19-gauge conventional and Franseen needles for the diagnosis of lymphadenopathy and classification of malignant lymphoma using endoscopic ultrasound fine-needle aspiration
- Mitsuru Okuno, Keisuke Iwata, Tsuyoshi Mukai, Yusuke Kito, Takuji Tanaka, Naoki Watanabe, Senji Kasahara, Yuhei Iwasa, Akihiko Sugiyama, Youichi Nishigaki, Yuhei Shibata, Junichi Kitagawa, Takuji Iwashita, Eiichi Tomita, Masahito Shimizu
- Clin Endosc 2024;57(3):364-374. Published online September 8, 2023
- DOI: https://doi.org/10.5946/ce.2023.095
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub 
- Background
/Aims: Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) using a 19-gauge needle is an efficient sampling method for the diagnosis of lymphadenopathy. This study compared 19-gauge conventional and Franseen needles for the diagnosis of lymphadenopathy and classification of malignant lymphoma (ML).
Methods
Patient characteristics, number of needle passes, puncture route, sensitivity, specificity, and accuracy of cytology/histology for lymphadenopathy were analyzed in patients diagnosed with lymphadenopathy by EUS-FNA using conventional or Franseen needles.
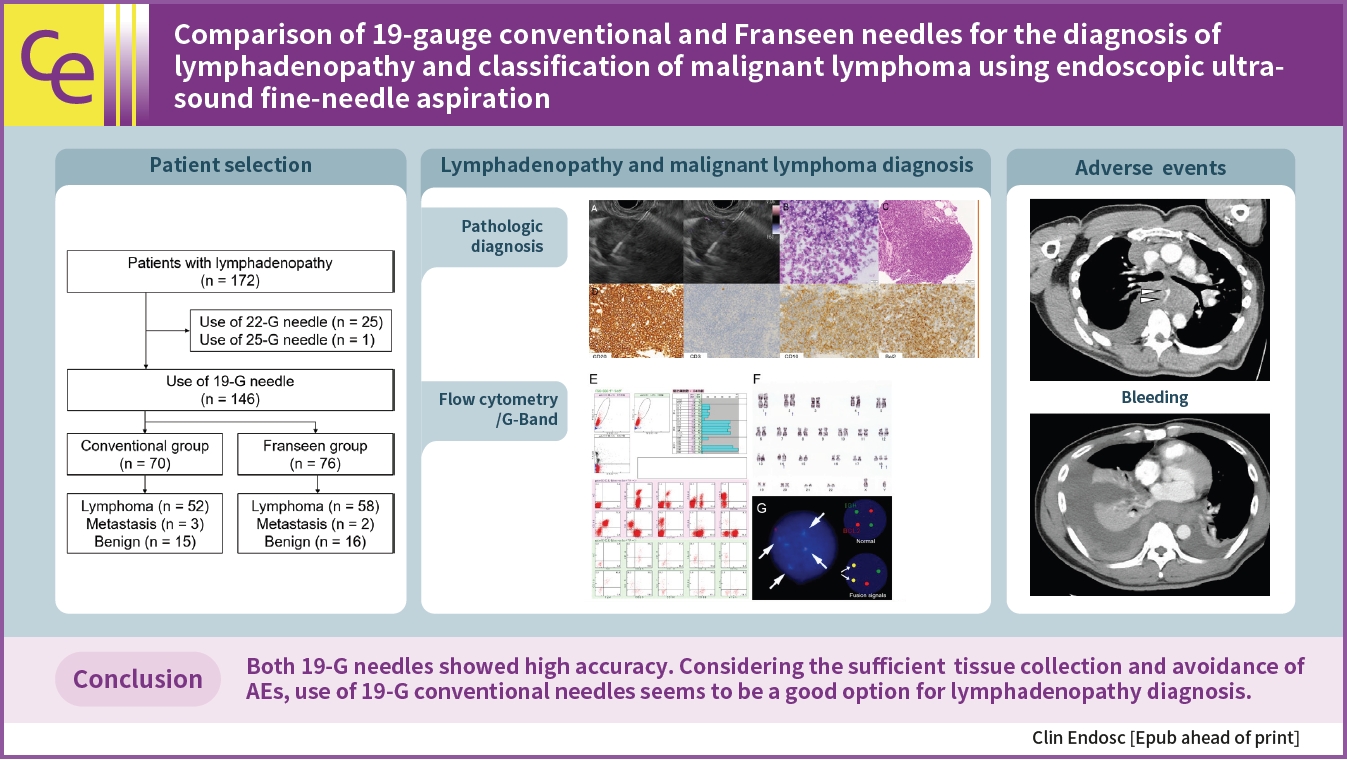
Results
Between 2012 and 2022, 146 patients met the inclusion criteria (conventional [n=70] and Franseen [n=76]). The median number of needle passes was significantly lower in the conventional group than in the Franseen group (3 [1–6] vs. 4 [1–6], p=0.023). There were no significant differences in cytological/histological diagnoses between the two groups. For ML, the immunohistochemical evaluation rate, sensitivity of flow cytometry, and cytogenetic assessment were not significantly different in either group. Bleeding as adverse events (AEs) were observed in three patients in the Franseen group.
Conclusions
Both the 19-gauge conventional and Franseen needles showed high accuracy in lymphadenopathy and ML classification. Considering sufficient tissue collection and the avoidance of AEs, the use of 19-gauge conventional needles seems to be a good option for the diagnosis of lymphadenopathy.
- 1,882 View
- 95 Download

-
Safety of endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction and ascites

- Tsukasa Yasuda, Kazuo Hara, Nobumasa Mizuno, Shin Haba, Takamichi Kuwahara, Nozomi Okuno, Yasuhiro Kuraishi, Takafumi Yanaidani, Sho Ishikawa, Masanori Yamada, Toshitaka Fukui
- Clin Endosc 2024;57(2):246-252. Published online September 7, 2023
- DOI: https://doi.org/10.5946/ce.2023.075

-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub 
- Background
/Aims: Endoscopic ultrasound (EUS)-guided hepaticogastrostomy (EUS-HGS) is useful for patients with biliary cannulation failure or inaccessible papillae. However, it can lead to serious complications such as bile peritonitis in patients with ascites; therefore, development of a safe method to perform EUS-HGS is important. Herein, we evaluated the safety of EUS-HGS with continuous ascitic fluid drainage in patients with ascites.
Methods
Patients with moderate or severe ascites who underwent continuous ascites drainage, which was initiated before EUS-HGS and terminated after the procedure at our institution between April 2015 and December 2022, were included in the study. We evaluated the technical and clinical success rates, EUS-HGS-related complications, and feasibility of re-intervention.
Results
Ten patients underwent continuous ascites drainage, which was initiated before EUS-HGS and terminated after completion of the procedure. Median duration of ascites drainage before and after EUS-HGS was 2 and 4 days, respectively. Technical success with EUS-HGS was achieved in all 10 patients (100%). Clinical success with EUS-HGS was achieved in 9 of the 10 patients (90 %). No endoscopic complications such as bile peritonitis were observed.
Conclusions
In patients with ascites, continuous ascites drainage, which is initiated before EUS-HGS and terminated after completion of the procedure, may prevent complications and allow safe performance of EUS-HGS. -
Citations
Citations to this article as recorded by- Management of iatrogenic perforations during endoscopic interventions in the hepato-pancreatico-biliary tract
Kirsten Boonstra, Rogier P. Voermans, Roy L.J. van Wanrooij
Best Practice & Research Clinical Gastroenterology.2024; : 101890. CrossRef
- Management of iatrogenic perforations during endoscopic interventions in the hepato-pancreatico-biliary tract
- 2,618 View
- 175 Download
- 1 Crossref

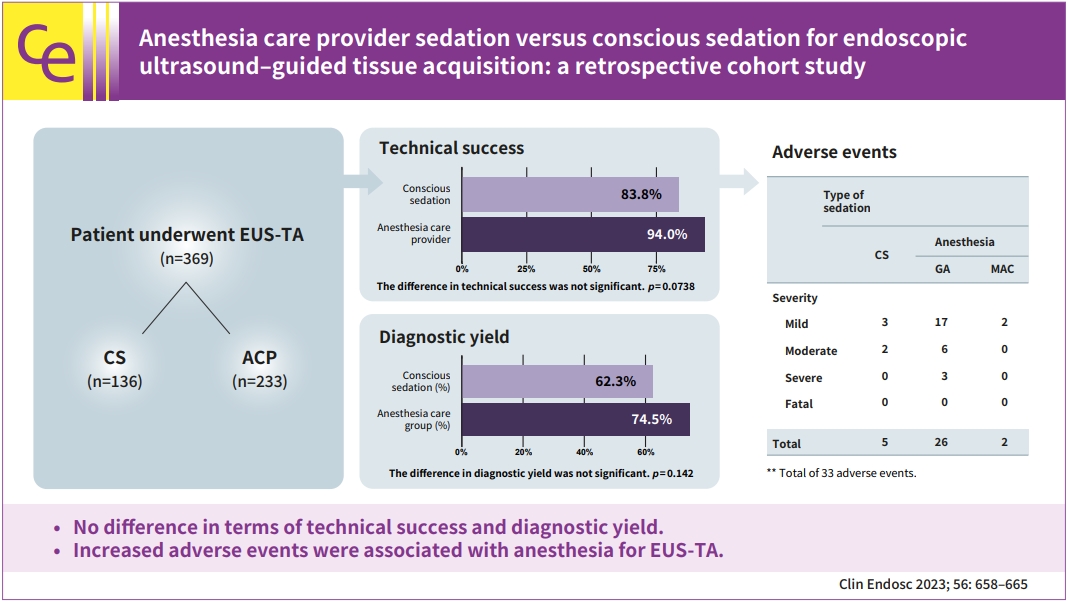
- Anesthesia care provider sedation versus conscious sedation for endoscopic ultrasound–guided tissue acquisition: a retrospective cohort study
- Sneha Shaha, Yinglin Gao, Jiahao Peng, Kendrick Che, John J. Kim, Wasseem Skef
- Clin Endosc 2023;56(5):658-665. Published online July 3, 2023
- DOI: https://doi.org/10.5946/ce.2023.006
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: We aimed to study the effects of sedation on endoscopic ultrasound–guided tissue acquisition.
Methods
We conducted a retrospective study evaluating the role of sedation in endoscopic ultrasound–guided tissue acquisition by comparing two groups: anesthesia care provider (ACP) sedation and endoscopist-directed conscious sedation (CS).
Results
Technical success was achieved in 219/233 (94.0%) in the ACP group and 114/136 (83.8%) in the CS group (p=0.0086). In multivariate analysis, the difference in technical success between the two groups was not significant (adjusted odds ratio [aOR], 0.5; 95% confidence interval [CI], 0.234–1.069; p=0.0738). A successful diagnostic yield was present in 146/196 (74.5%) in the ACP group and 66/106 (62.3%) in the CS group, respectively (p=0.0274). In multivariate analysis, the difference in diagnostic yield between the two groups was not significant (aOR, 0.643; 95% CI, 0.356–1.159; p=0.142). A total of 33 adverse events (AEs) were observed. The incidence of AEs was significantly lower in the CS group (5/33 CS vs. 28/33 ACP; OR, 0.281; 95% CI, 0.095–0.833; p=0.022).
Conclusions
CS provided equivalent technical success and diagnostic yield for malignancy in endoscopic ultrasound–guided tissue acquisition. Increased AEs were associated with anesthesia for the endoscopic ultrasound–guided tissue acquisition.
- 1,910 View
- 57 Download

Systematic Review and Meta-analysis
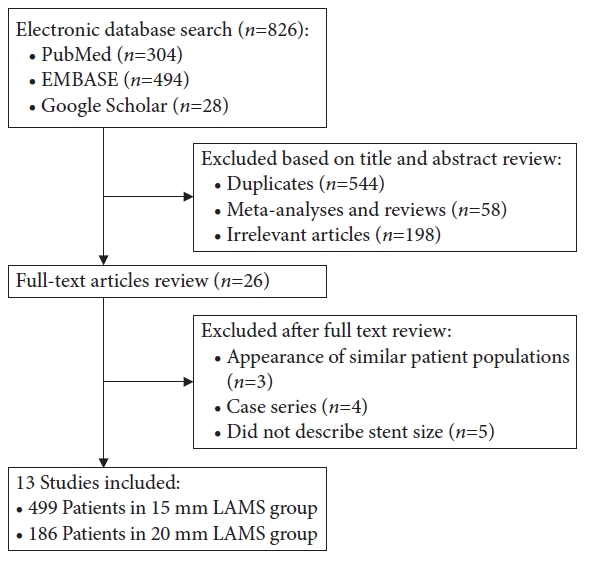
- No difference in outcomes with 15 mm vs. 20 mm lumen-apposing metal stents for endoscopic ultrasound-guided gastroenterostomy for gastric outlet obstruction: a meta-analysis
- Shyam Vedantam, Rahil Shah, Sean Bhalla, Shria Kumar, Sunil Amin
- Clin Endosc 2023;56(3):298-307. Published online May 22, 2023
- DOI: https://doi.org/10.5946/ce.2022.299
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: We compared outcomes between use of 15 vs. 20 mm lumen-apposing metal stents (LAMSs) in endoscopic ultrasound-guided gastroenterostomy (EUS-GE) for gastric outlet obstruction.
Methods
Databases were queried for studies that used LAMS for EUS-GE to relieve gastric outlet obstruction, and a proportional meta-analysis was performed.
Results
Thirteen studies were included. The 15 mm and 20 mm LAMS had pooled technical success rates of 93.2% (95% confidence interval [CI], 90.5%–95.2%) and 92.1% (95% CI, 68.4%–98.4%), clinical success rates of 88.6% (95% CI, 85.4%–91.1%) and 89.6% (95% CI, 79.0%–95.1%), adverse event rates of 11.4% (95% CI, 8.1%–15.9%) and 14.7% (95% CI, 4.4%–39.1%), and reintervention rates of 10.3% (95% CI, 6.7%–15.4%) and 3.5% (95% CI, 1.6%–7.6%), respectively. Subgroup analysis revealed no significant differences in technical success, clinical success, or adverse event rates. An increased need for reintervention was noted in the 15 mm stent group (pooled odds ratio, 3.59; 95% CI, 1.40–9.18; p=0.008).
Conclusions
No differences were observed in the technical, clinical, or adverse event rates between 15 and 20 mm LAMS use in EUS-GE. An increased need for reintervention is possible when using a 15 mm stent compared to when using a 20 mm stent. -
Citations
Citations to this article as recorded by- Endoscopic gastrointestinal bypass anastomosis using deformable self-assembled magnetic anastomosis rings (DSAMARs) in a pig model
Miaomiao Zhang, Jianqi Mao, Jia Ma, Shuqin Xu, Yi Lyu, Xiaopeng Yan
BMC Gastroenterology.2024;[Epub] CrossRef - Revealing Insights: A Comprehensive Overview of Gastric Outlet Obstruction Management, with Special Emphasis on EUS-Guided Gastroenterostomy
Dimitrios Ziogas, Thomas Vasilakis, Christina Kapizioni, Eleni Koukoulioti, Georgios Tziatzios, Paraskevas Gkolfakis, Antonio Facciorusso, Ioannis S. Papanikolaou
Medical Sciences.2024; 12(1): 9. CrossRef - Lumen-apposing metal stents: A primer on indications and technical tips
Sridhar Sundaram, Suprabhat Giri, Kenneth Binmoeller
Indian Journal of Gastroenterology.2024;[Epub] CrossRef - The Role of Luminal Apposing Metal Stents on the Treatment of Malignant and Benign Gastric Outlet Obstruction
Mihai Rimbaș, Kar Wai Lau, Giulia Tripodi, Gianenrico Rizzatti, Alberto Larghi
Diagnostics.2023; 13(21): 3308. CrossRef
- Endoscopic gastrointestinal bypass anastomosis using deformable self-assembled magnetic anastomosis rings (DSAMARs) in a pig model
- 2,380 View
- 116 Download
- 4 Web of Science
- 4 Crossref

Case Reports
- Refractory benign biliary stricture due to chronic pancreatitis in two patients treated using endoscopic ultrasound-guided choledochoduodenostomy fistula creation: case reports
- Sho Ishikawa, Nozomi Okuno, Kazuo Hara, Nobumasa Mizuno, Shin Haba, Takamichi Kuwahara, Yasuhiro Kuraishi, Takafumi Yanaidani
- Clin Endosc 2024;57(1):122-127. Published online May 16, 2023
- DOI: https://doi.org/10.5946/ce.2022.149
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Benign biliary stricture (BBS) is a complication of chronic pancreatitis (CP). Despite endoscopic biliary stenting, some patients do not respond to treatment, and they experience recurrent cholangitis. We report two cases of CP with refractory BBS treated using endoscopic ultrasound-guided choledochoduodenostomy (EUS-CDS) fistula creation. A 50-year-old woman and a 60-year-old man both presented with obstructive jaundice secondary to BBS due to alcoholic CP. They underwent repeated placement of a fully covered self-expandable metal stent for biliary strictures. However, the strictures persisted, causing repeated episodes of cholangitis. Therefore, an EUS-CDS was performed. The stents were eventually removed and the patients became stent-free. These fistulas have remained patent without cholangitis for more than 2.5 years. Fistula creation using EUS-CDS is an effective treatment option for BBS.
-
Citations
Citations to this article as recorded by- Forward viewing liner echoendoscopy for therapeutic interventions
Kazuo Hara, Nozomi Okuno, Shin Haba, Takamichi Kuwahara
Clinical Endoscopy.2024; 57(2): 175. CrossRef
- Forward viewing liner echoendoscopy for therapeutic interventions
- 1,766 View
- 144 Download
- 1 Web of Science
- 1 Crossref

- Endoscopic ultrasound-guided hepaticogastrostomy by puncturing both B2 and B3: a single center experience
- Moaz Elshair, Kazuo Hara, Nozomi Okuno, Shin Haba, Takamichi Kuwahara, Asmaa Bakr, Abdou Elshafei, Mohamed Z. Abu-Amer
- Received August 12, 2022 Accepted November 23, 2022 Published online May 3, 2023
- DOI: https://doi.org/10.5946/ce.2022.209 [Epub ahead of print]

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic ultrasound-guided hepaticogastrostomy (EUS-HGS) through ducts B2 or B3 is effective in most patients with biliary obstruction, because B2 and B3 commonly join together. However, in some patients, B2 and B3 do not join each other due to invasive hilar tumors; therefore, single-route drainage is insufficient. Here, we investigated the feasibility and efficacy of EUS-HGS through both B2 and B3 simultaneously in seven patients. We decided to perform EUS-HGS through both B2 and B3 to achieve adequate biliary drainage because these two ducts were separate from each other. Here, we report a 100% technical and overall clinical success rate. Early adverse effects were closely monitored. Minimal bleeding was reported in one patient (1/7) and mild peritonitis in one patient (1/7). None of the patients experienced stent dysfunction, fever, or bile leakage after the procedure. EUS-HGS through both B2 and B3 simultaneously is safe, feasible, and effective for biliary drainage in patients with separated ducts.
- 1,363 View
- 63 Download

Original Articles
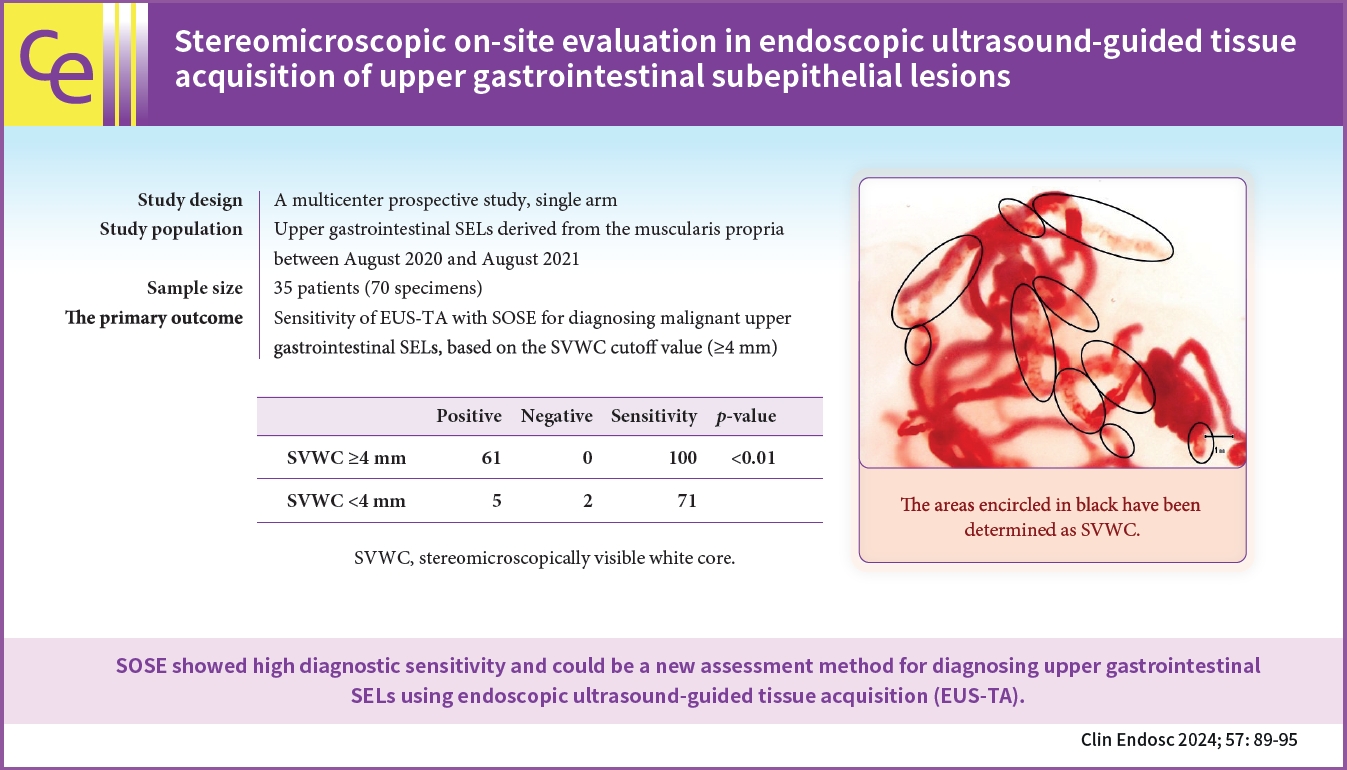
- Stereomicroscopic on-site evaluation in endoscopic ultrasound-guided tissue acquisition of upper gastrointestinal subepithelial lesions
- Seigo Nakatani, Kosuke Okuwaki, Masafumi Watanabe, Hiroshi Imaizumi, Tomohisa Iwai, Takaaki Matsumoto, Rikiya Hasegawa, Hironori Masutani, Takahiro Kurosu, Akihiro Tamaki, Junro Ishizaki, Ayana Ishizaki, Mitsuhiro Kida, Chika Kusano
- Clin Endosc 2024;57(1):89-95. Published online April 18, 2023
- DOI: https://doi.org/10.5946/ce.2022.288
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: In stereomicroscopic sample isolation processing, the cutoff value (≥4 mm) of stereomicroscopically visible white cores indicates high diagnostic sensitivity. We aimed to evaluate endoscopic ultrasound-guided tissue acquisition (EUS-TA) using a simplified stereomicroscopic on-site evaluation of upper gastrointestinal subepithelial lesions (SELs).
Methods
In this multicenter prospective trial, we performed EUS-TA using a 22-gauge Franseen needle in 34 participants with SELs derived from the upper gastrointestinal muscularis propria, requiring pathological diagnosis. The presence of stereomicroscopically visible white core (SVWC) in each specimen was assessed using stereomicroscopic on-site evaluation. The primary outcome was EUS-TA’s diagnostic sensitivity with stereomicroscopic on-site evaluation based on the SVWC cutoff value (≥4 mm) for malignant upper gastrointestinal SELs.
Results
The total number of punctures was 68; 61 specimens (89.7%) contained stereomicroscopically visible white cores ≥4 mm in size. The final diagnoses were gastrointestinal stromal tumor, leiomyoma, and schwannoma in 76.5%, 14.7%, and 8.8% of the cases, respectively. The sensitivity of EUS-TA with stereomicroscopic on-site evaluation based on the SVWC cutoff value for malignant SELs was 100%. The per-lesion accuracy of histological diagnosis reached the highest level (100%) at the second puncture.
Conclusions
Stereomicroscopic on-site evaluation showed high diagnostic sensitivity and could be a new method for diagnosing upper gastrointestinal SELs using EUS-TA. -
Citations
Citations to this article as recorded by- What method can we choose if rapid on-site evaluation is not available for the endoscopic ultrasound-guided tissue acquisition of upper gastrointestinal subepithelial lesions?
Yu Kyung Cho
Clinical Endoscopy.2024; 57(1): 53. CrossRef - Endoscopic Ultrasound-Guided Tissue Acquisition Using Fork-Tip Needle for Subepithelial Lesions: A Single-Center Validation Study
Masafumi Watanabe, Kosuke Okuwaki, Tomohisa Iwai, Mitsuhiro Kida, Hiroshi Imaizumi, Kai Adachi, Akihiro Tamaki, Junro Ishizaki, Taro Hanaoka, Chika Kusano
Digestive Diseases and Sciences.2024;[Epub] CrossRef - An Esophageal Leiomyoma with Cystic Degeneration Mimicking a Malignant Neoplasm
Gwang Ha Kim, Dong Chan Joo, Moon Won Lee, Bong Eun Lee, Kyungbin Kim
The Ewha Medical Journal.2023;[Epub] CrossRef
- What method can we choose if rapid on-site evaluation is not available for the endoscopic ultrasound-guided tissue acquisition of upper gastrointestinal subepithelial lesions?
- 1,837 View
- 140 Download
- 3 Web of Science
- 3 Crossref

- Covered self-expandable metallic stents versus plastic stents for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction
- Taro Shibuki, Kei Okumura, Masanari Sekine, Ikuhiro Kobori, Aki Miyagaki, Yoshihiro Sasaki, Yuichi Takano, Yusuke Hashimoto
- Clin Endosc 2023;56(6):802-811. Published online April 5, 2023
- DOI: https://doi.org/10.5946/ce.2022.211

-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: Covered self-expandable metallic stents (cSEMS) have become popular for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting (EUS-HGS). We compared the time to recurrent biliary obstruction (TRBO), complications, and reintervention rates between EUS-HGS using plastic stent (PS) and cSEMS in patients with unresectable malignancies at multicenter institutions in Japan.
Methods
Patients with unresectable malignant biliary obstruction who underwent EUS-HGS between April 2015 and July 2020 at any of the six participating facilities were enrolled. Primary endpoint: TRBO; secondary endpoints: rate of complications other than recurrent biliary obstruction and technical success rate of reintervention were evaluated.
Results
PS and cSEMS were used for EUS-HGS in 109 and 43 patients, respectively. The TRBO was significantly longer in the cSEMS group than in the PS group (646 vs. 202 days). Multivariate analysis identified two independent factors associated with a favorable TRBO: combined EUS-guided antegrade stenting with EUS-HGS and the use of cSEMS. No significant difference was observed in the rate of complications other than recurrent biliary obstruction between the two groups. The technical success rate of reintervention was 85.7% for PS and 100% for cSEMS (p=0.309).
Conclusions
cSEMS might be a better option for EUS-HGS in patients with unresectable malignancies, given the longer TRBO. -
Citations
Citations to this article as recorded by- The writing on the wall: self-expandable stents for endoscopic ultrasound-guided hepaticogastrostomy?
Hyung Ku Chon, Shayan Irani, Tae Hyeon Kim
Clinical Endoscopy.2023; 56(6): 741. CrossRef - Therapeutic Endoscopic Ultrasound for Complications of Pancreatic Cancer
Samuel Han, Georgios I. Papachristou
Cancers.2023; 16(1): 29. CrossRef
- The writing on the wall: self-expandable stents for endoscopic ultrasound-guided hepaticogastrostomy?
- 2,200 View
- 173 Download
- 2 Web of Science
- 2 Crossref

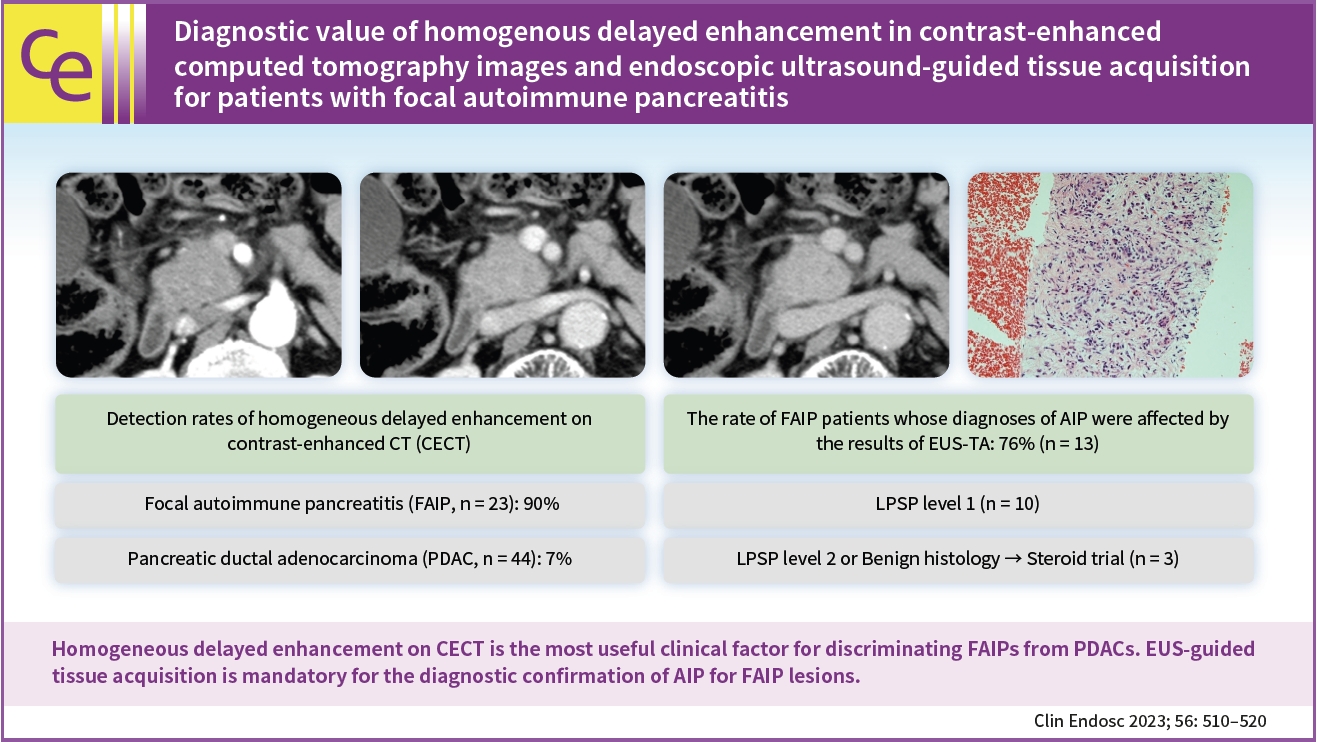
- Diagnostic value of homogenous delayed enhancement in contrast-enhanced computed tomography images and endoscopic ultrasound-guided tissue acquisition for patients with focal autoimmune pancreatitis
- Keisuke Yonamine, Shinsuke Koshita, Yoshihide Kanno, Takahisa Ogawa, Hiroaki Kusunose, Toshitaka Sakai, Kazuaki Miyamoto, Fumisato Kozakai, Hideyuki Anan, Haruka Okano, Masaya Oikawa, Takashi Tsuchiya, Takashi Sawai, Yutaka Noda, Kei Ito
- Clin Endosc 2023;56(4):510-520. Published online April 5, 2023
- DOI: https://doi.org/10.5946/ce.2022.142
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: We aimed to investigate (1) promising clinical findings for the recognition of focal type autoimmune pancreatitis (FAIP) and (2) the impact of endoscopic ultrasound (EUS)-guided tissue acquisition (EUS-TA) on the diagnosis of FAIP.
Methods
Twenty-three patients with FAIP were involved in this study, and 44 patients with resected pancreatic ductal adenocarcinoma (PDAC) were included in the control group.
Results
(1) Multivariate analysis revealed that homogeneous delayed enhancement on contrast-enhanced computed tomography was a significant factor indicative of FAIP compared to PDAC (90% vs. 7%, p=0.015). (2) For 13 of 17 FAIP patients (76.5%) who underwent EUS-TA, EUS-TA aided the diagnostic confirmation of AIPs, and only one patient (5.9%) was found to have AIP after surgery. On the other hand, of the six patients who did not undergo EUS-TA, three (50.0%) underwent surgery for pancreatic lesions.
Conclusions
Homogeneous delayed enhancement on contrast-enhanced computed tomography was the most useful clinical factor for discriminating FAIPs from PDACs. EUS-TA is mandatory for diagnostic confirmation of FAIP lesions and can contribute to a reduction in the rate of unnecessary surgery for patients with FAIP. -
Citations
Citations to this article as recorded by- A multidisciplinary approach is essential for differentiating autoimmune pancreatitis from pancreatic adenocarcinoma
Sung-Hoon Moon
Clinical Endoscopy.2023; 56(4): 457. CrossRef
- A multidisciplinary approach is essential for differentiating autoimmune pancreatitis from pancreatic adenocarcinoma
- 1,803 View
- 75 Download
- 1 Web of Science
- 1 Crossref

Case Report
-
Single-pigtail plastic stent made from endoscopic nasobiliary drainage tubes in endoscopic ultrasound-guided gallbladder drainage: a retrospective case series

- Koichi Soga
- Clin Endosc 2024;57(2):263-267. Published online April 4, 2023
- DOI: https://doi.org/10.5946/ce.2022.213
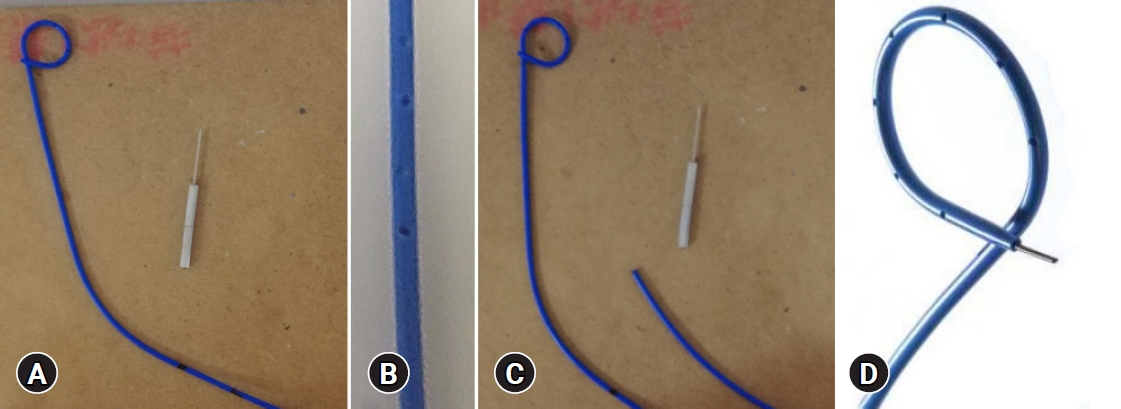
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Technical failure of endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) is often attributed to device failure. To rectify this problem, we developed a single-pigtail plastic stent (SPPS) for EUS-GBD. We retrospectively reviewed the cases of four patients who underwent EUS-GBD for acute cholecystitis. To prepare the SPPS, a 7.5-Fr endoscopic nasobiliary drainage tube was cut to an appropriate length. The use of SPPS during EUS-GBD was successful from both technical and clinical standpoints. The SPPS spontaneously detached 57 days after the procedure in patient 4 and 412 days after the procedure in patient 1. Patient 1 developed cholecystitis after 426 days and was managed with antibiotics. The other three patients did not develop any complications after surgery. In conclusion, we designed a new SPPS dedicated to EUS-GBD and established its technical feasibility and clinical effectiveness.
-
Citations
Citations to this article as recorded by- Usefulness of inserting a modified single‐pigtail plastic stent into a metallic stent in endoscopic ultrasound-guided gallbladder drainage
Koichi Soga
Endoscopy.2023; 55(S 01): E1081. CrossRef
- Usefulness of inserting a modified single‐pigtail plastic stent into a metallic stent in endoscopic ultrasound-guided gallbladder drainage
- 2,413 View
- 175 Download
- 1 Crossref

Original Article
- Clinical utility of endoscopic ultrasound-guided tissue acquisition for comprehensive genomic profiling of pancreatic cancer
- Nozomi Okuno, Kazuo Hara, Nobumasa Mizuno, Shin Haba, Takamichi Kuwahara, Yasuhiro Kuraishi, Daiki Fumihara, Takafumi Yanaidani
- Clin Endosc 2023;56(2):221-228. Published online March 7, 2023
- DOI: https://doi.org/10.5946/ce.2022.086

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic ultrasound-guided tissue acquisition (EUS-TA) is essential for the diagnosis of pancreatic cancer. The feasibility of comprehensive genomic profiling (CGP) using samples obtained by EUS-TA has been under recent discussion. This study aimed to evaluate the utility of EUS-TA for CGP in a clinical setting.
Methods
CGP was attempted in 178 samples obtained from 151 consecutive patients with pancreatic cancer at the Aichi Cancer Center between October 2019 and September 2021. We evaluated the adequacy of the samples for CGP and determined the factors associated with the adequacy of the samples obtained by EUS-TA retrospectively.
Results
The overall adequacy for CGP was 65.2% (116/178), which was significantly different among the four sampling methods (EUS-TA vs. surgical specimen vs. percutaneous biopsy vs. duodenal biopsy, 56.0% [61/109] vs. 80.4% [41/51] vs. 76.5% [13/17] vs. 100.0% [1/1], respectively; p=0.022). In a univariate analysis, needle gauge/type was associated with adequacy (22 G fine-needle aspiration vs. 22 G fine-needle biopsy [FNB] vs. 19 G-FNB, 33.3% (5/15) vs. 53.5% (23/43) vs. 72.5% (29/40); p=0.022). The sample adequacy of 19 G-FNB for CGP was 72.5% (29/40), and there was no significant difference between 19 G-FNB and surgical specimens (p=0.375).
Conclusions
To obtain adequate samples for CGP with EUS-TA, 19 G-FNB was shown to be the best in clinical practice. However, 19 G-FNB was not still sufficient, so further efforts are required to improve adequacy for CGP. -
Citations
Citations to this article as recorded by- Tissue acquisition for comprehensive genomic profiling of gallbladder cancer using a forward-viewing echoendoscope in a patient who underwent Roux-en-Y reconstruction
Michihiro Ono, Shutaro Oiwa, Atsushi Uesugi, Seiya Saito, Ryota Yokoyama, Makoto Usami, Tomoyuki Abe, Miri Fujita, Kohichi Takada, Masahiro Maeda
Clinical Journal of Gastroenterology.2024; 17(1): 164. CrossRef - Endoscopic ultrasound-guided tissue acquisition for comprehensive genomic profiling
Nozomi Okuno, Kazuo Hara
Journal of Medical Ultrasonics.2024; 51(2): 253. CrossRef - Oil blotting paper for formalin fixation increases endoscopic ultrasound‐guided tissue acquisition‐collected sample volumes on glass slides
Takuo Yamai, Kenji Ikezawa, Yusuke Seiki, Ko Watsuji, Yasuharu Kawamoto, Takeru Hirao, Kazuma Daiku, Shingo Maeda, Makiko Urabe, Yugo Kai, Ryoji Takada, Kaori Mukai, Tasuku Nakabori, Hiroyuki Uehara, Sayoko Tsuzaki, Ayumi Ryu, Satoshi Tanada, Shigenori Na
Cancer Medicine.2024;[Epub] CrossRef - Endoscopic ultrasound-guided tissue acquisition for personalized treatment in pancreatic adenocarcinoma
Sang Myung Woo
Clinical Endoscopy.2023; 56(2): 183. CrossRef - Comparison of the novel Franseen needle versus the fine‐needle aspiration needle in endoscopic ultrasound‐guided tissue acquisition for cancer gene panel testing: A propensity score‐matching analysis
Tomotaka Mori, Eisuke Ozawa, Akane Shimakura, Kosuke Takahashi, Satoshi Matsuo, Kazuaki Tajima, Yasuhiko Nakao, Masanori Fukushima, Ryu Sasaki, Satoshi Miuma, Hisamitsu Miyaaki, Shinji Okano, Kazuhiko Nakao
JGH Open.2023; 7(9): 652. CrossRef - Editorial: Endoscopic ultrasound‐guided tissue acquisition in the era of precision medicine
Tiing Leong Ang, James Weiquan Li, Lai Mun Wang
Journal of Gastroenterology and Hepatology.2023; 38(10): 1677. CrossRef
- Tissue acquisition for comprehensive genomic profiling of gallbladder cancer using a forward-viewing echoendoscope in a patient who underwent Roux-en-Y reconstruction
- 1,940 View
- 157 Download
- 4 Web of Science
- 6 Crossref

Review
- Endoscopic ultrasound-guided intervention for inaccessible papilla in advanced malignant hilar biliary obstruction
- Partha Pal, Sundeep Lakhtakia
- Clin Endosc 2023;56(2):143-154. Published online February 17, 2023
- DOI: https://doi.org/10.5946/ce.2022.198

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Advanced malignant hilar biliary obstruction (MHBO) with inaccessible papilla poses a significant challenge to endoscopists, as drainage of multiple liver segments may be warranted. Transpapillary drainage may not be feasible in patients with surgically altered anatomy, duodenal stenosis, prior duodenal self-expanding metal stent, and after initial transpapillary drainage, but require re-intervention for draining separated liver segments. Endoscopic ultrasound-guided biliary drainage (EUS-BD) and percutaneous trans-hepatic biliary drainage are the feasible options in this scenario. The major advantages of EUS-BD over percutaneous trans-hepatic biliary drainage include a reduction in patient discomfort and internal drainage away from the tumor, thus reducing the possibility of tissue or tumor ingrowth. With innovations, EUS-BD is helpful not only for bilateral communicating MHBO but also for non-communicating systems with bridging hilar stents or isolated right intra-hepatic duct drainage by hepatico-duodenostomy. EUS-guided multi-stent drainage with specially designed cannulas and guidewires has become a reality. A combined approach with endoscopic retrograde cholangiopancreatography for re-intervention, interventional radiology, and intraductal tumor ablative therapies has been reported. Stent migration and bile leakage can be minimized with proper stent selection and technique, and stent blocks can be managed with EUS-guided interventions in a majority of cases. Future comparative studies are required to establish the role of EUS-guided interventions in MHBO as rescue or primary therapy.
-
Citations
Citations to this article as recorded by- Endoscopic Management of Malignant Biliary Obstruction
Woo Hyun Paik, Do Hyun Park
Gastrointestinal Endoscopy Clinics of North America.2024; 34(1): 127. CrossRef - The Lambda stenting technique: a new approach to address EUS-guided biliary drainage–associated adverse events
Hiroki Sato, Hidemasa Kawabata, Mikihiro Fujiya
VideoGIE.2024; 9(2): 107. CrossRef
- Endoscopic Management of Malignant Biliary Obstruction
- 3,060 View
- 235 Download
- 2 Crossref

Systematic Review and Meta-analysis
- Influence of biliary stents on the diagnostic outcome of endoscopic ultrasound–guided tissue acquisition from solid pancreatic lesions: a systematic review and meta-analysis
- Suprabhat Giri, Shivaraj Afzalpurkar, Sumaswi Angadi, Jijo Varghese, Sridhar Sundaram
- Clin Endosc 2023;56(2):169-179. Published online February 15, 2023
- DOI: https://doi.org/10.5946/ce.2022.282
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: This meta-analysis analyzed the effect of an indwelling biliary stent on endoscopic ultrasound (EUS)–guided tissue acquisition from pancreatic lesions.
Methods
A literature search was performed to identify studies published between 2000 and July 2022 comparing the diagnostic outcomes of EUS-TA in patients with or without biliary stents. For non-strict criteria, samples reported as malignant or suspicious for malignancy were included, whereas for strict criteria, only samples reported as malignant were included in the analysis.
Results
Nine studies were included in this analysis. The odds of an accurate diagnosis were significantly lower in patients with indwelling stents using both non-strict (odds ratio [OR], 0.68; 95% confidence interval [CI], 0.52–0.90) and strict criteria (OR, 0.58; 95% CI, 0.46–0.74). The pooled sensitivity with and without stents were similar (87% vs. 91%) using non-strict criteria. However, patients with stents had a lower pooled sensitivity (79% vs. 88%) when using strict criteria. The sample inadequacy rate was comparable between groups (OR, 1.12; 95% CI, 0.76–1.65). The diagnostic accuracy and sample inadequacy were comparable between plastic and metal biliary stents.
Conclusions
The presence of a biliary stent may negatively affect the diagnostic outcome of EUS-TA for pancreatic lesions. -
Citations
Citations to this article as recorded by- Role of Endoscopic Ultrasound in Diagnosis of Pancreatic Ductal Adenocarcinoma
Abhirup Chatterjee, Jimil Shah
Diagnostics.2023; 14(1): 78. CrossRef
- Role of Endoscopic Ultrasound in Diagnosis of Pancreatic Ductal Adenocarcinoma
- 2,131 View
- 109 Download
- 1 Crossref

Original Articles
- Role of interventional endoscopic ultrasound in a developing country
- Hasan Maulahela, Nagita Gianty Annisa, Achmad Fauzi, Kaka Renaldi, Murdani Abdullah, Marcellus Simadibrata, Dadang Makmun, Ari Fahrial Syam
- Clin Endosc 2023;56(1):100-106. Published online January 17, 2023
- DOI: https://doi.org/10.5946/ce.2022.058
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic ultrasound (EUS) has become an essential diagnostic and therapeutic tool. EUS was introduced in 2013 in Indonesia and is considered relatively new. This study aimed to describe the current role of interventional EUS at our hospital as a part of the Indonesian tertiary health center experience.
Methods
This retrospective study included all patients who underwent interventional EUS (n=94) at our center between January 2015 and December 2020. Patient characteristics, technical success, clinical success, and adverse events associated with each type of interventional EUS procedure were evaluated.
Results
Altogether, 94 interventional EUS procedures were performed at our center between 2015 and 2020 including 75 cases of EUS-guided biliary drainage (EUS-BD), 14 cases of EUS-guided pancreatic fluid drainage, and five cases of EUS-guided celiac plexus neurolysis. The technical and clinical success rates of EUS-BD were 98.6% and 52%, respectively. The technical success rate was 100% for both EUS-guided pancreatic fluid drainage and EUS-guided celiac plexus neurolysis. The adverse event rates were 10.6% and 7.1% for EUS-BD and EUS-guided pancreatic fluid drainage, respectively.
Conclusions
EUS is an effective and safe tool for the treatment of gastrointestinal and biliary diseases. It has a low rate of adverse events, even in developing countries. -
Citations
Citations to this article as recorded by- EUS-guided interventional therapies for pancreatic diseases
Rongmin Xu, Kai Zhang, Nan Ge, Siyu Sun
Frontiers in Medicine.2024;[Epub] CrossRef
- EUS-guided interventional therapies for pancreatic diseases
- 2,055 View
- 135 Download
- 1 Web of Science
- 1 Crossref

- A multicenter comparative study of endoscopic ultrasound-guided fine-needle biopsy using a Franseen needle versus conventional endoscopic ultrasound-guided fine-needle aspiration to evaluate microsatellite instability in patients with unresectable pancreatic cancer
- Tadayuki Takagi, Mitsuru Sugimoto, Hidemichi Imamura, Yosuke Takahata, Yuki Nakajima, Rei Suzuki, Naoki Konno, Hiroyuki Asama, Yuki Sato, Hiroki Irie, Jun Nakamura, Mika Takasumi, Minami Hashimoto, Tsunetaka Kato, Ryoichiro Kobashi, Yuko Hashimoto, Goro Shibukawa, Shigeru Marubashi, Takuto Hikichi, Hiromasa Ohira
- Clin Endosc 2023;56(1):107-113. Published online January 16, 2023
- DOI: https://doi.org/10.5946/ce.2022.019

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Immune checkpoint blockade has recently been reported to be effective in treating microsatellite instability (MSI)-high tumors. Therefore, sufficient sampling of histological specimens is necessary in cases of unresectable pancreatic cancer (UR-PC). This multicenter study investigated the efficacy of endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB) using a Franseen needle for MSI evaluation in patients with UR-PC.
Methods
A total of 89 patients with UR-PC who underwent endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) or EUS-FNB using 22-G needles at three hospitals in Japan (2018–2021) were enrolled. Fifty-six of these patients (FNB 23 and FNA 33) were followed up or evaluated for MSI. Patient characteristics, UR-PC data, and procedural outcomes were compared between patients who underwent EUS-FNB and those who underwent EUS-FNA.
Results
No significant difference in terms of sufficient tissue acquisition for histology was observed between patients who underwent EUS-FNB and those who underwent EUS-FNA. MSI evaluation was possible significantly more with tissue samples obtained using EUS-FNB than with tissue samples obtained using EUS-FNA (82.6% [19/23] vs. 45.5% [15/33], respectively; p<0.01). In the multivariate analysis, EUS-FNB was the only significant factor influencing the possibility of MSI evaluation.
Conclusions
EUS-FNB using a Franseen needle is desirable for ensuring sufficient tissue acquisition for MSI evaluation. -
Citations
Citations to this article as recorded by- Oil blotting paper for formalin fixation increases endoscopic ultrasound‐guided tissue acquisition‐collected sample volumes on glass slides
Takuo Yamai, Kenji Ikezawa, Yusuke Seiki, Ko Watsuji, Yasuharu Kawamoto, Takeru Hirao, Kazuma Daiku, Shingo Maeda, Makiko Urabe, Yugo Kai, Ryoji Takada, Kaori Mukai, Tasuku Nakabori, Hiroyuki Uehara, Sayoko Tsuzaki, Ayumi Ryu, Satoshi Tanada, Shigenori Na
Cancer Medicine.2024;[Epub] CrossRef
- Oil blotting paper for formalin fixation increases endoscopic ultrasound‐guided tissue acquisition‐collected sample volumes on glass slides
- 2,159 View
- 125 Download
- 1 Crossref

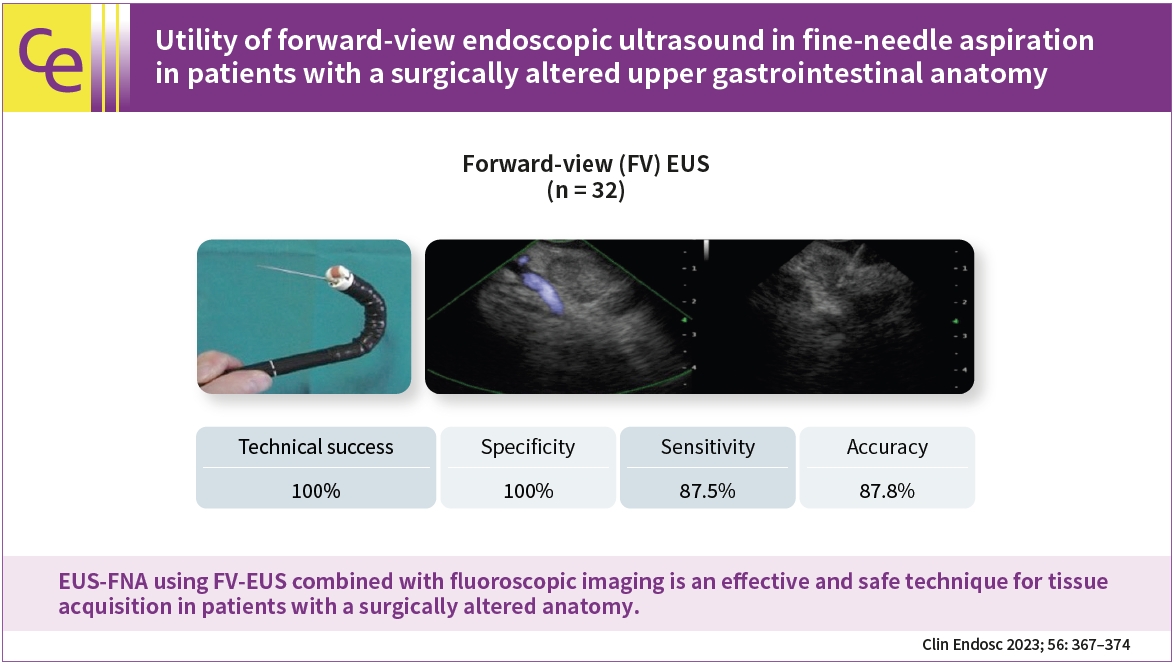
- Utility of forward-view endoscopic ultrasound in fine-needle aspiration in patients with a surgically altered upper gastrointestinal anatomy
- Asmaa Bakr, Kazuo Hara, Moaz Elshair, Shin Haba, Takamichi Kuwahara, Nozomi Okuno, Daiki Fumihara, Takafumi Yanaidani, Samy Zaky, Hanaa Omar
- Clin Endosc 2023;56(3):367-374. Published online January 5, 2023
- DOI: https://doi.org/10.5946/ce.2021.238
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: Endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA) using oblique-view EUS in patients with a surgically altered anatomy (SAA) of the upper gastrointestinal tract is limited because of difficult scope insertion due to the disturbed anatomy. This study aimed to investigate the efficiency of forward-view (FV)-EUS in performing FNA in patients with a SAA.
Methods
We retrospectively investigated 32 patients with a SAA of the upper gastrointestinal tract who visited Aichi Cancer Center Hospital in Nagoya, Japan, between January 2014 and December 2020. We performed-upper gastrointestinal EUS-FNA using FV-EUS combined with fluoroscopic imaging to confirm tumor recurrence or to make a decision before chemotherapy or after a failure of diagnosis by radiology.
Results
We successfully performed EUS-FNA in all studied patients (100% technical success), with the specificity, sensitivity, and accuracy of 100%, 87.5%, and 87.8%, respectively, with no complications.
Conclusions
EUS-FNA using FV-EUS combined with fluoroscopic imaging is an effective and safe technique for tissue acquisition in patients with a SAA.
- 1,696 View
- 167 Download

-
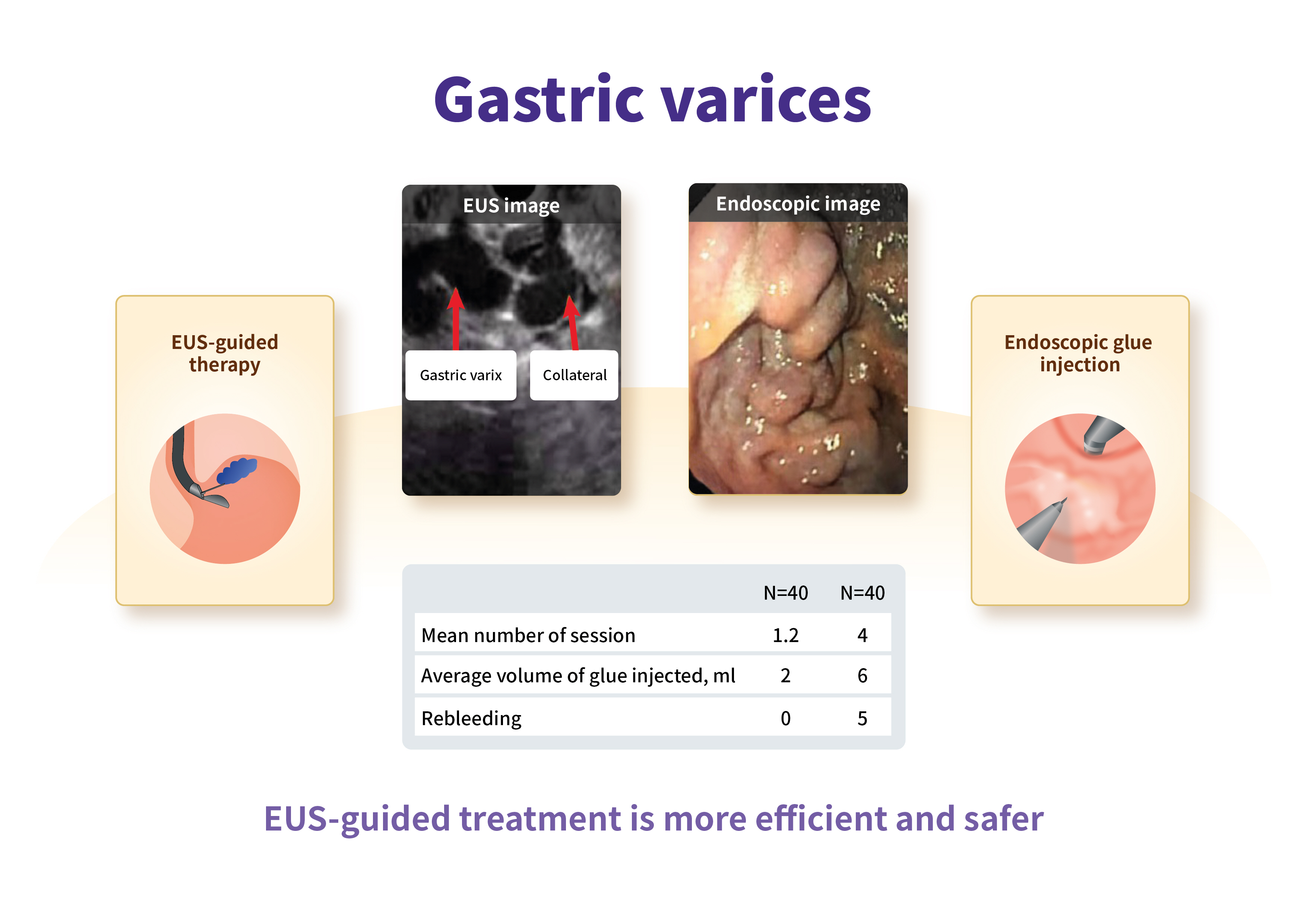
Endoscopic ultrasound-guided coiling and glue is safe and superior to endoscopic glue injection in gastric varices with severe liver disease: a retrospective case control study

- Kapil D. Jamwal, Rajesh K. Padhan, Atul Sharma, Manoj K. Sharma
- Clin Endosc 2023;56(1):65-74. Published online January 3, 2023
- DOI: https://doi.org/10.5946/ce.2021.119
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub 
- Background
/Aims: Gastric varices (GV) are present in 25% of cirrhotic patients with high rates of rebleeding and mortality. Data on endoscopic ultrasound (EUS)-guided treatment in severe liver disease (model for end stage liver disease sodium [MELD-Na] >18 and Child-Turcotte-Pugh [CTP] C with GV) are scarce. Thus, we performed a retrospective comparison of endoscopic glue injection with EUS-guided therapy in cirrhotic patients with large GV.
Methods
A retrospective study was performed in the tertiary hospitals of India. A total of 80 patients were recruited. The inclusion criteria were gastroesophageal varices type 2, isolated gastric varices type 1, bleeding within 6 weeks, size of GV >10 mm, and a MELD-Na >18. Treatment outcomes and complications of endoscopic glue injection and EUS-guided GV therapy were compared.
Results
In this study, the patients’ age, sex, liver disease severity (CTP, MELD-Na) and clinical parameters were comparable. The median number of procedures, injected glue volume, complications, and GV obturation were better in the EUS group, respectively. On subgroup analysis of the EUS method (e.g., direct gastric fundus vs. paragastric collateral [PGC] coil placement), PGC coil placement showed decreased coil requirement, less injected glue volume, decreased luminal coil extrusion, and increased successful GV obturation.
Conclusions
EUS-guided treatment is more efficient and safer, and requires a smaller number of treatment sessions, as compared to endoscopic treatment in severe liver disease patients with large GV. Furthermore, PGC coil placement increases the complete obliteration of GV. -
Citations
Citations to this article as recorded by- Efficacy of clip-assisted endoscopic cyanoacrylate injection therapy for gastric varices: A Meta-analysis
Yong-Cai Lv, Yan-Hua Yao, Jing-Jing Lei
World Chinese Journal of Digestology.2024; 32(2): 158. CrossRef - Advances in the endoscopic management of gastric varices
Xin‐Tong Chi, Ting‐Ting Lian, Ze‐Hao Zhuang
Digestive Endoscopy.2024;[Epub] CrossRef - Trends in endovascular treatment and prevention of portal bleeding
S.V. Mikhin, P.V. Mozgovoy, A.V. Kitaeva, D.E. Gorbunov, I.V. Mikhin
Khirurgiya. Zhurnal im. N.I. Pirogova.2024; (3): 38. CrossRef - Role of endoscopic ultrasound in the secondary prevention of gastric varices
Joung-Ho Han
Clinical Endoscopy.2023; 56(1): 50. CrossRef - Management of Gastric Varices: GI Perspective
Catherine Vozzo, Vibhu Chittajallu, Brooke Glessing, Ashley Faulx, Amitabh Chak, Richard C.K. Wong
Digestive Disease Interventions.2023; 07(04): 266. CrossRef
- Efficacy of clip-assisted endoscopic cyanoacrylate injection therapy for gastric varices: A Meta-analysis
- 3,755 View
- 203 Download
- 2 Web of Science
- 5 Crossref

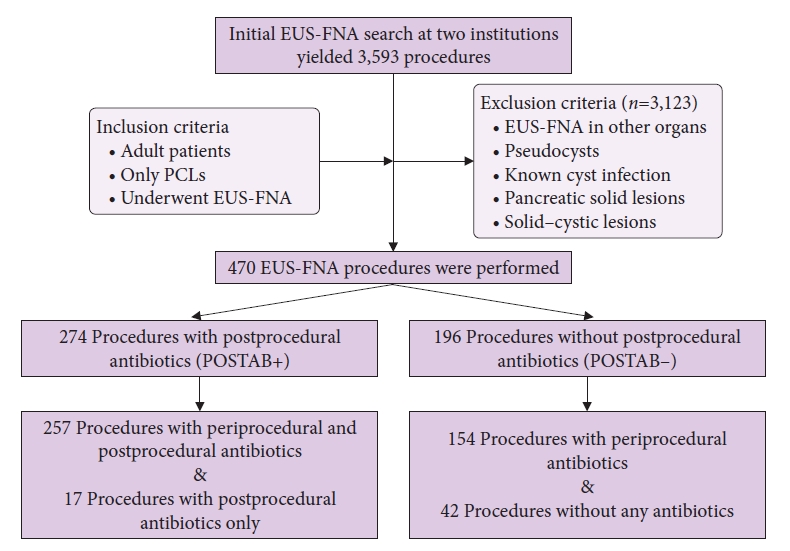
- Is antibiotic prophylaxis necessary after endoscopic ultrasound–guided fine-needle aspiration of pancreatic cysts?
- Seifeldin Hakim, Mihajlo Gjeorgjievski, Zubair Khan, Michael E. Cannon, Kevin Yu, Prithvi Patil, Roy Tomas DaVee, Sushovan Guha, Ricardo Badillo, Laith Jamil, Nirav Thosani, Srinivas Ramireddy
- Clin Endosc 2022;55(6):801-809. Published online November 10, 2022
- DOI: https://doi.org/10.5946/ce.2021.150
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Current society guidelines recommend antibiotic prophylaxis for 3 to 5 days after endoscopic ultrasound–guided fine-needle aspiration (EUS-FNA) of pancreatic cystic lesions (PCLs). The overall quality of the evidence supporting this recommendation is low. In this study, we aimed to assess cyst infection and adverse event rates after EUS-FNA of PCLs among patients treated with or without postprocedural prophylactic antibiotics.
Methods
We retrospectively reviewed all patients who underwent EUS-FNA of PCLs between 2015 and 2019 at two large-volume academic medical centers with different practice patterns of postprocedural antibiotic prophylaxis. Data on patient demographics, cyst characteristics, fine-needle aspiration technique, periprocedural and postprocedural antibiotic prophylaxis, and adverse events were retrospectively extracted.
Results
A total of 470 EUS-FNA procedures were performed by experienced endosonographers for the evaluation of PCLs in 448 patients, 58.7% of whom were women. The mean age was 66.3±12.8 years. The mean cyst size was 25.7±16.9 mm. Postprocedural antibiotics were administered in 274 cases (POSTAB+ group, 58.3%) but not in 196 cases (POSTAB– group, 41.7%). None of the patients in either group developed systemic or localized infection within the 30-day follow-up period. Procedure-related adverse events included mild abdominal pain (8 patients), intra-abdominal hematoma (1 patient), mild pancreatitis (1 patient), and perforation (1 patient). One additional case of pancreatitis was recorded; however, the patient also underwent endoscopic retrograde cholangiopancreatography.
Conclusions
The incidence of infection after EUS-FNA of PCLs is negligible. Routine use of postprocedural antibiotics does not add a significant benefit.
- 2,907 View
- 129 Download

Review
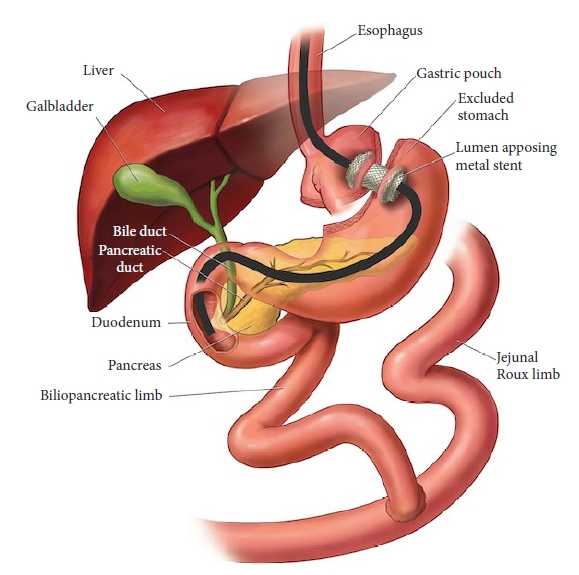
- Endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography for patients with Roux-en-Y gastric bypass anatomy: technical overview
- Hirokazu Honda, Jeffrey D. Mosko, Ryosuke Kobayashi, Andras Fecso, Bong Sik Kim, Schoeman Scott, Gary R. May
- Clin Endosc 2022;55(6):736-741. Published online October 5, 2022
- DOI: https://doi.org/10.5946/ce.2022.114
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic retrograde cholangiopancreatography (ERCP) in patients with Roux-en-Y gastric bypass anatomy is a well-documented challenge. Traditionally, this problem has been overcome with adjunctive techniques, such as device-assisted ERCP, including double-balloon or single-balloon enteroscopy and laparoscopy-assisted transgastric ERCP. Endoscopic ultrasound-directed transgastric ERCP (EDGE) is a novel technique that enables access to the ampulla using a duodenoscope without surgical intervention and has shown high clinical and technical success rates in recent studies. However, this approach is technically demanding, necessitating a thorough understanding of the gastrointestinal anatomy as well as high operator experience. In this review, we provide a technical overview of EDGE in parallel with our personal experience at our center and propose a simple algorithm to select patients for its appropriate application. In conjunction, the outcomes of EDGE compared with those of device-assisted and laparoscopy-assisted transgastric ERCP will be discussed.
-
Citations
Citations to this article as recorded by- Endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography procedure for choledocholithiasis after sleeve gastrectomy and Roux-en-Y
Reid D. Wasserman, Varun Kesar, Vivek Kesar, Paul Yeaton, Shehriyar Mehershahi
Endoscopy.2024; 56(S 01): E390. CrossRef - A novel endoscopic ultrasound-guided transluminal anchor device
Abhishek Agnihotri, Alexander Schlachterman
Endoscopy.2023; 55(S 01): E775. CrossRef
- Endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography procedure for choledocholithiasis after sleeve gastrectomy and Roux-en-Y
- 2,018 View
- 138 Download
- 2 Web of Science
- 2 Crossref

Systematic Review and Meta-Analysis
- Mucosal incision-assisted biopsy versus endoscopic ultrasound-assisted tissue acquisition for subepithelial lesions: a systematic review and meta-analysis
- Suprabhat Giri, Shivaraj Afzalpurkar, Sumaswi Angadi, Sridhar Sundaram
- Clin Endosc 2022;55(5):615-625. Published online August 4, 2022
- DOI: https://doi.org/10.5946/ce.2022.133
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Mucosal incision-assisted biopsy (MIAB) for tissue acquisition (TA) from subepithelial lesions (SELs) is emerging as an alternative to endoscopic ultrasound (EUS)-guided TA. Only a limited number of studies compared the diagnostic utility of MIAB and EUS for upper gastrointestinal (GI) SELs; therefore, we conducted this systematic review and meta-analysis.
Methods
A comprehensive literature search from January 2020 to January 2022 was performed to compare the diagnostic accuracy and safety of MIAB and EUS-guided TA for upper GI SELs.
Results
Seven studies were included in this meta-analysis. The pooled technical success rate (risk ratio [RR], 0.96; 95% confidence interval [CI], 0.89–1.04) and procedural time (mean difference=–4.53 seconds; 95% CI, –22.38 to 13.31] were comparable between both the groups. The overall chance of obtaining a positive diagnostic yield was lower with EUS than with MIAB for all lesions (RR, 0.83; 95% CI, 0.71–0.98) but comparable when using a fine-needle biopsy needle (RR, 0.93; 95% CI, 0.83–1.04). The positive diagnostic yield of MIAB was higher for lesions <20 mm (RR, 0.75; 95% CI, 0.63–0.89). Six studies reported no adverse events.
Conclusions
MIAB can be considered an effective alternative to EUS-guided TA for upper GI SELs without an increased risk of adverse events. -
Citations
Citations to this article as recorded by- Technical outcomes and postprocedural courses of mucosal incision‐assisted biopsy for possible gastric gastrointestinal stromal tumors: A series of 48 cases (with video)
Eriko Koizumi, Osamu Goto, Shun Nakagome, Tsugumi Habu, Yumiko Ishikawa, Kumiko Kirita, Hiroto Noda, Kazutoshi Higuchi, Takeshi Onda, Teppei Akimoto, Jun Omori, Naohiko Akimoto, Katsuhiko Iwakiri
DEN Open.2024;[Epub] CrossRef - The Diagnostic Approach of Benign Esophageal Tumors: A Narrative Review
Alex R. Jones, Preksha Vankawala, Tarek Sawas
Current Treatment Options in Gastroenterology.2024; 22(2): 44. CrossRef - Unroofing of subepithelial lesions in the upper gastrointestinal tract using cold snare: an easy and efficient technique for diagnosis
Bernhard Morell, Frans Olivier The, Christoph Gubler, Fritz Ruprecht Murray
Clinical Endoscopy.2024; 57(2): 274. CrossRef - Diagnostic yield of endoscopic and EUS-guided biopsy techniques in subepithelial lesions of the upper GI tract: a systematic review
Cynthia A. Verloop, Jacqueline A.C. Goos, Marco J. Bruno, Rutger Quispel, Lydi M.J.W. van Driel, Lieke Hol
Gastrointestinal Endoscopy.2024; 99(6): 895. CrossRef - Small gastric subepithelial lesions: A sand in the eye
Tanyaporn Chantarojanasiri, Nikhil Sonthalia, Rashid N. Lui
Journal of Gastroenterology and Hepatology.2024;[Epub] CrossRef - Approach to Small Gastric Subepithelial Lesions
Moon Won Lee, Bong Eun Lee
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2023; 23(1): 28. CrossRef - Is the canalization to obtain deep biopsy of gastrointestinal subepithelial tumors miniprobe-guidded as an alternative to conventional known techniques?
Modesto Varas Lorenzo, Ramón Abad Belando
Revista Española de Enfermedades Digestivas.2023;[Epub] CrossRef - Role of Advanced Gastrointestinal Endoscopy in the Comprehensive Management of Neuroendocrine Neoplasms
Harishankar Gopakumar, Vinay Jahagirdar, Jagadish Koyi, Dushyant Singh Dahiya, Hemant Goyal, Neil R. Sharma, Abhilash Perisetti
Cancers.2023; 15(16): 4175. CrossRef
- Technical outcomes and postprocedural courses of mucosal incision‐assisted biopsy for possible gastric gastrointestinal stromal tumors: A series of 48 cases (with video)
- 2,335 View
- 132 Download
- 4 Web of Science
- 8 Crossref

Case Reports
-
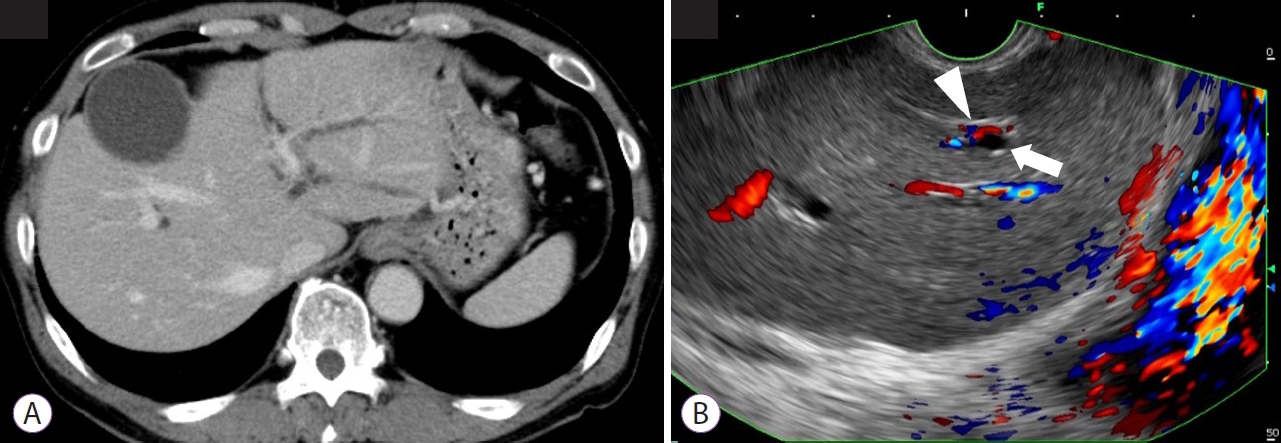
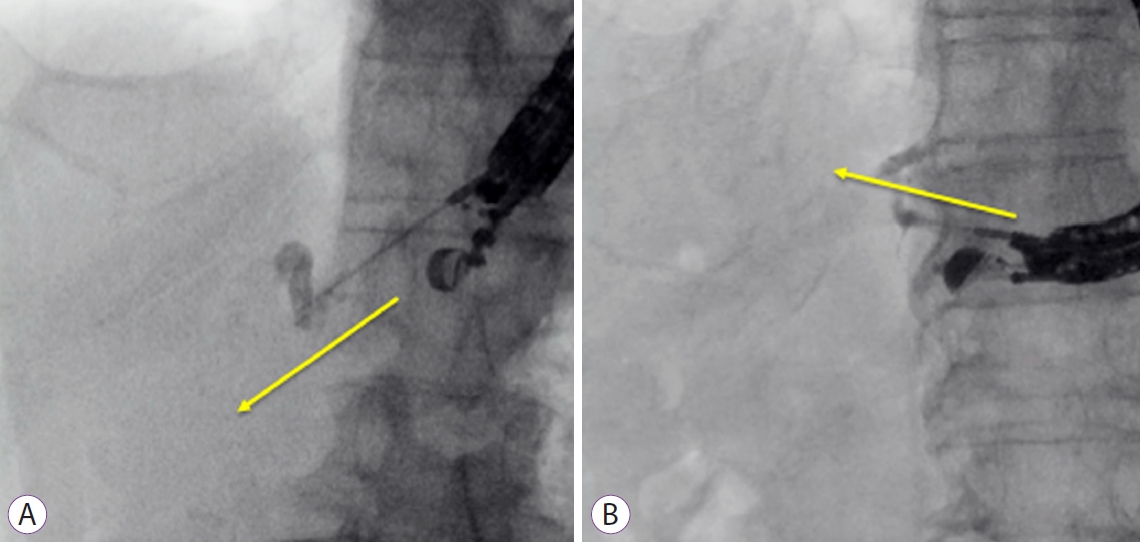
Endoscopic ultrasound-guided portal vein coiling: troubleshooting interventional endoscopic ultrasonography

- Shin Haba, Kazuo Hara, Nobumasa Mizuno, Takamichi Kuwahara, Nozomi Okuno, Akira Miyano, Daiki Fumihara, Moaz Elshair
- Clin Endosc 2022;55(3):458-462. Published online November 30, 2021
- DOI: https://doi.org/10.5946/ce.2021.114
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Endoscopic ultrasound (EUS)-guided hepaticogastrostomy (HGS) is widely performed not only as an alternative to transpapillary biliary drainage, but also as primary drainage for malignant biliary obstruction. For anatomical reasons, this technique carries an unavoidable risk of mispuncturing intrahepatic vessels. We report a technique for troubleshooting EUS-guided portal vein coiling to prevent bleeding from the intrahepatic portal vein after mispuncture during interventional EUS. EUS-HGS was planned for a 59-year-old male patient with unresectable pancreatic cancer. The dilated bile duct (lumen diameter, 2.8 mm) was punctured with a 19-gauge needle, and a guidewire was inserted. After bougie dilation, the guidewire was found to be inside the intrahepatic portal vein. Embolizing coils were placed to prevent bleeding. Embolization coils were successfully inserted under stabilization of the catheter using a double-lumen cannula with a guidewire. Following these procedures, the patient was asymptomatic. Computed tomography performed the next day revealed no complications.
- 3,352 View
- 170 Download

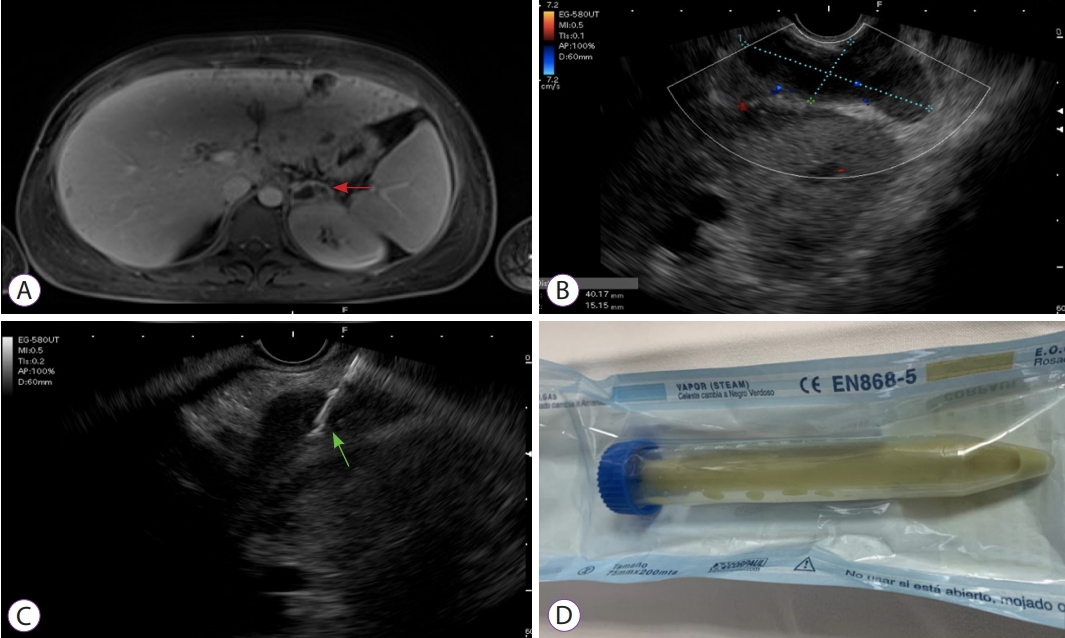
- Endoscopic Ultrasound-Guided Transgastric Puncture and Drainage of an Adrenal Abscess in an Immunosuppressed Patient
- Carlos Andrés Regino, Jean Paul Gómez, Gabriel Mosquera-Klinger
- Clin Endosc 2022;55(2):302-304. Published online November 16, 2021
- DOI: https://doi.org/10.5946/ce.2021.090
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Adrenal gland infection is a clinical entity of great importance, but it is a largely unrecognized pathology. Immunosuppressed individuals are at a higher risk of presentation. Herein, we describe a young female patient, recently diagnosed with HIV, who presented with severe sepsis due to methicillin-resistant Staphylococcus aureus, associated with a left adrenal abscess. She was initially treated with antibiotics; however, due to the persistence of the systemic inflammatory response and bacteremia, endoscopic ultrasound-guided drainage was performed. This procedure was successful in resolving the clinical situation. Endoscopic ultrasound-guided adrenal gland drainage can be a safe, efficacious, and minimally invasive option for managing antibiotic-refractory adrenal abscesses in immunosuppressed patients.
- 3,091 View
- 128 Download

Focused Review Series: Endoscopic Management of Postoperative Gastrointestinal Complication: What’s New?
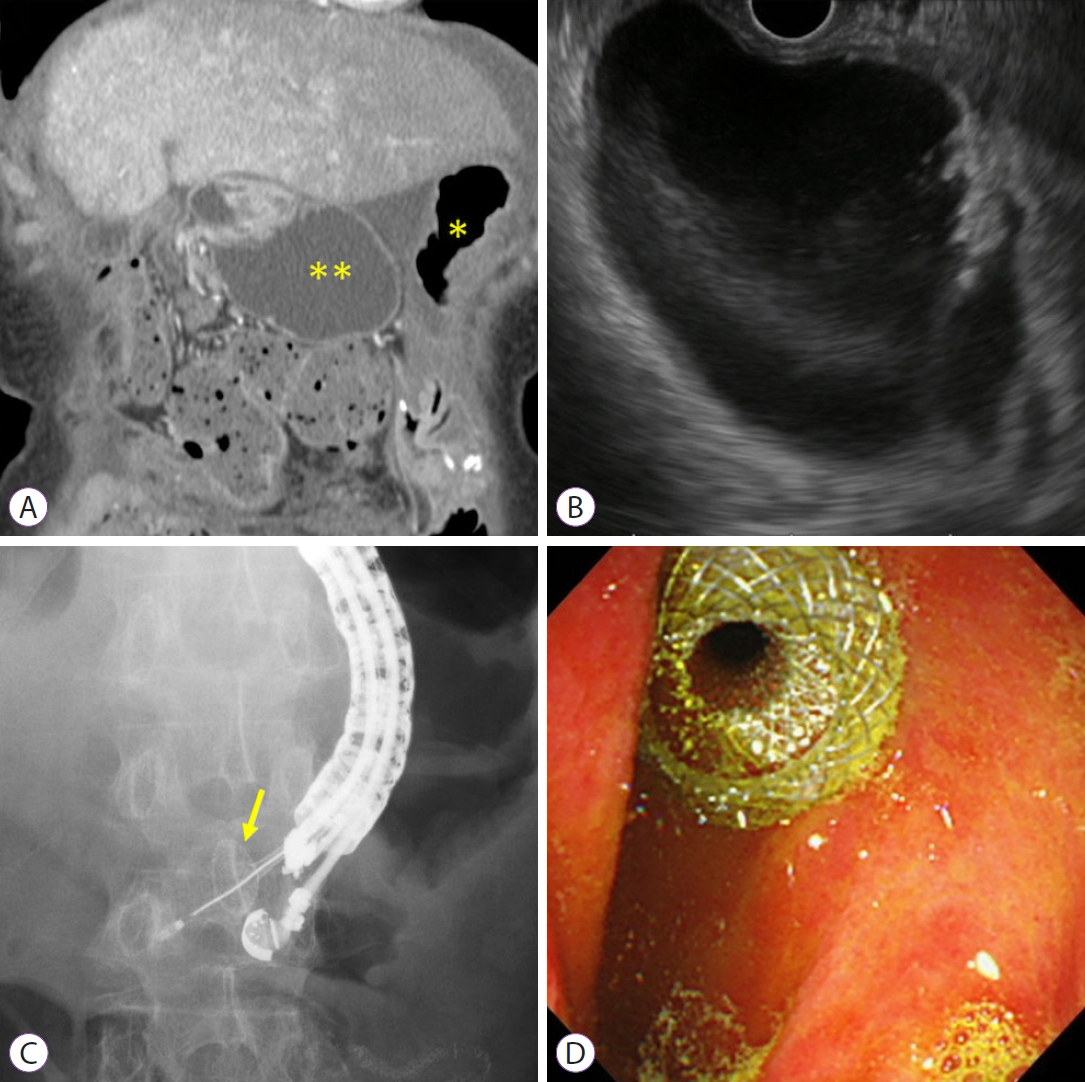
- Endoscopic Ultrasound-Guided Gastroenterostomy for Afferent Loop Syndrome
- Hideyuki Shiomi, Arata Sakai, Ryota Nakano, Shogo Ota, Takashi Kobayashi, Atsuhiro Masuda, Hiroko Iijima
- Clin Endosc 2021;54(6):810-817. Published online November 15, 2021
- DOI: https://doi.org/10.5946/ce.2021.234
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Afferent loop syndrome (ALS) is a mechanical obstruction of the afferent limbs after gastrectomy with gastrojejunostomy reconstruction. Patients with cancer recurrence require immediate and less invasive treatment because of their poor condition. Percutaneous transhepatic/transluminal drainage (PTD) and endoscopic enteral stenting offer reasonable palliative treatment for malignant ALS but are not fully satisfactory in terms of patient quality of life (QoL) and stent patency. Endoscopic ultrasound-guided gastroenterostomy (EUS-GE) using a lumen-apposing metal stent may address these shortcomings. Clinical data from 11 reports showed that all patients who had undergone EUS-GE had positive technical and clinical outcomes. The adverse event rate was 11.4%, including only mild or moderate abdominal pain, with no severe adverse events. Indirect comparative studies indicated that patients who had undergone EUS-GE had a significantly superior QoL, a higher clinical success rate, and a lower reintervention rate than those who had undergone PTD or endoscopic enteral stenting. Although the evidence is limited, EUS-GE may be considered as a first-line treatment for malignant ALS because it has better clinical outcomes than other less invasive treatments, such as PTD or endoscopic enteral stenting. Further prospective randomized control trials are necessary to establish EUS-GE as a standard treatment for ALS.
-
Citations
Citations to this article as recorded by- Endoscopic ultrasound‐guided gastrointestinal anastomosis: Are we there yet?
Vinay Dhir, Cesar Jaurrieta‐Rico, Vivek Kumar Singh
Digestive Endoscopy.2024;[Epub] CrossRef - Endoscopic ultrasound-guided gastroenterostomy using a novel dumbbell-shaped fully covered metal stent for afferent loop syndrome with long interluminal distance
Hideyuki Shiomi, Ryota Nakano, Shogo Ota, Hiroko Iijima
Endoscopy.2023; 55(S 01): E362. CrossRef - Endoscopic Ultrasound-Guided Gastrojejunostomy for Malignant Afferent Loop Syndrome Using a Fully Covered Metal Stent: A Multicenter Experience
Saburo Matsubara, Sho Takahashi, Naminatsu Takahara, Keito Nakagawa, Kentaro Suda, Takeshi Otsuka, Yousuke Nakai, Hiroyuki Isayama, Masashi Oka, Sumiko Nagoshi
Journal of Clinical Medicine.2023; 12(10): 3524. CrossRef - Endoscopic ultrasound guided gastroenterostomy: Technical details updates, clinical outcomes, and adverse events
Jian Wang, Jin-Long Hu, Si-Yu Sun
World Journal of Gastrointestinal Endoscopy.2023; 15(11): 634. CrossRef - Endoscopic ultrasound-guided intra-afferent loop entero-enterostomy using a forward-viewing echoendoscope and insertion of a metal stent
Yuki Kawasaki, Susumu Hijioka, Kosuke Maehara, Kiichi Tamada, Takuji Okusaka, Yutaka Saito
Endoscopy.2022; 54(S 02): E815. CrossRef - Endoscopic ultrasound‐guided gastrojejunostomy for malignant afferent loop syndrome with hemorrhage in a patient with recurrent peritoneal dissemination
Kenjiro Yamamoto, Takayoshi Tsuchiya, Ryosuke Tonozuka, Shuntaro Mukai, Hiroyuki Kojima, Noriyuki Hirakawa, Takao Itoi
Journal of Hepato-Biliary-Pancreatic Sciences.2022;[Epub] CrossRef - Current status of, and challenges posed by, endoscopic ultrasound‐guided anastomosis of the digestive tract in patients with afferent loop syndrome
Toshio Fujisawa, Hiroyuki Isayama
Digestive Endoscopy.2022; 34(7): 1440. CrossRef
- Endoscopic ultrasound‐guided gastrointestinal anastomosis: Are we there yet?
- 3,904 View
- 140 Download
- 7 Web of Science
- 7 Crossref

Reviews
- Technical Review of Developments in Endoscopic Ultrasound-Guided Hepaticogastrostomy
- Takeshi Ogura, Kazuhide Higuchi
- Clin Endosc 2021;54(5):651-659. Published online April 26, 2021
- DOI: https://doi.org/10.5946/ce.2021.020-KDDW
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic ultrasound-guided biliary drainage has been developed as an alternative method for biliary drainage. EUS-guided hepaticogastrostomy (EUS-HGS) can be attempted via the trans-gastric route. These procedures are technically complex for two reasons. First, puncture of the intrahepatic bile duct via the trans-gastric route can be more difficult than that by other approaches because of the small diameter of the target site, and guidewire insertion or manipulation is challenging during EUS-HGS. Second, critical adverse events, such as stent migration into the abdominal cavity, could occur because of the greater mobility of the stomach compared to the duodenum. Therefore, endoscopists should be cautious when performing EUS-HGS. An advantage of EUS-HGS is that it can be performed in patients with complications such as duodenal bulb obstruction or surgically altered anatomy. Recent advances in technique and improvements in devices and stents for EUS-HGS have shown promise for improving the technical success rate of EUS-HGS and reducing the rate of adverse events. However, endoscopists should remain aware of the possibility of critical adverse events such as stent migration.
-
Citations
Citations to this article as recorded by- Propensity score matching analysis for clinical impact of braided-type versus laser-cut-type covered self-expandable metal stents for endoscopic ultrasound-guided hepaticogastrostomy
Mitsuki Tomita, Takeshi Ogura, Akitoshi Hakoda, Saori Ueno, Atsushi Okuda, Nobu Nishioka, Yoshitaro Yamamoto, Hiroki Nishikawa
Hepatobiliary & Pancreatic Diseases International.2024; 23(2): 181. CrossRef - Usefulness of endoscopic ultrasound‐guided transhepatic biliary drainage with a 22‐gauge fine‐needle aspiration needle and 0.018‐inch guidewire in the procedure's induction phase
Kei Yane, Masahiro Yoshida, Takayuki Imagawa, Kotaro Morita, Hideyuki Ihara, Kota Hanada, Sota Hirokawa, Yusuke Tomita, Takeyoshi Minagawa, Yutaka Okagawa, Tetsuya Sumiyoshi, Michiaki Hirayama, Hitoshi Kondo
DEN Open.2024;[Epub] CrossRef - EUS-guided hepaticogastrostomy versus EUS-guided hepaticogastrostomy with antegrade stent placement in patients with unresectable malignant distal biliary obstruction: a propensity score–matched case-control study
Hirotoshi Ishiwatari, Takeshi Ogura, Susumu Hijioka, Takuji Iwashita, Saburo Matsubara, Kazuma Ishikawa, Fumitaka Niiya, Junya Sato, Atsushi Okuda, Saori Ueno, Yoshikuni Nagashio, Yuta Maruki, Shinya Uemura, Akifumi Notsu
Gastrointestinal Endoscopy.2024;[Epub] CrossRef - Stent Deployment Without Tract Dilation in Endoscopic Ultrasound-Guided Hepaticogastrostomy Using a Novel Partially Covered Metal Stent With a Super-Slim Stent Delivery System: A Case Report
Koji Takahashi, Hiroshi Ohyama, Izumi Ohno, Naoya Kato
Cureus.2024;[Epub] CrossRef - TOKYO criteria 2024 for the assessment of clinical outcomes of endoscopic biliary drainage
Hiroyuki Isayama, Tsuyoshi Hamada, Toshio Fujisawa, Mitsuharu Fukasawa, Kazuo Hara, Atsushi Irisawa, Shigeto Ishii, Ken Ito, Takao Itoi, Yoshihide Kanno, Akio Katanuma, Hironari Kato, Hiroshi Kawakami, Hirofumi Kawamoto, Masayuki Kitano, Hirofumi Kogure,
Digestive Endoscopy.2024;[Epub] CrossRef - Efficacy of a dedicated plastic stent in endoscopic ultrasound-guided hepaticogastrostomy during the learning curve: cumulative multi-center experience
Koh Kitagawa, Akira Mitoro, Ryuki Minami, Shinsaku Nagamatsu, Takahiro Ozutsumi, Yukihisa Fujinaga, Norihisa Nishimura, Yasuhiko Sawada, Tadashi Namisaki, Takemi Akahane, Kosuke Kaji, Fumimasa Tomooka, Shohei Asada, Miki Kaneko, Hitoshi Yoshiji
Scandinavian Journal of Gastroenterology.2023; 58(3): 296. CrossRef - Removal of a small pancreatic stone in thin main pancreatic duct using an ultrafine balloon catheter (with video)
Saburo Matsubara, Keito Nakagawa, Kentaro Suda, Takeshi Otsuka, Masashi Oka, Sumiko Nagoshi
Journal of Hepato-Biliary-Pancreatic Sciences.2023;[Epub] CrossRef - Drainage of Afferent Limb Obstruction via the Trans-gastric-bile Duct Formed after Endoscopic Ultrasound-guided Hepaticogastrostomy in a Patient with Pancreatic Cancer
Masatoshi Mabuchi, Seiji Adachi, Yukari Uno, Hironori Nakamura, Makoto Shimazaki, Shinji Nishiwaki, Iwao Kumazawa, Takuji Iwashita, Masahito Shimizu
Internal Medicine.2023; 62(16): 2355. CrossRef - Clinical feasibility of endoscopic ultrasound‐guided biliary drainage for preoperative management of malignant biliary obstruction (with videos)
Shuntaro Mukai, Takao Itoi, Takayoshi Tsuchiya, Kentaro Ishii, Ryosuke Tonozuka, Yuichi Nagakawa, Shingo Kozono, Chie Takishita, Hiroaki Osakabe, Atsushi Sofuni
Journal of Hepato-Biliary-Pancreatic Sciences.2023; 30(7): 983. CrossRef - A retrospective multicenter study comparing the punctures to B2 and B3 in endoscopic ultrasound–guided hepaticogastrostomy
Masanari Sekine, Yusuke Hashimoto, Taro Shibuki, Kei Okumura, Ikuhiro Kobori, Aki Miyagaki, Yoshihiro Sasaki, Yuichi Takano, Keita Matsumoto, Hirosato Mashima
DEN Open.2023;[Epub] CrossRef - Stent‐in‐stent technique in a case of difficult removal of a EUS‐guided hepaticogastrostomy partially covered metal stent due to mucosal hyperplasia (with video)
Takeshi Ogura, Yuki Uba, Hiroki Nishikawa
Journal of Hepato-Biliary-Pancreatic Sciences.2023; 30(10): 1188. CrossRef - Loop technique for guidewire manipulation during endoscopic ultrasound‐guided hepaticogastrostomy
Haruo Miwa, Kazuya Sugimori, Yuto Matsuoka, Kazuki Endo, Ritsuko Oishi, Masaki Nishimura, Yuichiro Tozuka, Takashi Kaneko, Kazushi Numata, Shin Maeda
JGH Open.2023; 7(5): 358. CrossRef - Drainage Approach for Malignant Biliary Obstruction
Ian Eisenberg, Monica Gaidhane, Michel Kahaleh, Amy Tyberg
Journal of Clinical Gastroenterology.2023; 57(6): 546. CrossRef - Guidewire malposition outside the bile duct during endoscopic ultrasound-guided hepaticogastrostomy
Yoshimitsu Fukasawa, Mitsuharu Fukasawa, Shinichi Takano, Satoshi Kawakami, Hiroshi Hayakawa, Shota Harai, Nobuyuki Enomoto
Endoscopy.2023; 55(S 01): E894. CrossRef - Preloading guidewire method: EUS-guided hepaticogastrostomy
Hirotsugu Maruyama, Yuki Ishikawa-Kakiya, Kojiro Tanoue, Akira Higashimori, Yasuhiro Fujiwara
Arab Journal of Gastroenterology.2023; 24(3): 183. CrossRef - Guidewires in GI endoscopy
Samuel Han, Mohit Girotra, Venkata S. Akshintala, Dennis Chen, Yen-I Chen, Koushik K. Das, Allon Kahn, Girish Mishra, V. Raman Muthusamy, Jorge V. Obando, Frances U. Onyimba, Swati Pawa, Tarun Rustagi, Sonali Sakaria, Guru Trikudanathan, Ryan J. Law
iGIE.2023; 2(3): 386. CrossRef - EUS–guided transhepatic metal stent deployment technique without tract dilation using a 0.018-inch guidewire (with video)
Takeshi Ogura, Jyunichi Kawai, Kyohei Nishiguchi, Yoshitaro Yamamoto, Kazuhide Higuchi
Endoscopic Ultrasound.2023; 12(5): 431. CrossRef - The writing on the wall: self-expandable stents for endoscopic ultrasound-guided hepaticogastrostomy?
Hyung Ku Chon, Shayan Irani, Tae Hyeon Kim
Clinical Endoscopy.2023; 56(6): 741. CrossRef - Rescue technique after endoscopic ultrasound-guided hepaticogastrostomy stent dislocation
Takeshi Ogura, Taro Iwatsubo, Atsushi Okuda, Saori Ueno, Hiroki Nishikawa
Endoscopy.2023; 55(S 01): E1236. CrossRef - Endoscopic ultrasound-guided hepaticogastrostomy using a novel drill dilator
Masanori Yamada, Kazuo Hara, Shin Haba, Nobumasa Mizuno, Takamichi Kuwahara, Nozomi Okuno, Yasuhiro Kuraishi
Endoscopy.2022; 54(S 02): E856. CrossRef - Successful endoscopic treatment of huge infected biloma and hepatic abscess after endoscopic ultrasound-guided hepaticogastrostomy with brain abscess
Koji Takahashi, Hiroshi Ohyama, Hiroki Nagashima, Yotaro Iino, Yuko Kusakabe, Kohichiroh Okitsu, Izumi Ohno, Yuichi Takiguchi, Naoya Kato
Clinical Journal of Gastroenterology.2022; 15(5): 988. CrossRef - Prevention of Serious Complications during Endoscopic Ultrasound-Guided Biliary Drainage: A Case-Based Technical Review
Surinder Singh Rana, Jimil Shah, Harish Bhujade, Ujjwal Gorsi, Mandeep Kang, Rajesh Gupta
Journal of Digestive Endoscopy.2022; 13(02): 082. CrossRef - Endoscopic salvage therapy after failed biliary cannulation using advanced techniques: A concise review
Yung-Kuan Tsou, Kuang-Tse Pan, Mu Hsien Lee, Cheng-Hui Lin
World Journal of Gastroenterology.2022; 28(29): 3803. CrossRef - Usefulness of the double-guidewire technique for endoscopic procedures in the field of biliary and pancreatic diseases
Mamoru Takenaka, Masatoshi Kudo
Clinical Endoscopy.2022; 55(5): 605. CrossRef - New Stents for Endoscopic Ultrasound-Guided Procedures
Gunn Huh, Tae Jun Song
The Korean Journal of Pancreas and Biliary Tract.2021; 26(4): 248. CrossRef
- Propensity score matching analysis for clinical impact of braided-type versus laser-cut-type covered self-expandable metal stents for endoscopic ultrasound-guided hepaticogastrostomy
- 5,229 View
- 296 Download
- 21 Web of Science
- 25 Crossref

- Clinical and Technical Guideline for Endoscopic Ultrasound (EUS)-Guided Tissue Acquisition of Pancreatic Solid Tumor: Korean Society of Gastrointestinal Endoscopy (KSGE)
- Moon Jae Chung, Se Woo Park, Seong-Hun Kim, Chang Min Cho, Jun-Ho Choi, Eun Kwang Choi, Tae Hoon Lee, Eunae Cho, Jun Kyu Lee, Tae Jun Song, Jae Min Lee, Jun Hyuk Son, Jin Suk Park, Chi Hyuk Oh, Dong-Ah Park, Jeong-Sik Byeon, Soo Teik Lee, Ho Gak Kim, Hoon Jai Chun, Ho Soon Choi, Chan Guk Park, Joo Young Cho
- Clin Endosc 2021;54(2):161-181. Published online March 24, 2021
- DOI: https://doi.org/10.5946/ce.2021.069
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic ultrasound (EUS)-guided tissue acquisition of pancreatic solid tumor requires a strict recommendation for its proper use in clinical practice because of its technical difficulty and invasiveness. The Korean Society of Gastrointestinal Endoscopy (KSGE) appointed a Task Force to draft clinical practice guidelines for EUS-guided tissue acquisition of pancreatic solid tumor. The strength of recommendation and the level of evidence for each statement were graded according to the Minds Handbook for Clinical Practice Guideline Development 2014. The committee, comprising a development panel of 16 endosonographers and an expert on guideline development methodology, developed 12 evidence-based recommendations in 8 categories intended to help physicians make evidence-based clinical judgments with regard to the diagnosis of pancreatic solid tumor. This clinical practice guideline discusses EUS-guided sampling in pancreatic solid tumor and makes recommendations on circumstances that warrant its use, technical issues related to maximizing the diagnostic yield (e.g., needle type, needle diameter, adequate number of needle passes, sample obtaining techniques, and methods of specimen processing), adverse events of EUS-guided tissue acquisition, and learning-related issues. This guideline was reviewed by external experts and suggests best practices recommended based on the evidence available at the time of preparation. This guideline may not be applicable for all clinical situations and should be interpreted in light of specific situations and the availability of resources. It will be revised as necessary to cover progress and changes in technology and evidence from clinical practice.
-
Citations
Citations to this article as recorded by- Role of Endoscopic Ultrasound in the Management of Pancreatic Cancer
Balaji Musunuri, Shiran Shetty
Indian Journal of Surgical Oncology.2024; 15(S2): 269. CrossRef - Endoscopic ultrasound-guided tissue acquisition for personalized treatment in pancreatic adenocarcinoma
Sang Myung Woo
Clinical Endoscopy.2023; 56(2): 183. CrossRef - Pancreatic duct lavage cytology combined with a cell-block method for patients with possible pancreatic ductal adenocarcinomas, including pancreatic carcinoma in situ
Hiroaki Kusunose, Shinsuke Koshita, Yoshihide Kanno, Takahisa Ogawa, Toshitaka Sakai, Keisuke Yonamine, Kazuaki Miyamoto, Fumisato Kozakai, Hideyuki Anan, Kazuki Endo, Haruka Okano, Masaya Oikawa, Takashi Tsuchiya, Takashi Sawai, Yutaka Noda, Kei Ito
Clinical Endoscopy.2023; 56(3): 353. CrossRef - Anesthesia care provider sedation versus conscious sedation for endoscopic ultrasound–guided tissue acquisition: a retrospective cohort study
Sneha Shaha, Yinglin Gao, Jiahao Peng, Kendrick Che, John J. Kim, Wasseem Skef
Clinical Endoscopy.2023; 56(5): 658. CrossRef - Endoscopic ultrasound-guided tissue acquisition and gene panel testing for pancreatic cancer
Kentaro SUDO, Emiri KITA, Akiko TSUJIMOTO, Kazuyoshi NAKAMURA, Akiko ODAKA, Makiko ITAMI, Sana YOKOI, Hiroshi ISHII
Suizo.2022; 37(1): 8. CrossRef - Impact of rapid on-site evaluation on diagnostic accuracy of EUS-guided fine-needle aspiration of solid pancreatic lesions: experience from a single center
Irem Guvendir, Itir Ebru Zemheri, Kamil Ozdil
BMC Gastroenterology.2022;[Epub] CrossRef - Endoscopic Ultrasound Guided Fine Needle Aspiration and Biopsy for Pancreatic Disease
Kwang Hyuck Lee
The Korean Journal of Pancreas and Biliary Tract.2021; 26(4): 241. CrossRef
- Role of Endoscopic Ultrasound in the Management of Pancreatic Cancer
- 7,237 View
- 279 Download
- 4 Web of Science
- 7 Crossref

Original Article
- Role of Endoscopic Ultrasound-Guided Fine-Needle Aspiration of Portal Vein Thrombus in the Diagnosis and Staging of Hepatocellular Carcinoma
- Dina Eskandere, Hazem Hakim, Magdy Attwa, Wagdi Elkashef, Ahmed Youssef Altonbary
- Clin Endosc 2021;54(5):745-753. Published online March 15, 2021
- DOI: https://doi.org/10.5946/ce.2020.240
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Malignant portal vein thrombus (PVT) is found in up to 44% of patients with hepatocellular carcinoma (HCC). The nature of the thrombus influences treatment selection. The aim of this study was to assess the safety and efficacy of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in determining the nature of PVT in liver cirrhosis and/or HCC.
Methods
A prospective study was conducted in 34 patients with liver cirrhosis and/or HCC with PVT. Under EUS guidance, PVT was punctured using a 22 G FNA needle (Cook Medical, Bloomington, IN, USA) followed by monitoring of the puncture tract using color Doppler. Patients were followed for adverse events 2 hours after recovery.
Results
Throughout the 30-month study period, 34 patients, including 24 males with a mean age of 59±8 years, were enrolled. There were 8 patients with known HCC and 26 with no liver masses detected by computed tomography (CT). EUS-FNA from PVT was positive for malignancy in 3 patients (8.8%), of which only 1 patient was diagnosed with HCC by CT and 2 patients were newly diagnosed with HCC after EUS-FNA. No major complications were reported.
Conclusions
EUS-FNA is a safe and effective technique for determining the nature of PVT that does not fulfill the malignant criteria via imaging studies in patients with liver cirrhosis and/or HCC. -
Citations
Citations to this article as recorded by- Endoscopic ultrasound-guided vascular interventions: An overview of current and emerging techniques
Ahmed Youssef Altonbary
International Journal of Gastrointestinal Intervention.2023; 12(1): 16. CrossRef
- Endoscopic ultrasound-guided vascular interventions: An overview of current and emerging techniques
- 3,782 View
- 102 Download
- 1 Web of Science
- 1 Crossref


 KSGE
KSGE


 First
First Prev
Prev



