Search
- Page Path
- HOME > Search
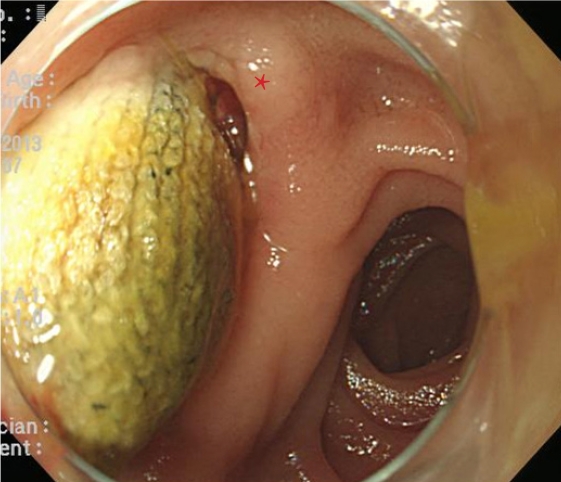
- Aortoduodenal fistula bleeding caused by an aortic stent graft
- Seunghyun Hong, Gwang Ha Kim
- Clin Endosc 2024;57(3):407-408. Published online February 2, 2024
- DOI: https://doi.org/10.5946/ce.2023.281
- 1,833 View
- 146 Download

-
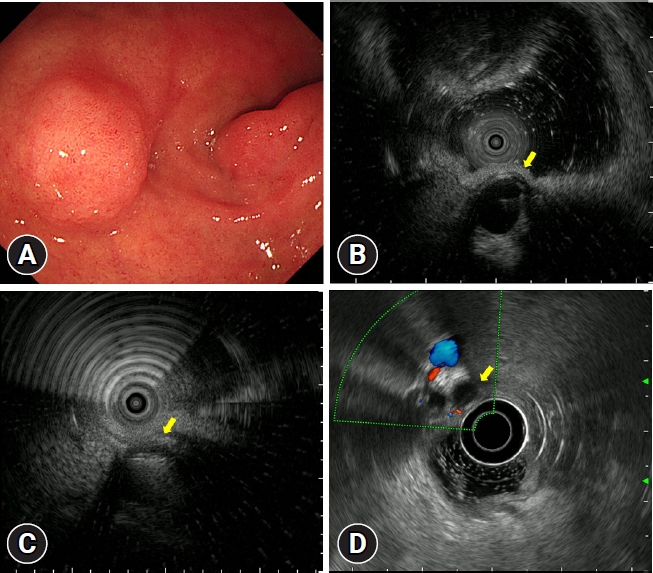
A remnant cystic duct presenting as a duodenal subepithelial tumor

- Gwang Ha Kim, Dong Chan Joo
- Clin Endosc 2024;57(2):268-269. Published online February 2, 2024
- DOI: https://doi.org/10.5946/ce.2023.275
- 2,245 View
- 209 Download

- Clinicians should be aware of proton pump inhibitor–related changes in the gastric mucosa
- Gwang Ha Kim
- Clin Endosc 2024;57(1):51-52. Published online January 10, 2024
- DOI: https://doi.org/10.5946/ce.2023.188

-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- Whitish gastric mucosa on upper gastrointestinal endoscopy
Eun Jeong Gong, Chang Seok Bang
Clinical Endoscopy.2024; 57(2): 277. CrossRef
- Whitish gastric mucosa on upper gastrointestinal endoscopy
- 2,071 View
- 169 Download
- 1 Web of Science
- 1 Crossref

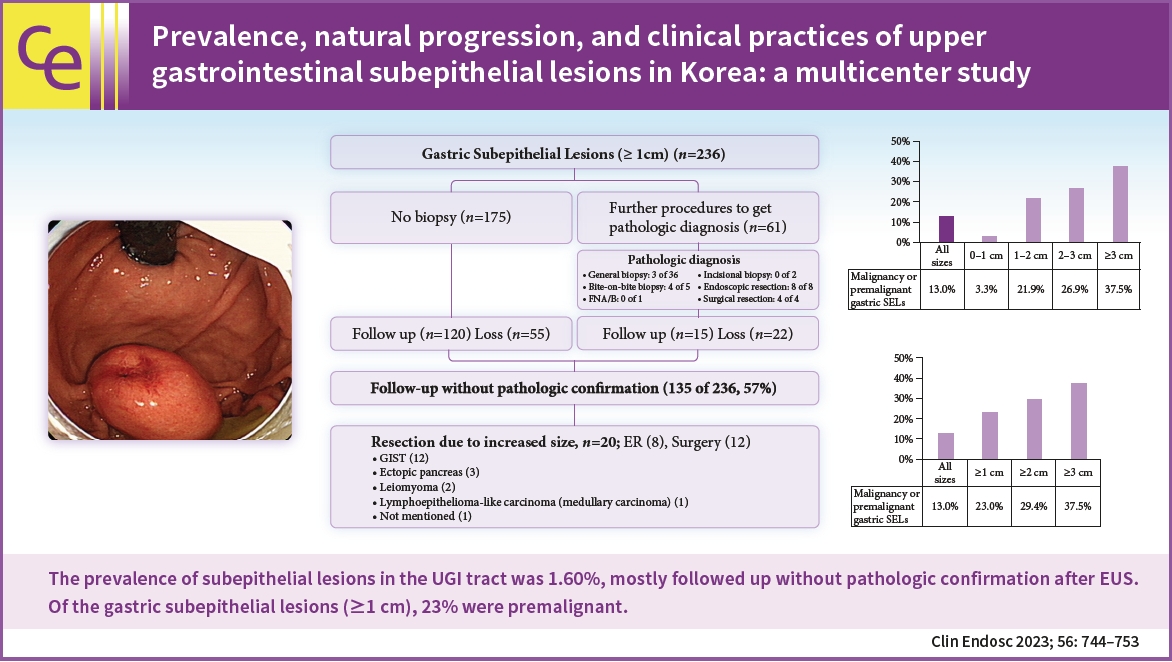
- Prevalence, natural progression, and clinical practices of upper gastrointestinal subepithelial lesions in Korea: a multicenter study
- Younghee Choe, Yu Kyung Cho, Gwang Ha Kim, Jun-Ho Choi, Eun Soo Kim, Ji Hyun Kim, Eun Kwang Choi, Tae Hyeon Kim, Seong-Hun Kim, Do Hoon Kim, The Research Group for Endoscopic Ultrasound in Korean Society of Gastrointestinal Endoscopy
- Clin Endosc 2023;56(6):744-753. Published online August 25, 2023
- DOI: https://doi.org/10.5946/ce.2023.005
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: This study aimed to evaluate the prevalence and natural progression of subepithelial lesions (SELs) in the upper gastrointestinal (UGI) tract.
Methods
The medical records of patients with UGI SELs who underwent endoscopic screening at eight university hospitals between January and December 2010 were retrospectively investigated. The follow-up evaluations were performed until December 2016.
Results
UGI SELs were found in 1,044 of the 65,233 participants screened (endoscopic prevalence, 1.60%; the total number of lesions, 1,062; mean age, 55.1±11.2 years; men, 53.6%). The median follow-up period was 48 (range, 8–74) months. SELs were most frequently found in the stomach (63.8%) and had a mean size of 9.9±6.1 mm. Endoscopic ultrasonography (EUS) was performed in 293 patients (28.1%). The most common lesions were leiomyomas, followed by gastrointestinal stromal tumors (GISTs), and ectopic pancreas. The proportions of SELs with malignant potential according to size were 3% (<1 cm), 22% (1–2 cm), 27% (2–3 cm), and 38% (≥3 cm). In gastric SELs larger than 1 cm, resections were performed in 20 patients because of an increase in size, of which 12 were found to be GISTs.
Conclusions
The prevalence of UGI SELs was 1.60%. Further, 23% of gastric SELs ≥1 cm were precancerous lesions, most followed by EUS and clinical decisions without initial pathological confirmation. -
Citations
Citations to this article as recorded by- A Case of Esophageal MALT Lymphoma Mimicking a Subepithelial Tumor
Ha Eun Lee, Gwang Ha Kim, Min Ji Kim, Kyung Bin Kim, Dong Chan Joo, Hye Kyung Jeon, Moon Won Lee, Bong Eun Lee
The Korean Journal of Gastroenterology.2024; 83(4): 157. CrossRef - Small gastric subepithelial lesions: A sand in the eye
Tanyaporn Chantarojanasiri, Nikhil Sonthalia, Rashid N. Lui
Journal of Gastroenterology and Hepatology.2024;[Epub] CrossRef - An Esophageal Leiomyoma with Cystic Degeneration Mimicking a Malignant Neoplasm
Gwang Ha Kim, Dong Chan Joo, Moon Won Lee, Bong Eun Lee, Kyungbin Kim
The Ewha Medical Journal.2023;[Epub] CrossRef
- A Case of Esophageal MALT Lymphoma Mimicking a Subepithelial Tumor
- 2,718 View
- 165 Download
- 4 Web of Science
- 3 Crossref

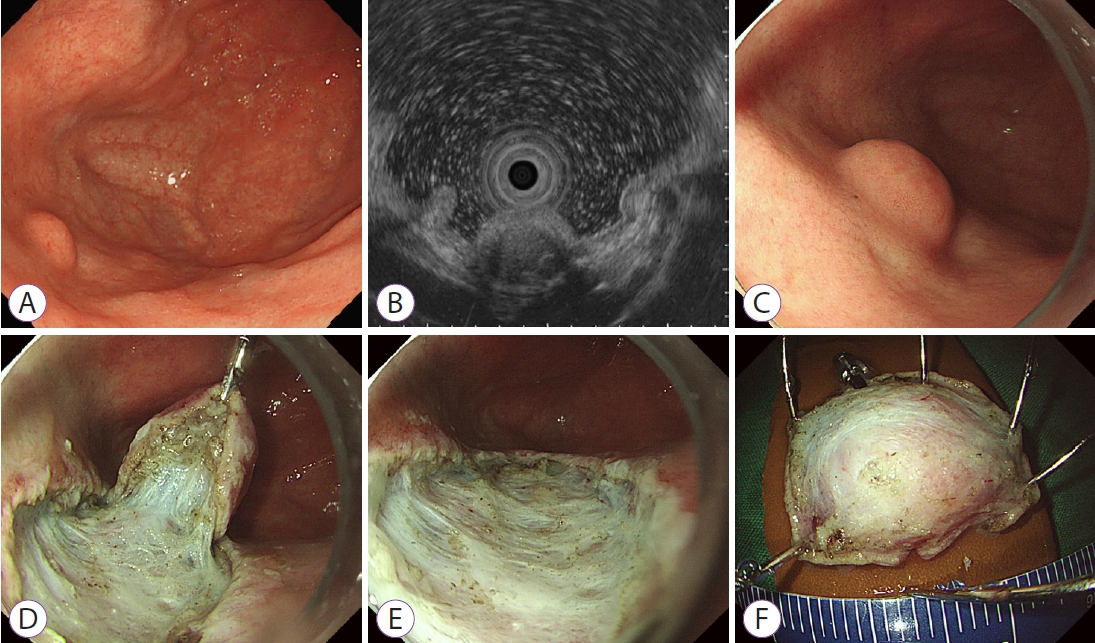
- A Rare Cause of Subepithelial Tumor in the Gastric Fundus
- Da Mi Kim, Gwang Ha Kim, Kyungbin Kim
- Clin Endosc 2022;55(2):313-314. Published online February 25, 2022
- DOI: https://doi.org/10.5946/ce.2022.039
- 4,164 View
- 188 Download

- Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
- Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
- Clin Endosc 2021;54(5):633-640. Published online September 13, 2021
- DOI: https://doi.org/10.5946/ce.2021.216
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - This is a special review to celebrate the 10th anniversary of Clinical Endoscopy. Each deputy editor has selected articles from one’s subspecialty that are significant in terms of the number of downloads, citations, and clinical importance. The articles included original articles, review articles, systematic reviews, and meta-analyses.
- 2,811 View
- 72 Download
- 1 Web of Science

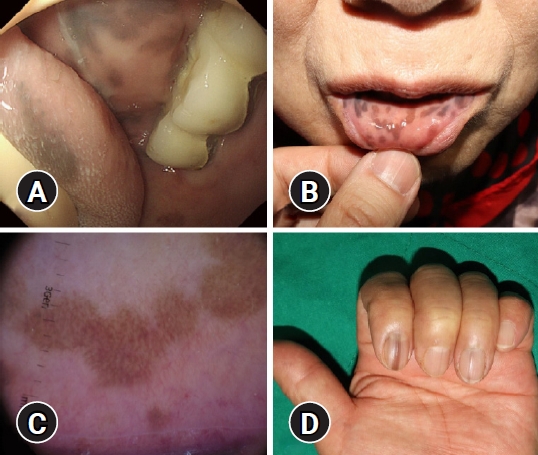
- Oral hyperpigmented macules observed during endoscopy intubation
- Da Mi Kim, Gwang Ha Kim, Moon-Bum Kim
- Clin Endosc 2022;55(4):574-575. Published online January 19, 2021
- DOI: https://doi.org/10.5946/ce.2020.286
- 3,523 View
- 214 Download

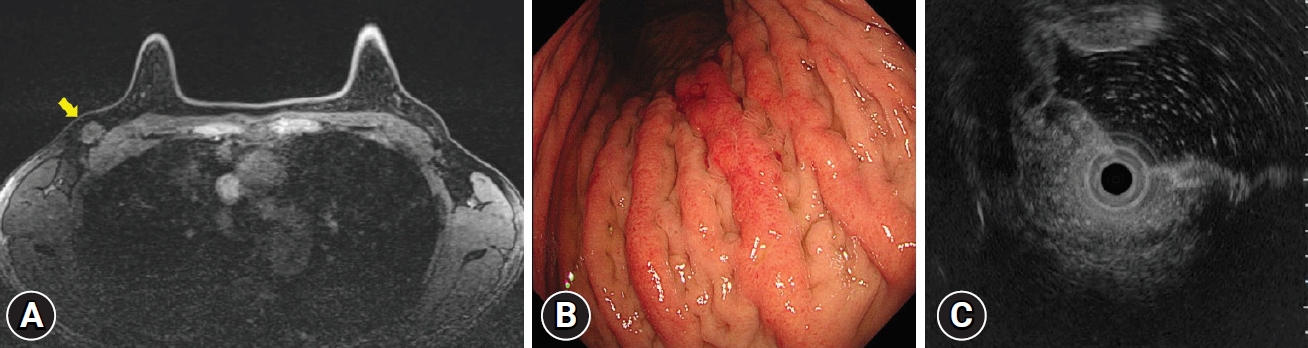
- Metastasis of breast cancer presenting as enlarged folds in the stomach
- So Eun Jeun, Gwang Ha Kim, Moon Won Lee, Sojeong Lee
- Clin Endosc 2022;55(3):463-464. Published online November 6, 2020
- DOI: https://doi.org/10.5946/ce.2020.239
- 3,097 View
- 179 Download

-
Large Jejunal Phytobezoar with Small Bowel Obstruction Treated by Single-Balloon Enteroscopy

- Eun Young Park, Dong Hoon Baek, Bong Eun Lee, Gwang Ha Kim, Geun Am Song
- Clin Endosc 2022;55(2):310-312. Published online November 6, 2020
- DOI: https://doi.org/10.5946/ce.2020.215

- 4,432 View
- 164 Download

-
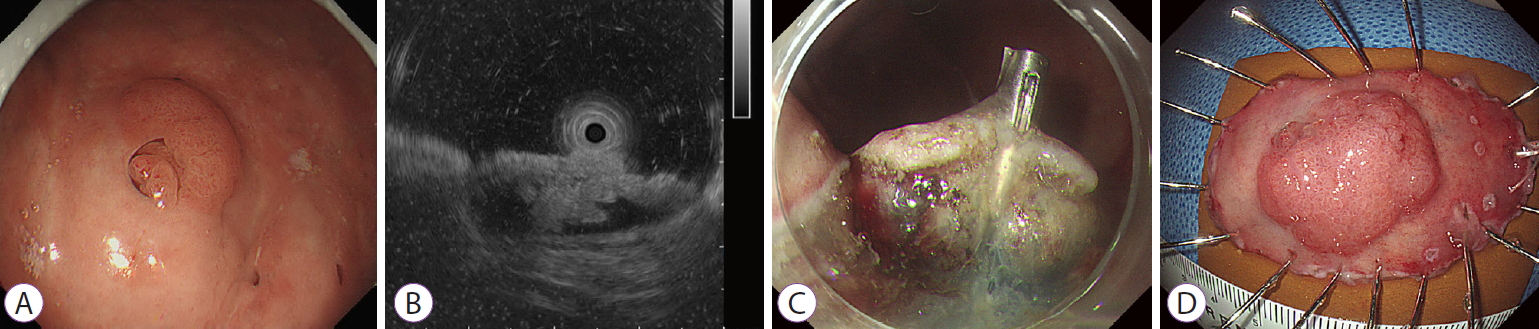
Endoscopic Submucosal Dissection of an Inverted Pyloric Gland Adenoma Using Dental Floss and Clip Traction

- Gwang Ha Kim, Moon Won Lee, Bong Eun Lee, Do Youn Park
- Clin Endosc 2021;54(6):935-936. Published online August 31, 2020
- DOI: https://doi.org/10.5946/ce.2020.164
-
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- Synchronous gastric MALT lymphoma and gastric adenocarcinoma of fundic gland type arising from a hamartomatous inverted polyp in a Helicobacter pylori naive patient
Ryo Miyamoto, Hidehiko Takigawa, Takahiro Kotachi, Hiroki Kadota, Ryo Yuge, Ryohei Hayashi, Yuji Urabe, Akira Ishikawa, Kazuhiro Sentani, Shiro Oka
Clinical Journal of Gastroenterology.2023; 16(4): 521. CrossRef - The Many Faces of Gastric Inverted Polyps: a case report
S.I. Kim, M.Y. Agapov, T.F. Savostyanov, A.A. Paratovskaya, I.A. Sokolova
Dokazatel'naya gastroenterologiya.2023; 12(2): 88. CrossRef - Pyloric Gastric Adenoma: Endoscopic Detection, Removal, and Echoendosonographic Characterization
Anabel Liyen Cartelle, Erik A. Holzwanger, Samuel Igbinedion, Sultan Mahmood, Harry J. Rosenberg, Tyler M. Berzin, Mandeep S. Sawhney, Moamen Gabr, Douglas K. Pleskow
ACG Case Reports Journal.2023; 10(12): e01229. CrossRef
- Synchronous gastric MALT lymphoma and gastric adenocarcinoma of fundic gland type arising from a hamartomatous inverted polyp in a Helicobacter pylori naive patient
- 4,483 View
- 158 Download
- 2 Web of Science
- 3 Crossref

-
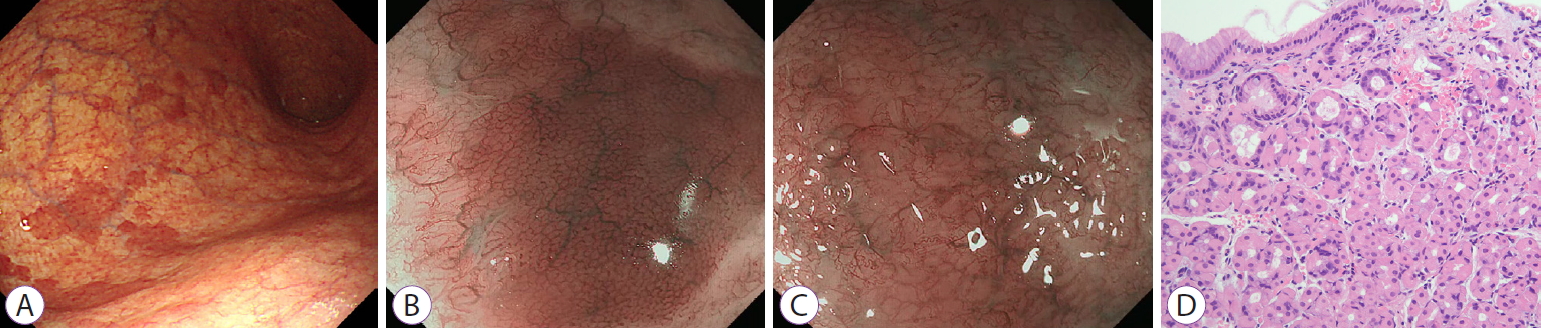
Gastric Oxyntic Mucosa Pseudopolyps

- Dong Chan Joo, Gwang Ha Kim
- Clin Endosc 2021;54(4):621-622. Published online August 11, 2020
- DOI: https://doi.org/10.5946/ce.2020.157
- 4,237 View
- 147 Download

- Diagnosing Gastric Mesenchymal Tumors by Digital Endoscopic Ultrasonography Image Analysis
- Moon Won Lee, Gwang Ha Kim
- Clin Endosc 2021;54(3):324-328. Published online June 18, 2020
- DOI: https://doi.org/10.5946/ce.2020.061

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Gastric mesenchymal tumors (GMTs) are incidentally discovered in national gastric screening programs in Korea. Endoscopic ultrasonography (EUS) is the most useful diagnostic modality for evaluating GMTs. The differentiation of gastrointestinal stromal tumors from benign mesenchymal tumors, such as schwannomas or leiomyomas, is important to ensure appropriate clinical management. However, this is difficult and operator dependent because of the subjective interpretation of EUS images. Digital image analysis computes the distribution and spatial variation of pixels using texture analysis to extract useful data, enabling the objective analysis of EUS images and decreasing interobserver and intraobserver agreement in EUS image interpretation. This review aimed to summarize the usefulness and future of digital EUS image analysis for GMTs based on published reports and our experience.
-
Citations
Citations to this article as recorded by- Schwannoma gástrico. Reporte de un caso
Darío Montes N, Nixon Cevallos R, Rubén Montes N
Oncología (Ecuador).2024; 34(1): 52. CrossRef - A combined radiomic model distinguishing GISTs from leiomyomas and schwannomas in the stomach based on endoscopic ultrasonography images
Xian‐Da Zhang, Ling Zhang, Ting‐Ting Gong, Zhuo‐Ran Wang, Kang‐Li Guo, Jun Li, Yuan Chen, Jian‐Tao Zhang, Ben‐Gong Ye, Jin Ding, Jian‐Wei Zhu, Feng Liu, Duan‐Min Hu, JianGang Chen, Chun‐Hua Zhou, Duo‐Wu Zou
Journal of Applied Clinical Medical Physics.2023;[Epub] CrossRef - An Esophageal Leiomyoma with Cystic Degeneration Mimicking a Malignant Neoplasm
Gwang Ha Kim, Dong Chan Joo, Moon Won Lee, Bong Eun Lee, Kyungbin Kim
The Ewha Medical Journal.2023;[Epub] CrossRef - Advancements in the Diagnosis of Gastric Subepithelial Tumors
Osamu Goto, Mitsuru Kaise, Katsuhiko Iwakiri
Gut and Liver.2022; 16(3): 321. CrossRef - Schwannoma gástrico. Diagnóstico diferencial de tumores submucosos
M. Reyes Busta Nistal, Noelia Alcaide Suarez, Luis Fernández Salazar, Daniel Corrales Cruz
Gastroenterología y Hepatología.2021;[Epub] CrossRef - Scoring systems for differentiating gastrointestinal stromal tumors and schwannomas from leiomyomas in the stomach
Shotaro Okanoue, Masaya Iwamuro, Takehiro Tanaka, Takuya Satomi, Kenta Hamada, Hiroyuki Sakae, Makoto Abe, Yoshiyasu Kono, Hiromitsu Kanzaki, Seiji Kawano, Yoshiro Kawahara, Hiroyuki Okada
Medicine.2021; 100(40): e27520. CrossRef
- Schwannoma gástrico. Reporte de un caso
- 5,389 View
- 217 Download
- 6 Web of Science
- 6 Crossref

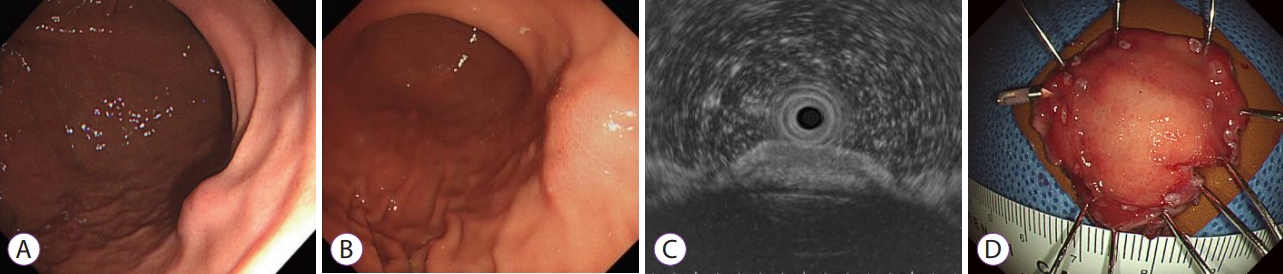
- A Rare Cause of Gastric Subepithelial Tumor
- Gwang Ha Kim, Do Youn Park
- Clin Endosc 2020;53(3):377-378. Published online May 29, 2020
- DOI: https://doi.org/10.5946/ce.2020.134
-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- A Rare Cause of Subepithelial Tumor in the Gastric Fundus
Da Mi Kim, Gwang Ha Kim, Kyungbin Kim
Clinical Endoscopy.2022; 55(2): 313. CrossRef
- A Rare Cause of Subepithelial Tumor in the Gastric Fundus
- 4,378 View
- 143 Download
- 1 Web of Science
- 1 Crossref

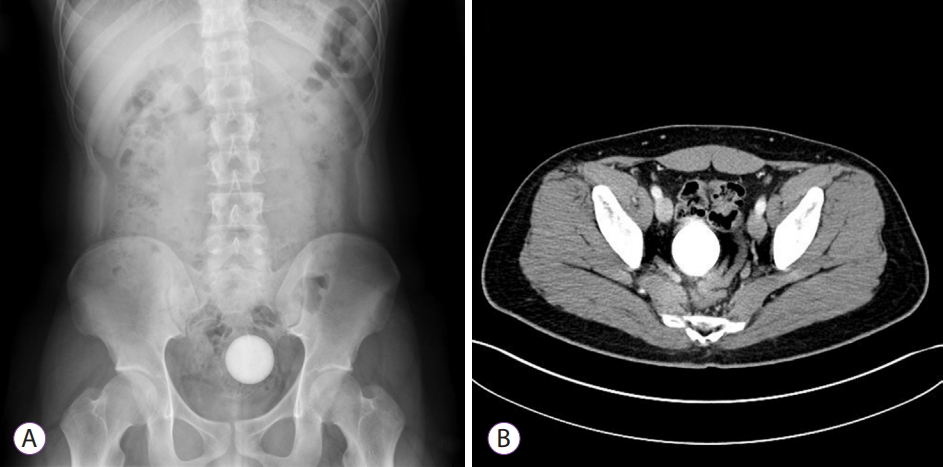
- Unusual Rectal Foreign Body: A Golf Ball
- Young Joo Park, Dong Hoon Baek, Eun Young Park, Gwang Ha Kim, Geun Am Song
- Clin Endosc 2021;54(2):291-292. Published online May 25, 2020
- DOI: https://doi.org/10.5946/ce.2020.097
-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- Successful Expulsion of a Golf Ball from the Sigmoid Colon Using Volume Laxatives
James P. Grantham, Amanda Hii, Tim Bright, David Liu, Neil Donald Merrett
Case Reports in Surgery.2023; 2023: 1. CrossRef
- Successful Expulsion of a Golf Ball from the Sigmoid Colon Using Volume Laxatives
- 8,706 View
- 126 Download
- 1 Web of Science
- 1 Crossref

- Diagnosis of Gastric Subepithelial Tumors Using Endoscopic Ultrasonography or Abdominopelvic Computed Tomography: Which is Better?
- Eun Young Park, Gwang Ha Kim
- Clin Endosc 2019;52(6):519-520. Published online November 14, 2019
- DOI: https://doi.org/10.5946/ce.2019.188
-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- Single-incision needle-knife biopsy for the diagnosis of GI subepithelial tumors: a systematic review and meta-analysis
Yassin Shams Eldien Naga, Banreet Singh Dhindsa, Smit Deliwala, Kyaw Min Tun, Amaninder Dhaliwal, Daryl Ramai, Ishfaq Bhat, Shailender Singh, Saurabh Chandan, Douglas G. Adler
Gastrointestinal Endoscopy.2023; 97(4): 640. CrossRef - Feasibility of endoscopic resection and impact of endoscopic ultrasound-based surveillance on colorectal subepithelial tumors
Eun Young Park, Dong Hoon Baek, Seung Min Hong, Bong Eun Lee, Moon Won Lee, Gwang Ha Kim, Geun Am Song
Surgical Endoscopy.2023; 37(9): 6867. CrossRef - Endoscopic ultrasound artificial intelligence-assisted for prediction of gastrointestinal stromal tumors diagnosis: A systematic review and meta-analysis
Rômulo Sérgio Araújo Gomes, Guilherme Henrique Peixoto de Oliveira, Diogo Turiani Hourneaux de Moura, Ana Paula Samy Tanaka Kotinda, Carolina Ogawa Matsubayashi, Bruno Salomão Hirsch, Matheus Oliveira Veras, João Guilherme Ribeiro Jordão Sasso, Roberto Pa
World Journal of Gastrointestinal Endoscopy.2023; 15(8): 528. CrossRef - Características endosonográficas de las lesiones subepiteliales del tracto digestivo superior: experiencia de un centro de referencia en Colombia
Ileana Rocío Bautista Parada, Angel Rojas Espinosa, Lazaro Antonio Arango Molano, Andrés Sánchez Gil, Claudia Díaz Tobar
Revista colombiana de Gastroenterología.2023; 38(3): 264. CrossRef - Convolutional neural network‐based object detection model to identify gastrointestinal stromal tumors in endoscopic ultrasound images
Chang Kyo Oh, Taewan Kim, Yu Kyung Cho, Dae Young Cheung, Bo‐In Lee, Young‐Seok Cho, Jin Il Kim, Myung‐Gyu Choi, Han Hee Lee, Seungchul Lee
Journal of Gastroenterology and Hepatology.2021; 36(12): 3387. CrossRef
- Single-incision needle-knife biopsy for the diagnosis of GI subepithelial tumors: a systematic review and meta-analysis
- 5,723 View
- 191 Download
- 3 Web of Science
- 5 Crossref

- A Rare Duodenal Subepithelial Tumor: Duodenal Schwannoma
- Dong Hwahn Kahng, Gwang Ha Kim, Sang Gyu Park, So Jeong Lee, Do Youn Park
- Clin Endosc 2018;51(6):587-590. Published online May 15, 2018
- DOI: https://doi.org/10.5946/ce.2018.050

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Schwannomas are uncommon neoplasms that arise from Schwann cells of the neural sheath. Gastrointestinal schwannomas are rare among mesenchymal tumors of the gastrointestinal tract, and only a few cases have been reported to date. Duodenal schwannomas are usually discovered incidentally and achieving a preoperative diagnosis is difficult. Schwannomas can be distinguished from other subepithelial tumors on endoscopic ultrasonography; however, any typical endosonographic features of duodenal schwannomas have not been reported due to the rarity of these tumors. Immunohistochemistry is essential to distinguish schwannomas from gastrointestinal stromal tumors and leiomyomas. We report a case of duodenal schwannoma found incidentally during a health check-up endoscopy. On endoscopic ultrasonography, this tumor was suspected as a gastrointestinal stromal tumor; therefore, the patient underwent laparoscopic wedge resection of the tumor. Histopathology and immunohistochemistry confirmed that the duodenal lesion was a benign schwannoma.
-
Citations
Citations to this article as recorded by- Ileal Schwannoma: A Rare Cause of Pelvic Mass
Martin Jezovit, Hasan Bakirli, Ifrat Bakirov, Khalid Hureibi, Gultakin Bakirova, Roman Okolicany, Pavol Janac, Iveta Meciarova, Nasser Alhwaymel, Ilkin Bakirli, Augustin Prochotsky, Muthukumaran Rangarajan
Case Reports in Surgery.2024; 2024: 1. CrossRef - Periampullary duodenal schwannoma mimicking ampullary neoplasm
Marly Pierina Rubio Sierra, Aydamir Alrakawi, Ahmad Alduaij, Dana AlNuaimi, Numan Cem Balci
Radiology Case Reports.2020; 15(11): 2085. CrossRef
- Ileal Schwannoma: A Rare Cause of Pelvic Mass
- 5,591 View
- 106 Download
- 1 Web of Science
- 2 Crossref

- Magnifying Endoscopy for Esophageal Ectopic Sebaceous Glands
- Mu Song Jeon, Gwang Ha Kim, Dong Young Jeong, Byeong Kyu Park, Moon Won Lee, So-Jeong Lee, Do Youn Park
- Clin Endosc 2018;51(5):495-497. Published online February 26, 2018
- DOI: https://doi.org/10.5946/ce.2017.187
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Ectopic sebaceous glands are found very rarely in the esophagus; heretofore, several cases have been reported. The sebaceous gland is originally a source of an endodermal origin; however, there have been controversies regarding whether the origin of the esophageal ectopic sebaceous gland is ectodermal or endodermal. Ectopic sebaceous glands of the esophagus usually do not cause symptoms; thus, they are often found incidentally on endoscopy for routine health screening. Endoscopic findings are characterized by single or multiple yellow patches or nodular lesions of various sizes, sometimes with small central openings. We report two cases of esophageal ectopic sebaceous glands found incidentally during endoscopy with magnifying endoscopic findings. The lesions were in the mid-esophagus and lower esophagus, respectively, and both endoscopic findings were similar as multiple yellowish patches or plaques. Magnifying endoscopy revealed the openings of the excretory ducts surrounded by circular microvessels in both cases.
-
Citations
Citations to this article as recorded by- Multiple heterotopic sebaceous glands in the oesophagus: A case report and literature review
Yuan Fang, Zhi Wang, Yong Qiang Yang, Bei Wen Song, Wen Bin Gou
Tropical Doctor.2024; 54(1): 49. CrossRef - Clinicopathologic Characteristics of Esophageal Ectopic Sebaceous Glands: Chronological Changes and Immunohistochemical Analysis
Hirotsugu Hashimoto, Hajime Horiuchi, Sakiko Miura, Shunya Takayanagi, Toshiaki Gunji, Teppei Morikawa
International Journal of Surgical Pathology.2021; 29(4): 378. CrossRef - The clinical and endoscopic features of esophageal ectopic sebaceous glands
Hui‐Fen Chen, Hsi‐Chang Lee, Min‐Kai Liao, Ting‐An Chang, Chih‐Lin Lin, Li‐Ying Liao, Kuan‐Yang Chen
Advances in Digestive Medicine.2020; 7(4): 179. CrossRef - Case Report of a Proposed, Novel, Endoscopic “Whitehead Pimple” Sign of Ectopic Esophageal Sebaceous Glands Based on Their Mimicking the Dermatologic and Histopathologic Characteristics of Cutaneous Whitehead Pimples/Closed Comedones
Amy Le, Mitual Amin, Mitchell S. Cappell
Digestive Diseases and Sciences.2019; 64(7): 2049. CrossRef - Ectopic Sebaceous Gland in Esophagus Presenting as Subepithelial Tumor
Dong Han Yeom, Han Seung Ryu
Chonnam Medical Journal.2019; 55(3): 168. CrossRef
- Multiple heterotopic sebaceous glands in the oesophagus: A case report and literature review
- 6,704 View
- 129 Download
- 4 Web of Science
- 5 Crossref

- Bile Duct Patency Maintained after Intraductal Radiofrequency Ablation in a Case of Hepatocellular Cholangiocarcinoma with Bile Duct Invasion
- Sung Yong Han, Geun Am Song, Dong Uk Kim, Dong Hoon Baek, Moon Won Lee, Gwang Ha Kim
- Clin Endosc 2018;51(2):201-205. Published online August 31, 2017
- DOI: https://doi.org/10.5946/ce.2017.097
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Combined hepatocellular-cholangiocarcinoma (HCC-CC) with bile duct invasion (BDI) is rare. In unresectable cases, biliary stent placement and photodynamic therapy (PDT) are used for resolving obstructive jaundice. However, stent occlusion remains problematic, and PDT is expensive and time-consuming. Intraductal radiofrequency ablation (RFA) is an emerging procedure for palliation in these patients. It has potential benefits including less expense, lower rates of severe complication, longer maintenance of ductal patency, and easier technique compared with PDT or stenting alone. We report a 67-year-old man who underwent repeated intraductal RFA for HCC-CC and HCC with BDI (HCC-BDI), for whom bile duct patency was maintained without additional biliary procedures.
-
Citations
Citations to this article as recorded by- Clinical and cost effectiveness of endoscopic bipolar radiofrequency ablation for the treatment of malignant biliary obstruction: a systematic review
Fiona Beyer, Stephen Rice, Giovany Orozco-Leal, Madeleine Still, Hannah O’Keefe, Nicole O’Connor, Akvile Stoniute, Dawn Craig, Stephen Pereira, Louise Carr, John Leeds
Health Technology Assessment.2023; : 1. CrossRef - Improving biliary stent patency for malignant obstructive jaundice using endobiliary radiofrequency ablation: experience in 150 patients
Ya-Lin Kong, Hong-Yi Zhang, Cheng-Li Liu, Xiao-Jun He, Gang Zhao, Cheng Wang, Ling-Hong Kong, Jing Zhao
Surgical Endoscopy.2022; 36(3): 1789. CrossRef
- Clinical and cost effectiveness of endoscopic bipolar radiofrequency ablation for the treatment of malignant biliary obstruction: a systematic review
- 5,633 View
- 156 Download
- 2 Web of Science
- 2 Crossref

- A Rare Case of Early Gastric Cancer Combined with Underlying Heterotopic Pancreas
- Jung Bin Yoon, Bong Eun Lee, Dae Hwan Kim, Do Youn Park, Hye Kyung Jeon, Dong Hoon Baek, Gwang Ha Kim, Geun Am Song
- Clin Endosc 2018;51(2):192-195. Published online August 31, 2017
- DOI: https://doi.org/10.5946/ce.2017.055
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Heterotopic pancreas in the stomach is usually asymptomatic and benign. Here, we presented a rare case of an early gastric cancer overlying a heterotopic pancreas. A 48-year-old woman underwent esophagogastroduodenoscopy, which revealed a subepithelial mass measuring 2.0×1.5 cm on the gastric antrum with a 1-cm erosive erythematous discoloration on the surface. A biopsy specimen showed moderately differentiated tubular adenocarcinoma. Endosonography showed a heterogeneous hypoechoic mass measuring 1.3×0.6 cm, with indistinct margins in the second and third layers of the gastric wall; anechoic tubular structures within the mass were suggestive of heterotopic pancreas. Distal gastrectomy was performed, which confirmed an early gastric cancer confined to the mucosa, and a separate underlying heterotopic pancreas. Although heterotopic pancreas is most likely benign, careful endoscopic observation of the mucosal surface is necessary to avoid overlooking a coincident early gastric cancer.
-
Citations
Citations to this article as recorded by- Duodenal Heterotopic Pancreas with a Large Retention Cyst: A Case Report and Literature Review
Shinya Kawaguchi, Akinori Murakami, Masato Nishida
Internal Medicine.2023; 62(5): 723. CrossRef - Gastric ectopic pancreas combined with synchronous multiple early gastric cancer: A rare case report
Zhen-Ya Zhao, Yue-Xing Lai, Ping Xu
World Journal of Clinical Cases.2023; 11(7): 1569. CrossRef - A rare case of enlarged gastric heterotopic pancreas with retention cysts: A case report and literature review
Keiso Matsubara, Michihiro Ishida, Toshiaki Morito, Tetsushi Kubota, Yasuhiro Choda, Masao Harano, Hiroyoshi Matsukawa, Hitoshi Idani, Shigehiro Shiozaki, Masazumi Okajima
International Journal of Surgery Case Reports.2020; 74: 284. CrossRef - A case of gastric heterotopic pancreas with gastroduodenal invagination
Shoko Iwahashi, Masaaki Nishi, Toshiaki Yoshimoto, Hideya Kashihara, Chie Takasu, Takuya Tokunaga, Tomohiko Miyatani, Jun Higashijima, Kozo Yoshikawa, Yuma Wada, Yoshimi Bando, Mitsuo Shimada
Surgical Case Reports.2019;[Epub] CrossRef
- Duodenal Heterotopic Pancreas with a Large Retention Cyst: A Case Report and Literature Review
- 6,704 View
- 182 Download
- 4 Web of Science
- 4 Crossref

- Is a Cytopathologist Always Needed during Endoscopic Ultrasonography-Guided Tissue Acquisition?
- Moon Won Lee, Gwang Ha Kim
- Clin Endosc 2017;50(4):311-312. Published online July 17, 2017
- DOI: https://doi.org/10.5946/ce.2017.103
- 4,464 View
- 91 Download

- Endoscopic Submucosal Dissection for Early Gastric Cancers with Uncommon Histology
- Gwang Ha Kim
- Clin Endosc 2016;49(5):434-437. Published online September 30, 2016
- DOI: https://doi.org/10.5946/ce.2016.127
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic submucosal dissection (ESD) enables en bloc curative resection of early gastric cancers (EGCs) with a negligible risk of lymph node metastasis (LNM). Although ESD for EGCs with absolute and expanded indications is safe, the results differ between EGCs with specialized and common histologies. EGC with papillary adenocarcinoma is a differentiated-type adenocarcinoma. At present, it is treated with ESD according to the same criteria as other differentiated-type adenocarcinomas. The LNM rate under the current indication criteria is high, and over half of the patients who undergo ESD as a primary treatment for EGC with papillary adenocarcinoma achieve an out-of-ESD result. Gastric carcinoma with lymphoid stroma in EGC has a low LNM rate and a favorable outcome, despite deep submucosal invasion. Patients with this gastric cancer subtype may be good candidates for ESD, even with deep submucosal invasion. Large-scale prospective multi-center studies with longer follow-up periods are needed to set proper ESD criteria for these tumors. Clinicians should be aware of these disease entities and ESD should be more carefully considered for EGCs with papillary adenocarcinoma and gastric carcinoma with lymphoid stroma.
-
Citations
Citations to this article as recorded by- Gastric carcinoma with lymphoid stroma derived from hamartomatous inverted polyp with osteoclast-like giant cells: a case report
Shoko Yamashita, Masaaki Nishi, Kozo Yoshikawa, Toshihiro Nakao, Takuya Tokunaga, Chie Takasu, Hideya Kashihara, Yuma Wada, Toshiaki Yoshimoto, Yosuke Iwakawa, Takeshi Oya, Koichi Tsuneyama, Mitsuo Shimada
International Cancer Conference Journal.2022; 11(3): 196. CrossRef - Endoscopic Submucosal Dissection of Papillary Gastric Adenocarcinoma; Systematic Review
Chang Seok Bang, Jae Jun Lee, Gwang Ho Baik
Journal of Clinical Medicine.2020; 9(5): 1465. CrossRef - Pitfalls in the Interpretation of Publications about Endoscopic Submucosal Dissection of Early Gastric Cancer with Undifferentiated-Type Histology
Chang Seok Bang, Gwang Ho Baik
Clinical Endoscopy.2019; 52(1): 30. CrossRef - A new Schiff base coordinated copper(II) compound induces apoptosis and inhibits tumor growth in gastric cancer
Yan Xia, Xingkai Liu, Luping Zhang, Jinzhu Zhang, Chaoying Li, Nan Zhang, Hong Xu, Yan Li
Cancer Cell International.2019;[Epub] CrossRef
- Gastric carcinoma with lymphoid stroma derived from hamartomatous inverted polyp with osteoclast-like giant cells: a case report
- 7,434 View
- 169 Download
- 7 Web of Science
- 4 Crossref

- Second-Look Endoscopy after Endoscopic Submucosal Dissection: Can We Obtain Valuable Information?
- Hye Kyung Jeon, Gwang Ha Kim
- Clin Endosc 2016;49(3):212-213. Published online May 9, 2016
- DOI: https://doi.org/10.5946/ce.2016.062
-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- Bleeding Risk Factors after Endoscopic Submucosal Dissection in Early Gastric Cancer and the Necessity of “Second-Look” Endoscopic Examination on the following Day
Rika Kobayashi, Ken Kawaura, Tohru Ito, Sadafumi Azukisawa, Hiroaki Kunou, Junji Kamai, Kazu Hamada, Tsuyoshi Mukai, Hidekazu Kitakata, Yasuhito Ishigaki
Journal of Clinical Medicine.2022; 11(4): 914. CrossRef
- Bleeding Risk Factors after Endoscopic Submucosal Dissection in Early Gastric Cancer and the Necessity of “Second-Look” Endoscopic Examination on the following Day
- 7,330 View
- 67 Download
- 1 Web of Science
- 1 Crossref

- Endosonographic Features of Gastric Schwannoma: A Single Center Experience
- Jong Min Yoon, Gwang Ha Kim, Do Youn Park, Na Ri Shin, Sangjeong Ahn, Chul Hong Park, Jin Sung Lee, Key Jo Lee, Bong Eun Lee, Geun Am Song
- Clin Endosc 2016;49(6):548-554. Published online March 15, 2016
- DOI: https://doi.org/10.5946/ce.2015.115
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Gastric schwannomas are rare benign mesenchymal tumors that are difficult to differentiate from other mesenchymal tumors with malignant potential, such as gastrointestinal stromal tumors. This study aimed to evaluate the characteristic findings of gastric schwannomas via endoscopic ultrasonography (EUS).
Methods
We retrospectively reviewed the EUS findings of 27 gastric schwannoma cases that underwent surgical excision at Pusan National University Hospital during 2007 to 2014.
Results
Gastric schwannomas were mainly located in the middle third of the stomach with a mean tumor size of 32 mm. All lesions exhibited hypoechoic echogenicity, and 24 lesions (88.9%) exhibited heterogeneous echogenicity. Seventeen lesions (63.0%) exhibited decreased echogenicity compared to the normal proper muscle layer. Distinct borders were observed in 24 lesions (88.9%), lobulated margins were observed in six lesions (22.2%), and marginal haloes were observed in 24 lesions (88.9%). Hyperechogenic spots were observed in 21 lesions (77.8%), calcifications were observed in one lesion (3.7%), and cystic changes were observed in two lesions (7.4%).
Conclusions
During EUS, gastric schwannomas appear as heterogeneously hypoechoic lesions with decreased echogenicity compared to the normal proper muscle layer. These features may be helpful for differentiating gastric schwannomas from other mesenchymal tumors. -
Citations
Citations to this article as recorded by- Gastric schwannoma: The gastrointestinal tumor simulator - case report and review of the literature
Amine Majdoubi, Anass El Achchi, Mohamed El Hammouti, Tareq Bouhout, Badr Serji
International Journal of Surgery Case Reports.2024; 116: 109389. CrossRef - Schwannoma gástrico. Reporte de un caso
Darío Montes N, Nixon Cevallos R, Rubén Montes N
Oncología (Ecuador).2024; 34(1): 52. CrossRef - Shwannoma of the stomach and synchronous cancer of the transverse colon: a clinical case report
A. B. Baychorov, M. A. Danilov, N. C. Karnaukhov, Z. M. Abdulatipova, A. V. Leontiev, G. G. Sahakyan
Surgery and Oncology.2023; 13(3): 38. CrossRef - Systematic Endoscopic Approach for Diagnosing Gastric Subepithelial Tumors
Gwang Ha Kim
Gut and Liver.2022; 16(1): 19. CrossRef - The Diagnosis of Small Gastrointestinal Subepithelial Lesions by Endoscopic Ultrasound-Guided Fine Needle Aspiration and Biopsy
Masanari Sekine, Takeharu Asano, Hirosato Mashima
Diagnostics.2022; 12(4): 810. CrossRef - Clinicopathological characteristics of gastrointestinal schwannomas: A retrospective analysis of 78 cases
Hailing Peng, Liu Han, Yuyong Tan, Yi Chu, Liang Lv, Deliang Liu, Hongyi Zhu
Frontiers in Oncology.2022;[Epub] CrossRef - What About Gastric Schwannoma? A Review Article
Sara Lauricella, Sergio Valeri, Gianluca Mascianà, Ida Francesca Gallo, Erica Mazzotta, Chiara Pagnoni, Saponaro Costanza, Lorenza Falcone, Domenico Benvenuto, Marco Caricato, Gabriella Teresa Capolupo
Journal of Gastrointestinal Cancer.2021; 52(1): 57. CrossRef - Diagnosing Gastric Mesenchymal Tumors by Digital Endoscopic Ultrasonography Image Analysis
Moon Won Lee, Gwang Ha Kim
Clinical Endoscopy.2021; 54(3): 324. CrossRef - Gastric schwannoma with high accumulation on fluorodeoxyglucose-positron emission tomography resected by non-exposed endoscopic wall-inversion surgery
Tomoya Sugiyama, Masahide Ebi, Tomoko Ochiai, Shintaro Kurahashi, Takuya Saito, Kentaro Onishi, Kazuhiro Yamamoto, Satoshi Inoue, Kazunori Adachi, Takashi Yoshimine, Yoshiharu Yamaguchi, Yasuhiro Tamura, Shinya Izawa, Yasutaka Hijikata, Yasushi Funaki, Na
Clinical Journal of Gastroenterology.2020; 13(1): 50. CrossRef - Schwannoma gástrico: una rareza entre los tumores mesenquimatosos del tracto gastrointestinal
G.E. Sánchez-Morales, A.M. Trolle-Silva, P. Moctezuma-Velázquez, J.H. Rodríguez-Quintero, R.J. Alcazar-Félix
Revista de Gastroenterología de México.2020; 85(1): 102. CrossRef - Gastric schwannoma: A rarity among mesenchymal tumors of the gastrointestinal tract
G.E. Sánchez-Morales, A.M. Trolle-Silva, P. Moctezuma-Velázquez, J.H. Rodríguez-Quintero, R.J. Alcazar-Félix
Revista de Gastroenterología de México (English Edition).2020; 85(1): 102. CrossRef - Clinical Characteristics and Surgical Management of Gastrointestinal Schwannomas
Xin Wu, Binglu Li, Chaoji Zheng, Xiaodong He
BioMed Research International.2020; 2020: 1. CrossRef - Periampullary duodenal schwannoma mimicking ampullary neoplasm
Marly Pierina Rubio Sierra, Aydamir Alrakawi, Ahmad Alduaij, Dana AlNuaimi, Numan Cem Balci
Radiology Case Reports.2020; 15(11): 2085. CrossRef - Gastric schwannoma: a case report and literature review
Changsheng Pu, Keming Zhang
Journal of International Medical Research.2020; 48(9): 030006052095782. CrossRef - Application of A Convolutional Neural Network in The Diagnosis of Gastric Mesenchymal Tumors on Endoscopic Ultrasonography Images
Yoon Ho Kim, Gwang Ha Kim, Kwang Baek Kim, Moon Won Lee, Bong Eun Lee, Dong Hoon Baek, Do Hoon Kim, Jun Chul Park
Journal of Clinical Medicine.2020; 9(10): 3162. CrossRef - Glomus Tumor of the Duodenum
Tae Kyoung Ha, Gwang Ha Kim, Moon Won Lee, Bong Eun Lee, Young Min Kwak, Guk Bin Park, Yong Bo Park
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2020; 20(4): 328. CrossRef - Simultaneous organ-sparing surgery in a patient with rare synchronous polyneoplasms of the gastrointestinal tract
D.V. Sidorov, I.V. Stepanyuk, I.A. Bakasov, M.V. Lozhkin, N.N. Volchenko, R.I. Moshurov, N.A. Grishin, E.V. Gameeva
Onkologiya. Zhurnal imeni P.A.Gertsena.2020; 9(6): 67. CrossRef - Digital image analysis-based scoring system for endoscopic ultrasonography is useful in predicting gastrointestinal stromal tumors
Moon Won Lee, Gwang Ha Kim, Kwang Baek Kim, Yoon Ho Kim, Do Youn Park, Chang In Choi, Dae Hwan Kim, Tae Yong Jeon
Gastric Cancer.2019; 22(5): 980. CrossRef - Gastric schwannoma misdiagnosed as a GIST
Roberto Peltrini, Paola Antonella Greco, Riccardo Aurelio Nasto, Alessandra D’Alessandro, Alessandro Iacobelli, Luigi Insabato, Luigi Bucci
Acta Chirurgica Belgica.2019; 119(6): 411. CrossRef - Clinical characteristics and surgical treatment of schwannomas of the esophagus and stomach: A case series and systematic review
Jesús Morales-Maza, Francisco Ulises Pastor-Sifuentes, Germán E Sánchez-Morales, Emilio Sanchez-Garcia Ramos, Oscar Santes, Uriel Clemente-Gutiérrez, Adriana Simoneta Pimienta-Ibarra, Heriberto Medina-Franco
World Journal of Gastrointestinal Oncology.2019; 11(9): 750. CrossRef - Gastric Schwannoma Mimicking Advanced Gastric Cancer
Woo Sun Rou, Ju Seok Kim, Sun Hyung Kang, Hee Seok Moon, Jae Kyu Sung, Hyun Yong Jeong
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2019; 19(4): 282. CrossRef - Gastrointestinal schwannomas: a rare but important differential diagnosis of mesenchymal tumors of gastrointestinal tract
Alexandros Mekras, Veit Krenn, Aristotelis Perrakis, Roland S Croner, Vasileios Kalles, Cem Atamer, Robert Grützmann, Nikolaos Vassos
BMC Surgery.2018;[Epub] CrossRef - Endosonographic Findings and the Natural Course of Chronic Gastric Anisakiasis: A Single-Center Experience
Eun Young Park, Dong Hoon Baek, Gwang Ha Kim, Bong Eun Lee, So-Jeong Lee, Do Youn Park
Gastroenterology Research and Practice.2018; 2018: 1. CrossRef - A Rare Duodenal Subepithelial Tumor: Duodenal Schwannoma
Dong Hwahn Kahng, Gwang Ha Kim, Sang Gyu Park, So Jeong Lee, Do Youn Park
Clinical Endoscopy.2018; 51(6): 587. CrossRef - Endoscopic ultrasonography diagnosis of subepithelial lesions
Mitsuhiro Kida, Yusuke Kawaguchi, Eiji Miyata, Rikiya Hasegawa, Toru Kaneko, Hiroshi Yamauchi, Shuko Koizumi, Kosuke Okuwaki, Shiro Miyazawa, Tomohisa Iwai, Hidehiko Kikuchi, Maya Watanabe, Hiroshi Imaizumi, Wasaburo Koizumi
Digestive Endoscopy.2017; 29(4): 431. CrossRef - Role of endoscopic ultrasound and endoscopic resection for the treatment of gastric schwannoma
Jinlong Hu, Xiang Liu, Nan Ge, Sheng Wang, Jintao Guo, Guoxin Wang, Siyu Sun
Medicine.2017; 96(25): e7175. CrossRef - Is Endoscopic Ultrasonography Adequate for the Diagnosis of Gastric Schwannomas?
Eun Jeong Gong, Kee Don Choi
Clinical Endoscopy.2016; 49(6): 498. CrossRef
- Gastric schwannoma: The gastrointestinal tumor simulator - case report and review of the literature
- 10,943 View
- 138 Download
- 20 Web of Science
- 27 Crossref

- Immunoglobulin G4-Related Inflammatory Pseudotumor Presenting as a Solitary Mass in the Stomach
- Hong Ryeol Cheong, Bong Eun Lee, Geun Am Song, Gwang Ha Kim, Sung Gyu An, Won Lim
- Clin Endosc 2016;49(2):197-201. Published online February 12, 2016
- DOI: https://doi.org/10.5946/ce.2015.074
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Immunoglobulin G4 (IgG4)-related disease (IgG4RD) is a relatively recently recognized entity that is histopathologically characterized by an extensive infiltration of lymphocytes and IgG4-positive plasma cells with dense fibrosis. IgG4RD is now known to affect any organ system, and a few cases of gastrointestinal lesions have also been reported. However, solitary IgG4RD of the stomach is still very rare. Furthermore, as it can mimic malignant conditions, it is important to recognize this disease to avoid unnecessary surgery. Herein, we present a case of IgG4RD presenting as an isolated subepithelial mass in the stomach.
-
Citations
Citations to this article as recorded by- Mass-forming immunoglobulin G4-related disease shows indolent clinical course after surgical resection, clinicopathological analysis of a series of 15 cases
Ruoyu Shi, Benjamin Livingston Farah, Chuanhui Xu, Joe Poh Sheng Yeong, Chik Hong Kuick, Jian Yuan Goh, Kenneth Tou En Chang, Angela Takano
Virchows Archiv.2022; 480(2): 383. CrossRef - Clinicopathological characteristics of gastric IgG4‐related disease: Systematic scoping review
Haruki Sawada, Torrey Czech, Krixie Silangcruz, Landon Kozai, Adham Obeidat, Eric Andrew Wien, Midori Filiz Nishimura, Asami Nishikori, Yasuharu Sato, Yoshito Nishimura
Journal of Gastroenterology and Hepatology.2022; 37(10): 1865. CrossRef - Utility of gastric biopsy in diagnosing IgG4‐related gastrointestinal disease
Kaori Uchino, Kenji Notohara, Takeshi Uehara, Yasuhiro Kuraishi, Junya Itakura, Akihiro Matsukawa
Pathology International.2021; 71(2): 124. CrossRef - Inflammatory Pseudotumor of Intestine Mimicking Lymphoma on 18F-FDG PET/CT
Qianqian Xue, Weibing Miao
Clinical Nuclear Medicine.2020; 45(5): 383. CrossRef - A reappraisal of sclerosing nodular and/or polypoid lesions of the gastrointestinal tract rich in IgG4‐positive plasma cells
Runjan Chetty
Histopathology.2020; 76(6): 832. CrossRef - Gastric IgG4-related disease presenting as a mass lesion and masquerading as a gastrointestinal stromal tumor
Banumathi Ramakrishna, Rohan Yewale, Kavita Vijayakumar, Patta Radhakrishna, Balakrishnan Siddartha Ramakrishna
Journal of Pathology and Translational Medicine.2020; 54(3): 258. CrossRef - IgG4-related Disease Manifesting as Gastroduodenal Ulcer Diagnosed by an Endoscopic Biopsy
Osamu Muto, Susumu Tamakawa, Kenji Takahashi, Shiro Yokohama, Ai Takasoe, Fuminori Hirano, Hideo Nishimura, Hiroki Saito
Internal Medicine.2020; 59(20): 2491. CrossRef - IgG4-related Sclerosing Disease Forming a Gastric Submucosal Tumor Diagnosed after Laparoscopic Endoscopic Cooperative Surgery—Report of a Case—
Tatsuki ISHIKAWA, Katsunori NAKANO, Masafumi OSAKA, Yayoi KADOTANI, Kaori OKUGAWA, Kiyokazu AKIOKA, Kenta SHIGEMORI, Yohei HOSOKAWA
Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association).2020; 81(2): 254. CrossRef - Immunoglobulin G4-related gastric inflammatory pseudotumor presenting with gastrointestinal bleeding
Betul Piyade, Gurhan Sisman, Serpil Yilmaz, Tayfun Karahasanoglu
European Journal of Gastroenterology & Hepatology.2020; 32(11): 1482. CrossRef - A Suspected Case of IgG4-Related Appendiceal Pseudotumor
Yudai Hojo, Yoshiharu Shirakata, Ai Izumi, Jun Matsui, Tokuyuki Yamashita, Hikaru Aoki, Makoto Kurimoto, Masaaki Hirata, Naoki Goda, Hiroaki Ito, Jun Tamura
The Japanese Journal of Gastroenterological Surgery.2020; 53(12): 976. CrossRef - Immunoglobulin G4-related gastric pseudotumor – An impostor: Case report
Manuel Santiago Mosquera, Andrea Suarez Gómez, Hugo Herrera, Karen Moreno-Medina, Alejandro González-Orozco, Carlos J-Perez Rivera
International Journal of Surgery Case Reports.2020; 75: 333. CrossRef - Imaging and pathological features of gastric lesion of immunoglobulin G4-related disease: A case report and review of the recent literature
Dai Inoue, Norihide Yoneda, Kotaro Yoshida, Hiromi Nuka, Jun Kinoshita, Sachio Fushida, Fumihito Toshima, Tetsuya Minami, Masayuki Takahira, Shoko Hamaoka, Hiroko Ikeda, Toshifumi Gabata, Mitsuhiro Kawano
Modern Rheumatology.2019; 29(2): 377. CrossRef - IgG4-Related Disease with Emphasis on Its Gastrointestinal Manifestation
Bijal Vashi, Arezou Khosroshahi
Gastroenterology Clinics of North America.2019; 48(2): 291. CrossRef - Gastrointestinal manifestation of immunoglobulin G4-related disease: clarification through a multicenter survey
Kenji Notohara, Terumi Kamisawa, Kazushige Uchida, Yoh Zen, Mitsuhiro Kawano, Satomi Kasashima, Yasuharu Sato, Masahiro Shiokawa, Takeshi Uehara, Hajime Yoshifuji, Hiroko Hayashi, Koichi Inoue, Keisuke Iwasaki, Hiroo Kawano, Hiroyuki Matsubayashi, Yukitos
Journal of Gastroenterology.2018; 53(7): 845. CrossRef - IgG4-related Disease in the Stomach which Was Confused with Gastrointestinal Stromal Tumor (GIST): Two Case Reports and Review of the Literature
Ho Seok Seo, Yoon Ju Jung, Cho Hyun Park, Kyo Young Song, Eun Sun Jung
Journal of Gastric Cancer.2018; 18(1): 99. CrossRef - IgG4-Related Disease Mimicking Crohn’s Disease: A Case Report and Review of Literature
Fabiana Ciccone, Antonio Ciccone, Mirko Di Ruscio, Filippo Vernia, Gianluca Cipolloni, Gino Coletti, Giuseppe Calvisi, Giuseppe Frieri, Giovanni Latella
Digestive Diseases and Sciences.2018; 63(4): 1072. CrossRef - Gastrointestinal and Extra-Intestinal Manifestations of IgG4–Related Disease
Katsuyuki Miyabe, Yoh Zen, Lynn D. Cornell, Govindarajan Rajagopalan, Vaidehi R. Chowdhary, Lewis R. Roberts, Suresh T. Chari
Gastroenterology.2018; 155(4): 990. CrossRef - A rare presentation of IgG4 related disease as a gastric antral lesion: Case report and review of the literature
Ali Bohlok, Melody El Khoury, Berenice Tulelli, Laurine Verset, Anthony Zaarour, Pieter Demetter, Pierre Eisendrath, Issam El Nakadi
International Journal of Surgery Case Reports.2018; 51: 244. CrossRef - IgG4-Related Sclerosing Disease Presenting as a Gastric Submucosal Tumor
Takashi Masuda, Toshifumi Matsumoto, Yushi Kaishakuji, Hirotada Tajiri, Akinori Egashira, Hirofumi Kawanaka
The Japanese Journal of Gastroenterological Surgery.2018; 51(10): 599. CrossRef
- Mass-forming immunoglobulin G4-related disease shows indolent clinical course after surgical resection, clinicopathological analysis of a series of 15 cases
- 7,680 View
- 101 Download
- 20 Web of Science
- 19 Crossref

- Endoscopic Management of Dieulafoy's Lesion
- Hye Kyung Jeon, Gwang Ha Kim
- Clin Endosc 2015;48(2):112-120. Published online March 27, 2015
- DOI: https://doi.org/10.5946/ce.2015.48.2.112
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub A Dieulafoy's lesion is a vascular abnormality consisting of a large caliber-persistent tortuous submucosal artery. A small mucosal defect with the eruption of this protruding vessel can cause bleeding. In fact, a Dieulafoy's lesion is a relatively rare but potentially life-threatening condition. It accounts for 1% to 2% of cases of acute gastrointestinal bleeding. Although there is no consensus on the treatment of Dieulafoy's lesions; treatment options depend on the mode of presentation, site of the lesion, and available expertise. Endoscopic therapy is usually successful in achieving primary hemostasis, with hemostasis success rates reaching 75% to 100%. Although various therapeutic endoscopic methods are used to control bleeding in Dieulafoy's lesions, the best method for endoscopic intervention is not clear. Combination endoscopic therapy is known to be superior to monotherapy because of a lower rate of recurrent bleeding. In addition, mechanical therapies including hemostatic clipping and endoscopic band ligation are more effective and successful in controlling bleeding than other endoscopic methods. Advances in endoscopic techniques have reduced mortality in patients with Dieulafoy's lesion-from 80% to 8%-and consequently, the need for surgical intervention has been reduced. Currently, surgical intervention is used for cases that fail therapeutic endoscopic or angiographic interventions.
-
Citations
Citations to this article as recorded by- Dieulafoy's lesion: Is there still a place for surgery? About 2 cases
Souhaib Atri, Mahdi Hammami, Yacine Ouadi, Amine Sebai, Youssef Chaker, Montassar Kacem
International Journal of Surgery Case Reports.2024; 114: 109166. CrossRef - Over-the-scope clip as a rescue treatment for massive bleeding due to Dieulafoy lesion at the colorectal anastomosis: A case report
Ping Han, Demin Li, Qiaozhen Guo, Yu Lei, Jingmei Liu, Dean Tian, Wei Yan
Medicine.2024; 103(16): e37871. CrossRef - Clinical, endoscopic and therapeutic features of bleeding Dieulafoy’s lesions: case series and literature review
Basma Aabdi, Ghizlane Kharrasse, Abdelkrim Zazour, Hajar Koulali, Ouiam Elmqaddem, Ismaili Zahi
BMJ Open Gastroenterology.2024; 11(1): e001299. CrossRef - Severe low gastrointestinal bleeding due to Dieulafoy's lesion: A report of two cases and review of literature
Cheng‐Chi Lee, Jen‐Chieh Huang, Jeng‐Shiann Shin
Advances in Digestive Medicine.2023; 10(4): 254. CrossRef - A bleeding duodenal lesion
Jhih‐Jie Lin, Ming‐Jen Chen
Advances in Digestive Medicine.2023; 10(3): 195. CrossRef - Dieulafoy’s Disease in Stomach and Duodenum-Recurrent Upper Gastrointestinal Bleeding
Guang-sheng Gao, Feng-qin Ren, Xin-xin Zhang, Xing-sheng Wang, Yun Li
Indian Journal of Surgery.2023; 85(6): 1464. CrossRef - Acquired Gastric Dieulafoy-Like Lesion due to Aberrant Blood Supply Diverted From the Left Phrenic Artery to an Enlarged Splenule
Benjamin Lew, Dennis E. Der, Brian S. Lim
ACG Case Reports Journal.2023; 10(4): e01032. CrossRef - Lesión de Dieulafoy en el recto: reporte de un caso
C.A. Martínez-Ortiz, E.D. Alvarez-Sores, U. Lara-Orozco, E. Murcio-Pérez
Revista de Gastroenterología de México.2023; 88(3): 301. CrossRef - Dieulafoy’s lesion of the rectum: A case report
C.A. Martínez-Ortiz, E.D. Alvarez-Sores, U. Lara-Orozco, E. Murcio-Pérez
Revista de Gastroenterología de México (English Edition).2023; 88(3): 301. CrossRef - Retrospective analysis of patients with Dieulafoy’s lesions
Bünyamin SARITAŞ, Şehmus ÖLMEZ, Adnan TAŞ, Nevin AKÇAER ÖZTÜRK, Banu KARA
Akademik Gastroenteroloji Dergisi.2023; 22(3): 136. CrossRef - Gastrointestinal Bleeding From a Transverse Colon Dieulafoy Lesion
Xinyu Xie, Jian Qin, Xiaojua Ma, Shanshan Liu
Cureus.2023;[Epub] CrossRef - Dieulafoy's lesion: a rare but potentially life-threatening cause of gastrointestinal bleeding
Eleanor Apthorp, Marta Mungai Ndungu, Kelum Rumwanpura, Muhammad JH Rahmani
British Journal of Hospital Medicine.2023; 84(11): 1. CrossRef - Intragastric Single-Port Surgery: An Innovative and Multipurpose Technique for the Therapy of Upper Digestive Tract Lesions
Renjie Li, Wilfried Veltzke-Schlieker, Andreas Adler, Mahmoud Ismail, Harun Badakhshi, Ricardo Zorron
Surgical Innovation.2022; 29(1): 56. CrossRef - Rectal Dieulafoy Lesion
Erika Koga, Satoshi Ashimine, Atsushi Iraha, Akira Hokama
Chonnam Medical Journal.2022; 58(1): 48. CrossRef - Therapeutic endoscopy of a Dieulafoy lesion in a 10-year-old girl: A case report
Ying Chen, Mei Sun, Xu Teng
World Journal of Clinical Cases.2022; 10(6): 1966. CrossRef - Diagnosis and Treatment of a Recurrent Bleeding Dieulafoy’s Lesion: A Case Report
Amanda R Levy, Sierra Broad, James R Loomis III, Julie A Thomas
Cureus.2022;[Epub] CrossRef - Severe lower gastrointestinal bleeding caused by rectal Dieulafoy’s lesion: Case reports and literature review
Ping Han, Yu Lei, Wei Hou, Nianjun Chen, Jingmei Liu, Dean Tian, Qiaozhen Guo, Wei Yan
Medicine.2022; 101(48): e32031. CrossRef - Jejunal Dieulafoy’s Lesion: A Systematic Review of Evaluation, Diagnosis, and Management
Adnan Malik, Faisal Inayat, Muhammad Hassan Naeem Goraya, Talal Almas, Rizwan Ishtiaq, Sohira Malik, Zahid Ijaz Tarar
Journal of Investigative Medicine High Impact Case Reports.2021; 9: 232470962098770. CrossRef - Role of imaging and endovascular radiology in endoscopically missed Dieulafoy’s lesion of stomach – A case report with review
Divyesh Dadhania, Jineesh Valakkada, Anoop Ayyappan, Santhosh Kannath
BJR|case reports.2021;[Epub] CrossRef - Incidental massive lower gastrointestinal hemorrhage caused by a rectal Dieulafoy’s lesion
Genesis Perez Del Nogal, Rangesh Modi, Ivania Salinas, Kalyan Chakrala
BMJ Case Reports.2021; 14(9): e244264. CrossRef - Massively bleeding Dieulafoy lesion and unique rescue: a video based case report from National Liver Institute, Menoufia University
Omkolsoum Alhaddad, Maha Elsabaawy, Ahmed Elfaioumy, Ashraf Eljaky
Egyptian Liver Journal.2021;[Epub] CrossRef - Modern management of acute non-variceal upper gastrointestinal bleeding
V. V. Darvin, A. Ya. Ilkanich, M. G. Ryzhikov, A. V. Oganian, A. V. Satinov
Сибирский научный медицинский журнал.2021; 41(6): 4. CrossRef - Trans‐ileostomy management to Dieulafoy's lesion
Bhavik Patel, Natasha R. Jeenah, Russell Canavan, Martin Wullschleger
ANZ Journal of Surgery.2020; 90(6): 1168. CrossRef - Hemorragia digestiva alta no varicosa
P. Cañamares Orbis, C. Borao Laguna, l. Sánchez Miguel, G. Hijos Mallada, A. Lanas Arbeloa
Medicine - Programa de Formación Médica Continuada Acreditado.2020; 13(3): 136. CrossRef - A case of the lower gastrointestinal bleeding due to Dieulafoy’s ulcer in the cecum
Keisuke Kinoshita, Osamu Matsunari, Akira Sonoda, Kensuke Fukuda, Kazuhisa Okamoto, Ryo Ogawa, Kazuhiro Mizukami, Tadayoshi Okimoto, Masaaki Kodama, Kazunari Murakami
Clinical Journal of Gastroenterology.2020; 13(4): 564. CrossRef - Tratamiento quirúrgico de la hemorragia digestiva alta por enfermedad de Dieulafoy
José Ángel Zamora-Soler, Vanesa Maturana-Ibáñez
Revista Colombiana de Cirugía .2020; 35(1): 113. CrossRef - Dieulafoy lesion: two pediatric case reports
Giovanni Di Nardo, Gianluca Esposito, Angela Mauro, Letizia Zenzeri, Gian Paolo Ciccarelli, Andrea Catzola, Alessandro Rossi, Vito Domenico Corleto
Italian Journal of Pediatrics.2020;[Epub] CrossRef - Massive Gastrointestinal Bleeding From a Jejunal Dieulafoy's Lesion
Palashkumar Jaiswal, Mairin Joseph-Talreja, John Anthony Teotico, Evan Grossman
ACG Case Reports Journal.2020; 7(6): e00400. CrossRef - Endoscopic full-thickness resection to treat active Dieulafoy's disease: A case report
Shan Yu, Xiao-Ming Wang, Xin Chen, Hong-Yan Xu, Guang-Jie Wang, Na Ni, Yu-Xin Sun
World Journal of Gastroenterology.2020; 26(30): 4557. CrossRef - Lesión de Dieulafoy rectal: una causa rara, pero potencialmente mortal de hemorragia del tubo digestivo bajo
Benjamín Gallo Arriaga, José Raúl Nieto Saucedo, Benjamín Gallo Chico, J Jesús Ibarra Rodríguez, Karla Edith Santibáñez Bedolla, Carlos Hidalgo Valadez
Acta Médica Grupo Ángeles.2020; 18(3): 302. CrossRef - Dieulafoy disease with gastric MALT lymphoma
Qin Zeng, Jin Feng Dai, Haijun Cao, Shuo Zhang
Medicine.2020; 99(41): e22651. CrossRef - Dieulafoy’s lesion: an unexpected and rare cause of upper gastrointestinal bleeding
Saravana Kumar Rajanthran, Harjit Chaal Singh, Da Jun Than, Firdaus Hayati
BMJ Case Reports.2020; 13(12): e240905. CrossRef - Endoscopic management of nonvariceal upper gastrointestinal bleeding
Pablo Cañamares-Orbís, Francis K.L. Chan
Best Practice & Research Clinical Gastroenterology.2019; 42-43: 101608. CrossRef - Finding the Dieulafoy’s Lesion: A Case of Recurrent Rectal Bleeding in an Immunosuppressed Patient
Caleb Hudspath, Dylan Russell, Ki Eum, Joel Guess, Jessica Bunin, Franklin Goldwire
Case Reports in Gastrointestinal Medicine.2019; 2019: 1. CrossRef - Hemorragia digestiva recurrente por lesión de Dieulafoy tratada con éxito mediante esclerosis guiada por ecoendoscopia
Irene García de la Filia, Nerea Hernanz, Enrique Vázquez Sequeiros, Eduardo Tavío Hernández
Gastroenterología y Hepatología.2018; 41(5): 319. CrossRef - Endoscopic Management of Nonvariceal, Nonulcer Upper Gastrointestinal Bleeding
Michael A. Chang, Thomas J. Savides
Gastrointestinal Endoscopy Clinics of North America.2018; 28(3): 291. CrossRef - A Retrospective Analysis of Cyanoacrylate Injection versus Hemoclip Placement for Bleeding Dieulafoy’s Lesion in Duodenum
Yu Jiang, Julong Hu, Ping Li, Wen Jiang, Wenyan Liang, Hongshan Wei
Gastroenterology Research and Practice.2018; 2018: 1. CrossRef - Recurrent gastrointestinal bleeding secondary to Dieulafoy's lesion successfully treated with endoscopic ultrasound-guided sclerosis
Irene García de la Filia, Nerea Hernanz, Enrique Vázquez Sequeiros, Eduardo Tavío Hernández
Gastroenterología y Hepatología (English Edition).2018; 41(5): 319. CrossRef - Case 1: Hematemesis in a 30-month-old Boy
Sudarshawn Damodharan, Istvan Danko
Pediatrics In Review.2018; 39(11): 560. CrossRef - Dieulafoy’s lesion of the duodenum: a comparative review of 37 cases
Faisal Inayat, Waseem Amjad, Qulsoom Hussain, Abu Hurairah
BMJ Case Reports.2018; : bcr-2017-223246. CrossRef - Postural Syncope and Constipation: An Unusual Presentation of a Duodenal Dieulafoy’s Lesion
Ahmed Dirweesh, Alvarez Chikezie, Muhammad Yasir Khan, Sana Zia, Muhammad Tahir
Case Reports in Gastrointestinal Medicine.2017; 2017: 1. CrossRef - Dieulafoy of cecum: A rare cause of a refractory gastrointestinal bleeding in an uncommon location
Hamzeh Saraireh, Muhannad Al Hanayneh, Habeeb Salameh, Sreeram Parupudi
Digestive and Liver Disease.2017; 49(9): 1062. CrossRef - Gastric cirsoid aneurysm: Uncommon cause of death from upper GI bleed
Tatiana Bihun, James Ribe
Human Pathology: Case Reports.2017; 10: 89. CrossRef - Massive upper gastrointestinal bleeding due to a Dieulafoys lesion inside a duodenal diverticulum
Lucía Relea Pérez, Marta Magaz Martínez, Fernando Pons Renedo
Revista Española de Enfermedades Digestivas.2017;[Epub] CrossRef - Dieulafoy’s lesion the uncommon cause of upper gastrointestinal bleeding
Ayman Alsebaey
Egyptian Liver Journal.2017; 7(3 and 4): 41. CrossRef - Dieulafoy’s lesion of the colon and rectum: a case series and literature review
Faisal Inayat, Waqas Ullah, Qulsoom Hussain, Hafez Mohammad Ammar Abdullah
BMJ Case Reports.2017; : bcr-2017-220431. CrossRef - Part 4: Clinical management of an undiagnosed systemic condition
LaurenE Stone, MarkW Fegley, Rodrigo Duarte-Chavez, Sudip Nanda
International Journal of Academic Medicine.2017; 3(1): 151. CrossRef - Lower Gastrointestinal Bleeding in Children
Benjamin Sahn, Samuel Bitton
Gastrointestinal Endoscopy Clinics of North America.2016; 26(1): 75. CrossRef - Dieulafoy lesion: the little known sleeping giant of gastrointestinal bleeds
Ramy Saleh, Alan Lucerna, James Espinosa, Victor Scali
The American Journal of Emergency Medicine.2016; 34(12): 2464.e3. CrossRef - Successful Endoscopic Treatment of an Actively Bleeding Jejunal Dieulafoy's Lesion
Taiki Aoyama, Akira Fukumoto, Shinichi Mukai, Hiroyuki Ueda, Shigeru Kimura, Shinji Nagata
Internal Medicine.2016; 55(13): 1739. CrossRef - Hypertension and Clinical Outcomes of Patients With Gastrointestinal Submucosal Vascular (Dieulafoy) Lesional Hemorrhage
Maen Kamal, Prasanna Santhanam, Yaser M. Rayyan
The Journal of Clinical Hypertension.2016; 18(7): 710. CrossRef - Endoscopic submucosal dissection for silent gastric Dieulafoy lesions mimicking gastrointestinal stromal tumors
Xue Chen, Hailong Cao, Sinan Wang, Dan Wang, Mengque Xu, Meiyu Piao, Bangmao Wang
Medicine.2016; 95(36): e4829. CrossRef - Distinctive aspects of peptic ulcer disease, Dieulafoy's lesion, and Mallory-Weiss syndrome in patients with advanced alcoholic liver disease or cirrhosis
Borko Nojkov
World Journal of Gastroenterology.2016; 22(1): 446. CrossRef - Culprit for recurrent acute gastrointestinal massive bleeding: “Small bowel Dieulafoy’s lesions” - a case report and literature review
Anjana Sathyamurthy, Jessica N Winn, Jamal A Ibdah, Veysel Tahan
World Journal of Gastrointestinal Pathophysiology.2016; 7(3): 296. CrossRef - Bleeding “Dieulafoy’s-like” lesion resembling the duodenal papilla: a case report
Mohammad Bilal, Anastasios Kapetanos, Haider Ali Khan, Shyam Thakkar
Journal of Medical Case Reports.2015;[Epub] CrossRef
- Dieulafoy's lesion: Is there still a place for surgery? About 2 cases
- 18,096 View
- 278 Download
- 51 Web of Science
- 55 Crossref

- Lymph Node Metastases in Esophageal Carcinoma: An Endoscopist's View
- Jin Woong Cho, Suck Chei Choi, Jae Young Jang, Sung Kwan Shin, Kee Don Choi, Jun Haeng Lee, Sang Gyun Kim, Jae Kyu Sung, Seong Woo Jeon, Il Ju Choi, Gwang Ha Kim, Sam Ryong Jee, Wan Sik Lee, Hwoon-Yong Jung, Korean ESD Study Group
- Clin Endosc 2014;47(6):523-529. Published online November 30, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.6.523
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub One of the most important prognostic factors in esophageal carcinoma is lymph node metastasis, and in particular, the number of affected lymph nodes, which influences long-term outcomes. The esophageal lymphatic system is connected longitudinally and transversally; thus, the pattern of lymph node metastases is very complex. Early esophageal cancer frequently exhibits skipped metastasis, and minimal surgery using sentinel node navigation cannot be performed. In Korea, most esophageal cancer cases are squamous cell carcinoma (SCC), although the incidence of adenocarcinoma has started to increase recently. Most previous reports have failed to differentiate between SCC and adenocarcinoma, despite the fact that the Union for International Cancer Control (7th edition) and American Joint Committee on Cancer staging systems both consider these separately because they differ in cause, biology, lymph node metastasis, and outcome. Endoscopic tumor resection is an effective and safe treatment for lesions with no associated lymph node metastasis. Esophageal mucosal cancer confined to the lamina propria is an absolute indication for endoscopic resection, and a lesion that has invaded the muscularis mucosae can be cured by local resection if invasion to the lymphatic system has not occurred.
-
Citations
Citations to this article as recorded by- The association between histological subtypes and lymph node metastasis and prognosis in early esophageal cancer: a population-based study
Jun-Peng Lin, Xiao-Feng Chen, Hang Zhou, Feng-Nian Zhuang, Hao He, Wei-Jie Chen, Feng Wang, Shuo-Yan Liu
European Journal of Cancer Prevention.2024; 33(2): 152. CrossRef - Application of the convolution neural network in determining the depth of invasion of gastrointestinal cancer: a systematic review and meta-analysis
Ruo Wu, Kaiwen Qin, Yuxin Fang, Yuyuan Xu, Haonan Zhang, Wenhua Li, Xiaobei Luo, Zelong Han, Side Liu, Qingyuan Li
Journal of Gastrointestinal Surgery.2024; 28(4): 538. CrossRef - Esophageal cancer
Daniel C. Eisner
JAAPA.2024; 37(4): 19. CrossRef - Gliomagenesis is orchestrated by the Oct3/4 regulatory network
Tatyana N. IGNATOVA, Hersh J. CHAITIN, Nickolay V. KUKEKOV, Oleg N. SUSLOV, Galina I. DULATOVA, Khalid A. HANAFY, Frank D. VRIONIS
Journal of Neurosurgical Sciences.2024;[Epub] CrossRef - Management of early oesophageal cancer: An overview
Gavin G Calpin, Matthew G Davey, Noel E Donlon
World Journal of Gastrointestinal Surgery.2024; 16(5): 1255. CrossRef - Surgical Approach to Esophagectomy Post CheckMate 577
Nikhil Panda, Lana Schumacher
Thoracic Surgery Clinics.2023; 33(2): 209. CrossRef - Mechanisms of esophageal cancer metastasis and treatment progress
Yusheng Wang, Wei Yang, Qianyun Wang, Yong Zhou
Frontiers in Immunology.2023;[Epub] CrossRef - Ten-year follow-up of endoscopic mucosal resection versus esophagectomy for esophageal intramucosal adenocarcinoma in the setting of Barrett’s esophagus: a Canadian experience
Alisha Fernandes, Chao Li, Daniel French, James Ellsmere
Surgical Endoscopy.2023; 37(11): 8735. CrossRef - Advanced Endoscopic Resection Techniques: Endoscopic Submucosal Dissection and Endoscopic Full-Thickness Resection
Phillip S. Ge, Hiroyuki Aihara
Digestive Diseases and Sciences.2022; 67(5): 1521. CrossRef - A Novel Ferroptosis-Related Gene Signature to Predict Prognosis of Esophageal Carcinoma
Jian Wang, Ziming Guo, Fei Sun, Tian Xu, Jianlin Wang, Jingping Yu, Dong-Hua Yang
Journal of Oncology.2022; 2022: 1. CrossRef - Recent advances in multidisciplinary therapy for adenocarcinoma of the esophagus and esophagogastric junction
Yi-Han Zheng, En-Hao Zhao
World Journal of Gastroenterology.2022; 28(31): 4299. CrossRef - The relationship between esophageal cancer mortality‐to‐incidence ratios of countries and ranking of world's health system
Ming‐Hui Chang, Shih‐Ming Huang, Wen‐Wei Sung, Tzu‐Wei Yang, Hsuan‐Yi Chen, Chang‐Cheng Su, Wei‐Liang Chen, Ming‐Chang Tsai, Chi‐Chih Wang
Advances in Digestive Medicine.2021; 8(4): 234. CrossRef - Staging esophageal cancer: low EUS accuracy in t2n0 patients
Germana de Nucci, Maria Chiara Petrone, Nicola Imperatore, Emanuele Asti, Gemma Rossi, Giampiero Manes, Maurizio Vecchi, Luca Pastorelli, Luigi Bonavina, Paolo Giorgio Arcidiacono
Endoscopy International Open.2021; 09(03): E313. CrossRef - Long non-coding RNA SPRY4-IT1 as a promising indicator for three field lymph-node dissection of thoracic esophageal carcinoma
Peng Qie, Qifan Yin, Xuejiao Xun, Yongbin Song, Shaohui Zhou, Huining Liu, Junpeng Feng, Ziqiang Tian
Journal of Cardiothoracic Surgery.2021;[Epub] CrossRef - A clinically interpretable convolutional neural network for the real-time prediction of early squamous cell cancer of the esophagus: comparing diagnostic performance with a panel of expert European and Asian endoscopists
Martin A. Everson, Luis Garcia-Peraza-Herrera, Hsiu-Po Wang, Ching-Tai Lee, Chen-Shuan Chung, Ping-Hsin Hsieh, Chien-Chuan Chen, Cheng-Hao Tseng, Ming-Hung Hsu, Tom Vercauteren, Sebastien Ourselin, Sergey Kashin, Raf Bisschops, Oliver Pech, Laurence Lovat
Gastrointestinal Endoscopy.2021; 94(2): 273. CrossRef - Pitfalls and Pearls in Esophageal Carcinoma
Sonia L. Betancourt-Cuellar, Diana P. Palacio, Marcelo F. Kuperman Benveniste, Yasmeen Mawlawi, Jeremy J. Erasmus
Seminars in Ultrasound, CT and MRI.2021; 42(6): 535. CrossRef - Effect of perioperative flurbiprofen axetil on long‐term survival of patients with esophageal carcinoma who underwent thoracoscopic esophagectomy: A retrospective study
Yanhu Xie, Di Wang, Chen Gao, Jicheng Hu, Min Zhang, Wei Gao, Shuhua Shu, Xiaoqing Chai
Journal of Surgical Oncology.2021; 124(4): 540. CrossRef - Long-term outcomes of an esophagus-preserving chemoradiotherapy strategy for patients with endoscopically unresectable stage I thoracic esophageal squamous cell carcinoma
Tatsuya Suwa, Yuichi Ishida, Yoshiharu Negoro, Fusako Kusumi, Yoshio Kadokawa, Rihito Aizawa, Toshifumi Nakajima, Yoshiaki Okamoto, Yoshishige Okuno, Kazunari Yamada, Masakazu Ogura, Masao Murakami, Takashi Mizowaki
Clinical and Translational Radiation Oncology.2021; 30: 88. CrossRef - Treating esophageal squamous cell carcinoma with ablation: the fear of what lies beneath
Elizabeth Anne Montgomery, Rehan Haidry
Gastrointestinal Endoscopy.2021; 94(4): 843. CrossRef - Lymph Node Involvement in Oesophageal Carcinoma: A Single-Centre Observational Study From Western India
Ajay K Boralkar , Abdul Rafe, Bhushan Bhalgat
Cureus.2021;[Epub] CrossRef - A nomogram for predicting lymph node metastasis in superficial esophageal squamous cell carcinoma
Weifeng Zhang, Han Chen, Guoxin Zhang, Guangfu Jin
The Journal of Biomedical Research.2021; 35(5): 361. CrossRef - Predicting lymph node metastases with endoscopic resection in cT2N0M0 oesophageal cancer: A systematic review and meta‐analysis
Ali Al‐Kaabi, Rachel S van der Post, Jonathan Huising, Camiel Rosman, Iris D Nagtegaal, Peter D Siersema
United European Gastroenterology Journal.2020; 8(1): 35. CrossRef - Intrapapillary capillary loop classification in magnification endoscopy: open dataset and baseline methodology
Luis C. García-Peraza-Herrera, Martin Everson, Laurence Lovat, Hsiu-Po Wang, Wen Lun Wang, Rehan Haidry, Danail Stoyanov, Sébastien Ourselin, Tom Vercauteren
International Journal of Computer Assisted Radiology and Surgery.2020; 15(4): 651. CrossRef - Clinical impact of FDG PET/CT in alimentary tract malignancies: an updated review
Esma A. Akin, Zain N. Qazi, Murat Osman, Robert K. Zeman
Abdominal Radiology.2020; 45(4): 1018. CrossRef - Comparison of general anesthesia and conscious sedation in procedure-related complications during esophageal endoscopic submucosal dissection
Seung Hyun Kim, Yong Seon Choi, Sang Kil Lee, Hanseul Oh, Seung Ho Choi
Surgical Endoscopy.2020; 34(8): 3560. CrossRef - Indications, contraindications and limitations of endoscopic therapy for Barrett’s esophagus and early esophageal adenocarcinoma
Carol Rouphael, Mythri Anil Kumar, Madhusudhan R. Sanaka, Prashanthi N. Thota
Therapeutic Advances in Gastroenterology.2020; 13: 175628482092420. CrossRef - Importance of investigating high-risk human papillomavirus in lymph node metastasis of esophageal adenocarcinoma
Preeti Sharma, Shweta Dutta Gautam, Shanmugarajah Rajendra
World Journal of Gastroenterology.2020; 26(21): 2729. CrossRef - Inaccurate pretreatment staging can impact survival in early stage esophageal adenocarcinoma
Anthony J. Scholer, Abhineet Uppal, Shu‐Ching Chang, Debopriya Ghosh, Mary Garland‐ Kledzik, Juan Santamaria‐Barria, Adam Khader, Ahmed Dehal, Trevan Fischer, Melanie Goldfarb
Journal of Surgical Oncology.2020; 122(5): 914. CrossRef - Development of a Novel Serum Exosomal MicroRNA Nomogram for the Preoperative Prediction of Lymph Node Metastasis in Esophageal Squamous Cell Carcinoma
Tong Liu, Lu-Tao Du, Yun-Shan Wang, Shan-Yu Gao, Juan Li, Pei-Long Li, Zhao-Wei Sun, Helen Binang, Chuan-Xin Wang
Frontiers in Oncology.2020;[Epub] CrossRef - A comprehensive methylation signature identifies lymph node metastasis in esophageal squamous cell carcinoma
Roshni Roy, Raju Kandimalla, Fuminori Sonohara, Masahiko Koike, Yasuhiro Kodera, Naoki Takahashi, Yasuhide Yamada, Ajay Goel
International Journal of Cancer.2019; 144(5): 1160. CrossRef - Artificial intelligence for the real‐time classification of intrapapillary capillary loop patterns in the endoscopic diagnosis of early oesophageal squamous cell carcinoma: A proof‐of‐concept study
M Everson, LCGP Herrera, W Li, I Muntion Luengo, O Ahmad, M Banks, C Magee, D Alzoubaidi, HM Hsu, D Graham, T Vercauteren, L Lovat, S Ourselin, S Kashin, Hsiu-Po Wang, Wen-Lun Wang, RJ Haidry
United European Gastroenterology Journal.2019; 7(2): 297. CrossRef - Early detection and therapeutics
Wladyslaw Januszewicz, Rebecca C. Fitzgerald
Molecular Oncology.2019; 13(3): 599. CrossRef - Endoscopic Submucosal Dissection for Esophageal Adenocarcinoma: A North American Perspective
Philippe Bouchard, Juan-Carlos Molina, Jonathan Cools-Lartigue, Jonathan Spicer, Carmen L. Mueller, Lorenzo E. Ferri
Journal of Gastrointestinal Surgery.2019; 23(6): 1087. CrossRef - Improved prognosis with induction chemotherapy in pathological complete responders after trimodality treatment for esophageal squamous cell carcinoma: Hypothesis generating for adjuvant treatment
Shao-Lun Lu, Feng-Ming Hsu, Chiao-Ling Tsai, Jang-Ming Lee, Pei-Ming Huang, Chih-Hung Hsu, Chia-Chi Lin, Yih-Leong Chang, Min-Shu Hsieh, Jason Chia-Hsien Cheng
European Journal of Surgical Oncology.2019; 45(8): 1498. CrossRef - Initial Evaluation of Computer-Assisted Radiologic Assessment for Renal Mass Edge Detection as an Indication of Tumor Roughness to Predict Renal Cancer Subtypes
Rahul Rajendran, Kevan Iffrig, Deepak K Pruthi, Allison Wheeler, Brian Neuman, Dharam Kaushik, Ahmed M Mansour, Karen Panetta, Sos Agaian, Michael A. Liss
Advances in Urology.2019; 2019: 1. CrossRef - Membrane Metalloendopeptidase (MME) Suppresses Metastasis of Esophageal Squamous Cell Carcinoma (ESCC) by Inhibiting FAK-RhoA Signaling Axis
Mengqing Li, Ling Wang, Yuting Zhan, Tingting Zeng, Xu Zhang, Xin-Yuan Guan, Yan Li
The American Journal of Pathology.2019; 189(7): 1462. CrossRef - Barrett oesophagus
Yonne Peters, Ali Al-Kaabi, Nicholas J. Shaheen, Amitabh Chak, Andrew Blum, Rhonda F. Souza, Massimiliano Di Pietro, Prasad G. Iyer, Oliver Pech, Rebecca C. Fitzgerald, Peter D. Siersema
Nature Reviews Disease Primers.2019;[Epub] CrossRef - Early Esophageal Cancer: A Gastroenterologist’s Disease
Joseph Spataro, Alvin M. Zfass, Mitchell Schubert, Tilak Shah
Digestive Diseases and Sciences.2019; 64(11): 3048. CrossRef - Endoscopic Techniques for Early Detection of Esophageal Cancer
Jae Myung Park
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2019; 19(3): 149. CrossRef - Endoscopic Treatment for Esophageal Cancer
Eun Jeong Gong, Do Hoon Kim
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2019; 19(3): 156. CrossRef - Endoskopische Therapieoptionen beim Adenokarzinom am ösophagogastralen Übergang
Seung-Hun Chon, Isabel Bartella, Martin Bürger
Der Onkologe.2019; 25(12): 1073. CrossRef - Treatment of early stage (T1) esophageal adenocarcinoma: Personalizing the best therapy choice
Lindsay Danielle Kumble, Elisabeth Silver, Aaron Oh, Julian A Abrams, Joshua R Sonett, Chin Hur
World Journal of Meta-Analysis.2019; 7(9): 406. CrossRef - Clinical diagnostic value of digestive endoscopic narrow-band imaging in early esophageal cancer
Zhenhua Su, Liang Wang, Sichen Wei, Xinliang Wei, Yu Kong, Weiwei Wang, Ruixue Guo, Xiaomeng Shi
Oncology Letters.2019;[Epub] CrossRef - Role of Perioperative Chemotherapy in Lymph Node-negative Esophageal Cancer After Resection
Yang Yang, Xia Zhou, Luoyong Tang, Xiaoling Xu, Xianghui Du, Guoqin Qiu
American Journal of Clinical Oncology.2019; 42(12): 924. CrossRef - Endoscopic Management of Early Adenocarcinoma and Squamous Cell Carcinoma of the Esophagus: Screening, Diagnosis, and Therapy
Massimiliano di Pietro, Marcia I. Canto, Rebecca C. Fitzgerald
Gastroenterology.2018; 154(2): 421. CrossRef - Elucidation of the Anatomical Mechanism of Nodal Skip Metastasis in Superficial Thoracic Esophageal Squamous Cell Carcinoma
Yuji Kumakura, Takehiko Yokobori, Tomonori Yoshida, Keigo Hara, Makoto Sakai, Makoto Sohda, Tatsuya Miyazaki, Hideaki Yokoo, Tadashi Handa, Tetsunari Oyama, Hiroshi Yorifuji, Hiroyuki Kuwano
Annals of Surgical Oncology.2018; 25(5): 1221. CrossRef - Endoscopic Treatment of Early-Stage Esophageal Cancer
Mariam Naveed, Nisa Kubiliun
Current Oncology Reports.2018;[Epub] CrossRef - Role of endoscopic therapy in early esophageal cancer
Sonika Malik, Gautam Sharma, Madhusudhan R Sanaka, Prashanthi N Thota
World Journal of Gastroenterology.2018; 24(35): 3965. CrossRef - Loss of PAR-3 protein expression is associated with invasion, lymph node metastasis, and poor survival in esophageal squamous cell carcinoma
Tomoko Kitaichi, Kohichiroh Yasui, Yasuyuki Gen, Osamu Dohi, Naoto Iwai, Akira Tomie, Nobuhisa Yamada, Kei Terasaki, Atsushi Umemura, Taichiro Nishikawa, Kanji Yamaguchi, Michihisa Moriguchi, Yoshio Sumida, Hironori Mitsuyoshi, Yuji Naito, Yoh Zen, Yoshit
Human Pathology.2017; 62: 134. CrossRef - Oesophageal adenocarcinoma has a higher risk of lymph node metastasis than squamous cell carcinoma: a propensity score-matched study
Han-Yu Deng, Zhi-Qiang Wang, Yun-Cang Wang, Gang Li, Jun Luo, Long-Qi Chen, Lun-Xu Liu, Qing-Hua Zhou, Yi-Dan Lin
European Journal of Cardio-Thoracic Surgery.2017; 52(5): 958. CrossRef - Prognostic significance of tumor length in patients receiving esophagectomy for esophageal cancer
Alexander C. Hollis, Lauren M. Quinn, James Hodson, Emily Evans, James Plowright, Ruksana Begum, Harriet Mitchell, Mike T. Hallissey, John L. Whiting, Ewen A. Griffiths
Journal of Surgical Oncology.2017; 116(8): 1114. CrossRef - New magnifying endoscopic classification for superficial esophageal squamous cell carcinoma
Su Jin Kim, Gwang Ha Kim, Moon Won Lee, Hye Kyung Jeon, Dong Hoon Baek, Bong Eun Lee, Geun Am Song
World Journal of Gastroenterology.2017; 23(24): 4416. CrossRef - Is endoscopic ultrasound examination necessary in the management of esophageal cancer?
Tomas DaVee, Jaffer A Ajani, Jeffrey H Lee
World Journal of Gastroenterology.2017; 23(5): 751. CrossRef - Endoscopic ultrasound staging for early esophageal cancer: Are we denying patients neoadjuvant chemo-radiation?
Carrie Luu, Marisa Amaral, Jason Klapman, Cynthia Harris, Khaldoun Almhanna, Sarah Hoffe, Jessica Frakes, Jose M Pimiento, Jacques P Fontaine
World Journal of Gastroenterology.2017; 23(46): 8193. CrossRef - Decreased expression of CD63 tetraspanin protein predicts elevated malignant potential in human esophageal cancer
Xiaojing Lai, Qing Gu, Xia Zhou, Wei Feng, Xiao Lin, Yan He, Jinming Cao, Pengfei Liu, Huojun Zhang, Xiao Zheng
Oncology Letters.2017; 13(6): 4245. CrossRef - Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis
Hariruk Yodying, Akihisa Matsuda, Masao Miyashita, Satoshi Matsumoto, Nobuyuki Sakurazawa, Marina Yamada, Eiji Uchida
Annals of Surgical Oncology.2016; 23(2): 646. CrossRef - Endoscopic Submucosal Dissection for Superficial Esophageal Neoplasm: A Growing Body of Evidence
Eun Jeong Gong, Hwoon-Yong Jung
Clinical Endoscopy.2016; 49(2): 101. CrossRef - FDG-PET/CT lymph node staging after neoadjuvant chemotherapy in patients with adenocarcinoma of the esophageal–gastric junction
Pavel Fencl, Otakar Belohlavek, Tomas Harustiak, Milada Zemanova
Abdominal Radiology.2016; 41(11): 2089. CrossRef - MiR-106b promotes migration and invasion through enhancing EMT via downregulation of Smad 7 in Kazakh’s esophageal squamous cell carcinoma
Fang Dai, Tao Liu, Shutao Zheng, Qing Liu, Chenchen Yang, Jian Zhou, Yumei Chen, Ilyar Sheyhidin, Xiaomei Lu
Tumor Biology.2016; 37(11): 14595. CrossRef - A Risk Prediction Model Based on Lymph-Node Metastasis in Poorly Differentiated–Type Intramucosal Gastric Cancer
Jeung Hui Pyo, Hyuk Lee, Byung-Hoon Min, Jun Haeng Lee, Min Gew Choi, Jun Ho Lee, Tae Sung Sohn, Jae Moon Bae, Kyoung-Mee Kim, Hyeon Seon Ahn, Sin-Ho Jung, Sung Kim, Jae J. Kim, Xin-Yuan Guan
PLOS ONE.2016; 11(5): e0156207. CrossRef - MicroRNA-92b represses invasion-metastasis cascade of esophageal squamous cell carcinoma
Gang Ma, Chao Jing, Lin Li, Furong Huang, Fang Ding, Baona Wang, Dongmei Lin, Aiping Luo, Zhihua Liu
Oncotarget.2016; 7(15): 20209. CrossRef - DNA polymerase iota (Pol ι) promotes invasion and metastasis of esophageal squamous cell carcinoma
Shitao Zou, Zeng-Fu Shang, Biao Liu, Shuyu Zhang, Jinchang Wu, Min Huang, Wei-Qun Ding, Jundong Zhou
Oncotarget.2016; 7(22): 32274. CrossRef - CTL- vs Treg lymphocyte-attracting chemokines, CCL4 and CCL20, are strong reciprocal predictive markers for survival of patients with oesophageal squamous cell carcinoma
J Y Liu, F Li, L P Wang, X F Chen, D Wang, L Cao, Y Ping, S Zhao, B Li, S H Thorne, B Zhang, P Kalinski, Y Zhang
British Journal of Cancer.2015; 113(5): 747. CrossRef - Metadherin is required for the proliferation, migration, and invasion of esophageal squamous cell carcinoma and its meta-analysis
Chenchen Yang, Shutao Zheng, Qing Liu, Tao Liu, Mang Lu, Fang Dai, Xiangpeng Gao, Ilyar Sheyhidin, Xiaomei Lu
Translational Research.2015; 166(6): 614. CrossRef - A Comparison of Postoperative Early Enteral Nutrition with Delayed Enteral Nutrition in Patients with Esophageal Cancer
Gongchao Wang, Hongbo Chen, Jun Liu, Yongchen Ma, Haiyong Jia
Nutrients.2015; 7(6): 4308. CrossRef
- The association between histological subtypes and lymph node metastasis and prognosis in early esophageal cancer: a population-based study
- 10,733 View
- 131 Download
- 69 Web of Science
- 65 Crossref

- Stricture Occurring after Endoscopic Submucosal Dissection for Esophageal and Gastric Tumors
- Gwang Ha Kim, Sam Ryong Jee, Jae Young Jang, Sung Kwan Shin, Kee Don Choi, Jun Haeng Lee, Sang Gyun Kim, Jae Kyu Sung, Suck Chei Choi, Seong Woo Jeon, Byung Ik Jang, Kyu Chan Huh, Dong Kyung Chang, Sung-Ae Jung, Bora Keum, Jin Woong Cho, Il Ju Choi, Hwoon-Yong Jung, Korean ESD Study Group
- Clin Endosc 2014;47(6):516-522. Published online November 30, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.6.516
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Endoscopic submucosal dissection (ESD) is a widely accepted treatment for early gastric and esophageal cancer. Compared to endoscopic mucosal resection, ESD has the advantage of enabling
en bloc removal of tumors regardless of their size. However, ESD can result in a large artificial ulcer, which may lead to a considerable deformity. Circumferential mucosal defects of more than three-fourths the esophageal circumference, long longitudinal mucosal defects (>30 mm), and lesions in the upper esophagus are significant risk factors for the development of post-ESD strictures of the esophagus. In the stomach, a circumferential mucosal defects more than three-fourths in extent and longitudinal mucosal defects >5 cm are risk factors of post-ESD stricture. If scheduled early, regular endoscopic balloon dilation is effective in controlling and preventing post-ESD stricture. Moreover, intralesional steroid injections or oral steroids can achieve remission of dysphagia or reduce the need for repeated endoscopic balloon dilation. However, further study is needed to improve the prevention of stricture formation.-
Citations
Citations to this article as recorded by- A novel twin-grasper assisted mucosal inverted closure technique for closing large artificial gastric mucosal defects
Qinbo Cai, Huanjie Chen, Haobin Hou, Wenqing Dong, Lele Zhang, Minxuan Shen, Shaoxiong Yi, Rongman Xie, Xun Hou, Wentong Lan, Yulong He, Dongjie Yang
Surgical Endoscopy.2024; 38(1): 460. CrossRef - The efficacy and safety of snare traction-assisted endoscopic submucosal dissection for circumferential superficial esophageal cancer
Nan Dai, Saif Ullah, Jingwen Zhang, Xiaoyu Wan, Shanshan Zhu, Ping Liu, Changqing Guo, Xinguang Cao
Surgical Endoscopy.2024; 38(6): 3329. CrossRef - Risk factors of refractory post-endoscopic submucosal dissection esophageal strictures
Enrique Pérez-Cuadrado Robles, Tom G. Moreels , Hubert Piessevaux , Ralph Yeung, Tarik Aouattah , Pierre H. Deprez
Revista Española de Enfermedades Digestivas.2021;[Epub] CrossRef - Early Esophageal Cancer
Mike T. Wei, Shai Friedland
Gastroenterology Clinics of North America.2021; 50(4): 791. CrossRef - Comparison of general anesthesia and conscious sedation in procedure-related complications during esophageal endoscopic submucosal dissection
Seung Hyun Kim, Yong Seon Choi, Sang Kil Lee, Hanseul Oh, Seung Ho Choi
Surgical Endoscopy.2020; 34(8): 3560. CrossRef - Cyclodextrin Polymer Preserves Sirolimus Activity and Local Persistence for Antifibrotic Delivery over the Time Course of Wound Healing
Nathan A. Rohner, Steve J. Schomisch, Jeffrey M. Marks, Horst A. von Recum
Molecular Pharmaceutics.2019; 16(4): 1766. CrossRef - Endoscopic Treatment for Esophageal Cancer
Yang Won Min
The Korean Journal of Gastroenterology.2018; 71(3): 116. CrossRef - Endoscopic submucosal dissection under general anesthesia for superficial esophageal squamous cell carcinoma is associated with better clinical outcomes
Byeong Geun Song, Yang Won Min, Ra Ri Cha, Hyuk Lee, Byung-Hoon Min, Jun Haeng Lee, Poong-Lyul Rhee, Jae J. Kim
BMC Gastroenterology.2018;[Epub] CrossRef - Endoscopic Submucosal Dissection for Superficial Esophageal Neoplasm: A Growing Body of Evidence
Eun Jeong Gong, Hwoon-Yong Jung
Clinical Endoscopy.2016; 49(2): 101. CrossRef - Short-Term Outcomes of Endoscopic Submucosal Dissection in Patients with Early Gastric Cancer: A Prospective Multicenter Cohort Study
ll Ju Choi, Na Rae Lee, Sang Gyun Kim, Wan Sik Lee, Seun Ja Park, Jae J. Kim, Jun Haeng Lee, Jin-Won Kwon, Seung-Hee Park, Ji Hye You, Ji Hyun Kim, Chul-Hyun Lim, Joo Young Cho, Gwang Ha Kim, Yong Chan Lee, Hwoon-Yong Jung, Ji Young Kim, Hoon Jai Chun, Sa
Gut and Liver.2016; 10(5): 739. CrossRef - Complications of endoscopic resection techniques for upper GI tract lesions
D. Libânio, P. Pimentel-Nunes, M. Dinis-Ribeiro
Best Practice & Research Clinical Gastroenterology.2016; 30(5): 735. CrossRef - An Intractable Caustic Esophageal Stricture Successfully Managed with Sequential Treatment Comprising Incision with an Insulated-Tip Knife, Balloon Dilation, and an Oral Steroid
Woong Ki Lee, Byung Sun Kim, Min A Yang, So Hee Yun, Young Jae Lee, Ji Woong Kim, Jin Woong Cho
Clinical Endoscopy.2016; 49(6): 560. CrossRef - Transplantation of tissue‐engineered cell sheets for stricture prevention after endoscopic submucosal dissection of the oesophagus
Eduard Jonas, Sebastian Sjöqvist, Peter Elbe, Nobuo Kanai, Jenny Enger, Stephan L Haas, Ammar Mohkles‐Barakat, Teruo Okano, Ryo Takagi, Takeshi Ohki, Masakazu Yamamoto, Makoto Kondo, Katrin Markland, Mei Ling Lim, Masayuki Yamato, Magnus Nilsson, Johan Pe
United European Gastroenterology Journal.2016; 4(6): 741. CrossRef - Systematic review: the prevention of oesophageal stricture after endoscopic resection
M. Barret, B. Beye, S. Leblanc, F. Beuvon, S. Chaussade, F. Batteux, F. Prat
Alimentary Pharmacology & Therapeutics.2015; 42(1): 20. CrossRef
- A novel twin-grasper assisted mucosal inverted closure technique for closing large artificial gastric mucosal defects
- 8,427 View
- 122 Download
- 17 Web of Science
- 14 Crossref

- Acute Ectopic Pancreatitis Occurring after Endoscopic Biopsy in a Gastric Ectopic Pancreas
- Seong Jun Lee, Gwang Ha Kim, Do Youn Park, Sang A Choi, Sang Hee Lee, Yu Yi Choi, Moo Song Jeon, Geun Am Song
- Clin Endosc 2014;47(5):455-459. Published online September 30, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.5.455
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Ectopic pancreas is a congenital anomaly and the most common type of ectopic tissue in the gastrointestinal tract. Most patients with an ectopic pancreas are asymptomatic and rarely have complications. Ectopic pancreatitis after an endoscopic biopsy has not been reported. We report a patient who developed acute ectopic pancreatitis in the stomach after an endoscopic biopsy. A 71-year-old male patient presented with a subepithelial tumor (SET) in the stomach and had no symptoms. Endoscopic ultrasonography demonstrated a 30-mm hypoechoic mural mass, lobulated margins, and anechoic duct-like lesions. To obtain proper tissue specimen, endoscopic biopsy was performed through the opening on the surface of the mass. The pathologic results confirmed an ectopic pancreas. One day after the endoscopic biopsy, he developed persistent epigastric pain. His serum amylase and lipase elevated. Computed tomography of the abdomen showed swelling of the SET and diffuse edema of the gastric wall. His condition was diagnosed as acute ectopic pancreatitis occurring after endoscopic biopsy.
-
Citations
Citations to this article as recorded by- Gastric ectopic pancreas in magnetic resonance imaging: A review of 2 cases
Miguel Braga, António P. Matos, Pedro Pinto Marques, Miguel Ramalho
Radiology Case Reports.2023; 18(3): 1181. CrossRef - Clinicopathological features and management strategy for superficial nonampullary duodenal tumors: a multi-center retrospective study
Eun Young Kim, Dong Jin Kim, Han Hong Lee, Jun Hyun Lee, Jeong Goo Kim, Kyo Young Song, Jin Jo Kim, Hyung Min Chin, Wook Kim
Annals of Surgical Treatment and Research.2022; 102(5): 263. CrossRef - Pancreatitis in the developmentally anomalous pancreas
Cecil G. Wood, Camila Lopes Vendrami, Elizabeth Craig, Pardeep K. Mittal, Frank H. Miller
Abdominal Radiology.2020; 45(5): 1316. CrossRef - Minimally Invasive Management of Ectopic Pancreas
Gerardo A. Vitiello, Michael J. Cavnar, Cristina Hajdu, Inessa Khaykis, Elliot Newman, Marcovalerio Melis, H. Leon Pachter, Steven M. Cohen
Journal of Laparoendoscopic & Advanced Surgical Techniques.2017; 27(3): 277. CrossRef - Clinical picture: multiple sites of ectopic pancreatic tissue
J Straatman, R J Meester, N C T v. Grieken, M J A M Jacobs, P d. Graaf, G Kazemier, M A Cuesta
SpringerPlus.2015;[Epub] CrossRef
- Gastric ectopic pancreas in magnetic resonance imaging: A review of 2 cases
- 8,257 View
- 84 Download
- 4 Web of Science
- 5 Crossref

- Plastic and Biodegradable Stents for Complex and Refractory Benign Esophageal Strictures
- Young Hee Ham, Gwang Ha Kim
- Clin Endosc 2014;47(4):295-300. Published online July 28, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.4.295
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Endoscopic stent placement is a well-accepted and effective alternative treatment modality for complex and refractory esophageal strictures. Among the currently available types of stents, the partially covered self-expanding metal stent (SEMS) has a firm anchoring effect, preventing stent migration and ensuring effective covering of a narrowed segment. However, hyperplastic tissue reaction driven by the uncovered mesh may prevent easy and safe stent removal. As an alternative, a fully covered SEMS decreases the recurrence of dysphagia caused by hyperplastic tissue ingrowth; however, it has a high migration rate. Likewise, although a self-expanding plastic stent (SEPS) reduces reactive hyperplasia, the long-term outcome is disappointing because of the high rate of stent migration. A biodegradable stent has the main benefit of not requiring stent removal in comparison with SEMS and SEPS. However, it still has a somewhat high rate of hyperplastic reaction, and the long-term outcome does not satisfy expectations. Up to now, the question of which type of stent should be recommended for the effective treatment of complex and refractory benign strictures has no clear answer. Therefore, the selection of stent type for endoscopic treatment should be individualized, taking into consideration the endoscopist's experience as well as patient and stricture characteristics.
-
Citations
Citations to this article as recorded by- Lumen-apposing metal stents for the treatment of benign gastrointestinal tract strictures: a single-center experience and proposed treatment algorithm
Tala Mahmoud, Azizullah Beran, Fateh Bazerbachi, Reem Matar, Veeravich Jaruvongvanich, Farah Abdul Razzak, Donna Maria Abboud, Eric J. Vargas, John A. Martin, Todd A. Kellogg, Omar M. Ghanem, Bret T. Petersen, Michael J. Levy, Ryan J. Law, Vinay Chandrase
Surgical Endoscopy.2023; 37(3): 2133. CrossRef - Caustic esophageal stenosis: A case report of endoscopic dilatation with nasogastric tubes
Esau Ogei, Jackson Kakooza, Catherine R. Lewis
Clinical Case Reports.2023;[Epub] CrossRef - Comprehensive review of materials, applications, and future innovations in biodegradable esophageal stents
Yaochen Yang, Yuanyuan Yang, Zhipeng Hou, Tingting Wang, Peng Wu, Lufan Shen, Peng Li, Kai Zhang, Liqun Yang, Siyu Sun
Frontiers in Bioengineering and Biotechnology.2023;[Epub] CrossRef - Palliative Endoskopie
Benno Arnstadt, Hans-Dieter Allescher
Der Chirurg.2022; 93(3): 310. CrossRef - Safety and efficacy of esophageal stents for esophageal anastomotic strictures: A 10-year single-center experience
Sundus Bilal, Saad Muhammad Saeed, Zeeshan Siddique, Muhammad Saqib, Shahana Shahid, Muhammed Aasim Yusuf
International Journal of Gastrointestinal Intervention.2022; 11(2): 72. CrossRef - Benign Esophageal Stricture
Hyun Lim
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2022; 22(3): 197. CrossRef - Mechanical properties and degradation process of biliary self‐expandable biodegradable stents
Chang‐Il Kwon, Jun Sik Son, Kyu Seok Kim, Jong Pil Moon, Sehwan Park, Jinkyung Jeon, Gwangil Kim, Sung Hoon Choi, Kwang Hyun Ko, Seok Jeong, Don Haeng Lee
Digestive Endoscopy.2021; 33(7): 1158. CrossRef - Managing esophageal strictures following endoscopic resection of superficial neoplastic lesions
Alberto Álvarez Delgado, Maria Luis Pérez García
Revista Española de Enfermedades Digestivas.2021;[Epub] CrossRef - Biodegradable Stent/Tube for Pancreatic and Biliary Disease
Chang-Il Kwon
The Korean Journal of Pancreas and Biliary Tract.2020; 25(1): 18. CrossRef - An Overview of the Design, Development and Applications of Biodegradable Stents
Keerthana Nakka, Sri D. Nagarajan, Balamayilsamy Sundaravadivel, Subramanian Shankaravel, Christopher Vimalson
Drug Delivery Letters.2020; 10(1): 2. CrossRef - Alternative uses of lumen apposing metal stents
Prabin Sharma, Thomas R McCarty, Ankit Chhoda, Antonio Costantino, Caroline Loeser, Thiruvengadam Muniraj, Marvin Ryou, Christopher C Thompson
World Journal of Gastroenterology.2020; 26(21): 2715. CrossRef - Corrosive injury of the upper gastrointestinal tract: the evolving role of a radiologist
Ayushi Agarwal, Deep Narayan Srivastava, Kumble Seetharama Madhusudhan
The British Journal of Radiology.2020;[Epub] CrossRef - Improving stent efficiency by understanding stent-related adverse events
Daniel B. Maselli, Andrew C. Storm, Reem Matar, Barham K. Abu Dayyeh
Techniques and Innovations in Gastrointestinal Endoscopy.2020; 22(4): 232. CrossRef - Management of tracheo-oesophageal fistula in adults
Hyun S. Kim, Danai Khemasuwan, Javier Diaz-Mendoza, Atul C. Mehta
European Respiratory Review.2020; 29(158): 200094. CrossRef - 3D-printed flexible polymer stents for potential applications in inoperable esophageal malignancies
Maohua Lin, Negar Firoozi, Chi-Tay Tsai, Michael B. Wallace, Yunqing Kang
Acta Biomaterialia.2019; 83: 119. CrossRef - A Review of Self-Expanding Esophageal Stents for the Palliation Therapy of Inoperable Esophageal Malignancies
Yunqing Kang
BioMed Research International.2019; 2019: 1. CrossRef - Clinical outcomes of lumen-apposing metal stent in the management of benign gastrointestinal strictures: a systematic review and meta-analysis
Shali Tan, Chunyu Zhong, Shu Huang, Xujuan Luo, Jin Xu, Xiangsheng Fu, Yan Peng, Xiaowei Tang
Scandinavian Journal of Gastroenterology.2019; 54(7): 811. CrossRef - Novel Uses of Lumen-Apposing Metal Stents
Monica Saumoy, Clark Yarber, Michel Kahaleh
Gastrointestinal Endoscopy Clinics of North America.2018; 28(2): 197. CrossRef - Biodegradable Stents in Resistant Peptic Oesophageal Stricture: Is It the Right Way to Go?
Tom Richardson, Gerlin Naidoo, Namal Rupasinghe, Howard Smart, Sayantan Bhattacharya
Clinical Medicine Insights: Gastroenterology.2018; 11: 117955221881949. CrossRef - Current trends in the diagnosis and treatment of gastroesophageal reflux disease
Radek Kroupa, Štefan Konečný, Jiří Dolina
Vnitřní lékařství.2018; 64(6): 588. CrossRef - Technical feasibility and tissue reaction after silicone-covered biodegradable magnesium stent insertion in the oesophagus: a primary study in vitro and in vivo
Yue-Qi Zhu, Laura Edmonds, Li-Ming Wei, Rei-La Zheng, Ruo-Yu Cheng, Wen-Guo Cui, Ying-Sheng Cheng
European Radiology.2017; 27(6): 2546. CrossRef - Use of a lumen-apposing metal stent to treat GI strictures (with videos)
Shayan Irani, Sujai Jalaj, Andrew Ross, Michael Larsen, Ian S. Grimm, Todd H. Baron
Gastrointestinal Endoscopy.2017; 85(6): 1285. CrossRef - Use of self-expandable plastic stents (SEPS) in management of refractory benign esophageal strictures: a single center experience
Mohamed Abdel Fattah Selimah, Moustafa Ramadan Abo Elsoud
Esophagus.2017; 14(2): 159. CrossRef - Endoscopic treatment of benign esophageal strictures: a literature review
Laurent Poincloux, Olivier Rouquette, Armand Abergel
Expert Review of Gastroenterology & Hepatology.2017; 11(1): 53. CrossRef - Silicone-covered biodegradable magnesium-stent insertion in the esophagus: a comparison with plastic stents
Yue-Qi Zhu, Kai Yang, Laura Edmonds, Li-Ming Wei, Reila Zheng, Ruo-Yu Cheng, Wen-Guo Cui, Ying-Sheng Cheng
Therapeutic Advances in Gastroenterology.2017; 10(1): 11. CrossRef - Management of esophageal caustic injury
Mark Anthony A De Lusong, Aeden Bernice G Timbol, Danny Joseph S Tuazon
World Journal of Gastrointestinal Pharmacology and Therapeutics.2017; 8(2): 90. CrossRef - Stent-in-stent technique for removal of embedded partially covered self-expanding metal stents
Tomas DaVee, Shayan Irani, Cadman L. Leggett, Manuel Berzosa Corella, Karina V. Grooteman, Louis-Michel Wong Kee Song, Michael B. Wallace, Richard A. Kozarek, Todd H. Baron
Surgical Endoscopy.2016; 30(6): 2332. CrossRef - Caustic Esophageal Stenosis: A Case Report of Endoscopic Dilation With a Dynamic Stent
Marlene Abreu, Isabel Nunes, Susana Corujeira, Marta Tavares, Eunice Trindade, Jorge Amil Dias
GE Portuguese Journal of Gastroenterology.2016; 23(4): 218. CrossRef - A Comparison of a Fully Covered and an Uncovered Segmented Biodegradable Esophageal Stent in a Porcine Model: Preclinical Evaluation of Degradation, Complications, and Tissue Reactions
Yadong Feng, Chunhua Jiao, Yang Cao, Ye Zhao, Yanfang Chen, Lin Fang, Ruihua Shi
Gastroenterology Research and Practice.2016; 2016: 1. CrossRef - Therapy of caustic ingestion
Mitchell D. Shub
Current Opinion in Pediatrics.2015; 27(5): 609. CrossRef - Refractory Esophageal Strictures: What To Do When Dilation Fails
Petra G. A. van Boeckel, Peter D. Siersema
Current Treatment Options in Gastroenterology.2015; 13(1): 47. CrossRef - Foregut caustic injuries: results of the world society of emergency surgery consensus conference
Luigi Bonavina, Mircea Chirica, Ognjan Skrobic, Yoram Kluger, Nelson A. Andreollo, Sandro Contini, Aleksander Simic, Luca Ansaloni, Fausto Catena, Gustavo P. Fraga, Carlo Locatelli, Osvaldo Chiara, Jeffry Kashuk, Federico Coccolini, Yuri Macchitella, Mass
World Journal of Emergency Surgery.2015;[Epub] CrossRef - Caustic injury of the oesophagus
Alastair J. W. Millar, Sharon G. Cox
Pediatric Surgery International.2015; 31(2): 111. CrossRef - Efficacy and histopathological esophageal wall damage of biodegradable esophageal stents for treatment of severe refractory esophageal anastomotic stricture in a child with long gap esophageal atresia
Yuichi Okata, Chieko Hisamatsu, Yuko Bitoh, Akiko Yokoi, Eiji Nishijima, Kosaku Maeda, Makiko Yoshida, Tsukasa Ishida, Takeshi Azuma, Hiromu Kutsumi
Clinical Journal of Gastroenterology.2014; 7(6): 496. CrossRef
- Lumen-apposing metal stents for the treatment of benign gastrointestinal tract strictures: a single-center experience and proposed treatment algorithm
- 9,867 View
- 143 Download
- 38 Web of Science
- 34 Crossref

- A Case of Squamous Metaplasia of the Stomach
- Moo Song Jeon, Gwang Ha Kim, Do Youn Park, Jae Hoon Jeong, Dong Hwahn Kahng, Hye Yoon Jang, Jong Hyun Choi, Eun Kyoung Park
- Clin Endosc 2013;46(4):407-409. Published online July 31, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.4.407
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Intestinal metaplasia of the stomach is a common metaplastic lesion associated with chronic gastritis and mucosal atrophy. However, squamous metaplasia is a comparatively rare condition. On endoscopy, squamous metaplasia is usually observed as a whitish mucosal lesion in the lesser curvature of the cardiac region of the stomach. When Lugol's iodine solution is applied, the lesion stains brown in the same way as normal esophageal mucosa. We report a case of 79-year-old man with a whitish flat lesion in the lesser curvature of the cardiac region on surveillance endoscopy after endoscopic treatment of gastric adenoma. The endoscopic biopsy showed stratified squamous epithelial mucosa.
-
Citations
Citations to this article as recorded by- A rare cause of dyspeptic symptoms and anaemia in a young man
Hareem Rehman, Shahab Abid, Naila Kayani, Ali Haroon
Gut.2022; 71(3): 478. CrossRef - Squamous Metaplasia of the Stomach Associated with Lymphoma Infiltration
Masaya Iwamuro, Nobuharu Fujii, Takehiro Tanaka, Hiromitsu Kanzaki, Seiji Kawano, Yoshiro Kawahara, Hiroyuki Okada
Internal Medicine.2021; 60(14): 2229. CrossRef - Squamous Lesions of the Stomach
Badr AbdullGaffar, Hoda Quraishi
International Journal of Surgical Pathology.2020; 28(6): 647. CrossRef - An Unexpected Mucosal Metaplasia at the Gastric Cardia in Longstanding Pernicious Anemia
Mohammad H Derakhshan, Andrew Crumley, Matthew Forshaw, David R Mitchell, James J Going
American Journal of Gastroenterology.2015; 110(10): 1505. CrossRef - Gastric Squamous Papilloma in a 52-Year-Old Female Patient
Hyung Ha Jang, Hyung Wook Kim, Su Jin Kim, Choel Woong Choi, Su Bum Park, Byeong Jun Song, Dong Hoon Shin, Dae Hwan Kang
Clinical Endoscopy.2014; 47(6): 571. CrossRef
- A rare cause of dyspeptic symptoms and anaemia in a young man
- 8,166 View
- 84 Download
- 5 Crossref


 KSGE
KSGE


 First
First Prev
Prev



