Search
- Page Path
- HOME > Search
Original Articles
- Efficacy of hemostasis by gastroduodenal covered metal stent placement for hemorrhagic duodenal stenosis due to pancreatobiliary cancer invasion: a retrospective study
- Yasunari Sakamoto, Taku Sakamoto, Akihiro Ohba, Mitsuhito Sasaki, Shunsuke Kondo, Chigusa Morizane, Hideki Ueno, Yutaka Saito, Yasuaki Arai, Takuji Okusaka
- Received June 18, 2023 Accepted January 15, 2024 Published online June 14, 2024
- DOI: https://doi.org/10.5946/ce.2023.155 [Epub ahead of print]

-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: Advanced pancreatic and biliary tract cancers can invade the duodenum and cause duodenal hemorrhagic stenosis. This study aimed to evaluate the efficacy of covered self-expandable metal stents in the treatment of cancer-related duodenal hemorrhage with stenosis.
Methods
Between January 2014 and December 2016, metal stents were placed in 51 patients with duodenal stenosis. Among these patients, a self-expandable covered metal stent was endoscopically placed in 10 patients with hemorrhagic duodenal stenosis caused by pancreatobiliary cancer progression. We retrospectively analyzed the therapeutic efficacy of the stents by evaluating the technical and clinical success rates based on successful stent placement, degree of oral intake, hemostasis, stent patency, and overall survival.
Results
The technical and clinical success rates were 100%. All 10 patients achieved a Gastric Outlet Obstruction Scoring System score of three within two weeks after the procedure and had no recurrence of melena. The median stent patency duration and overall survival after stent placement were 52 days (range, 20–220 days) and 66.5 days (range, 31–220 days), respectively.
Conclusions
Endoscopic placement of a covered metal stent for hemorrhagic duodenal stenosis associated with pancreatic or biliary tract cancer resulted in duodenal hemostasis, recanalization, and improved quality of life.
- 427 View
- 13 Download

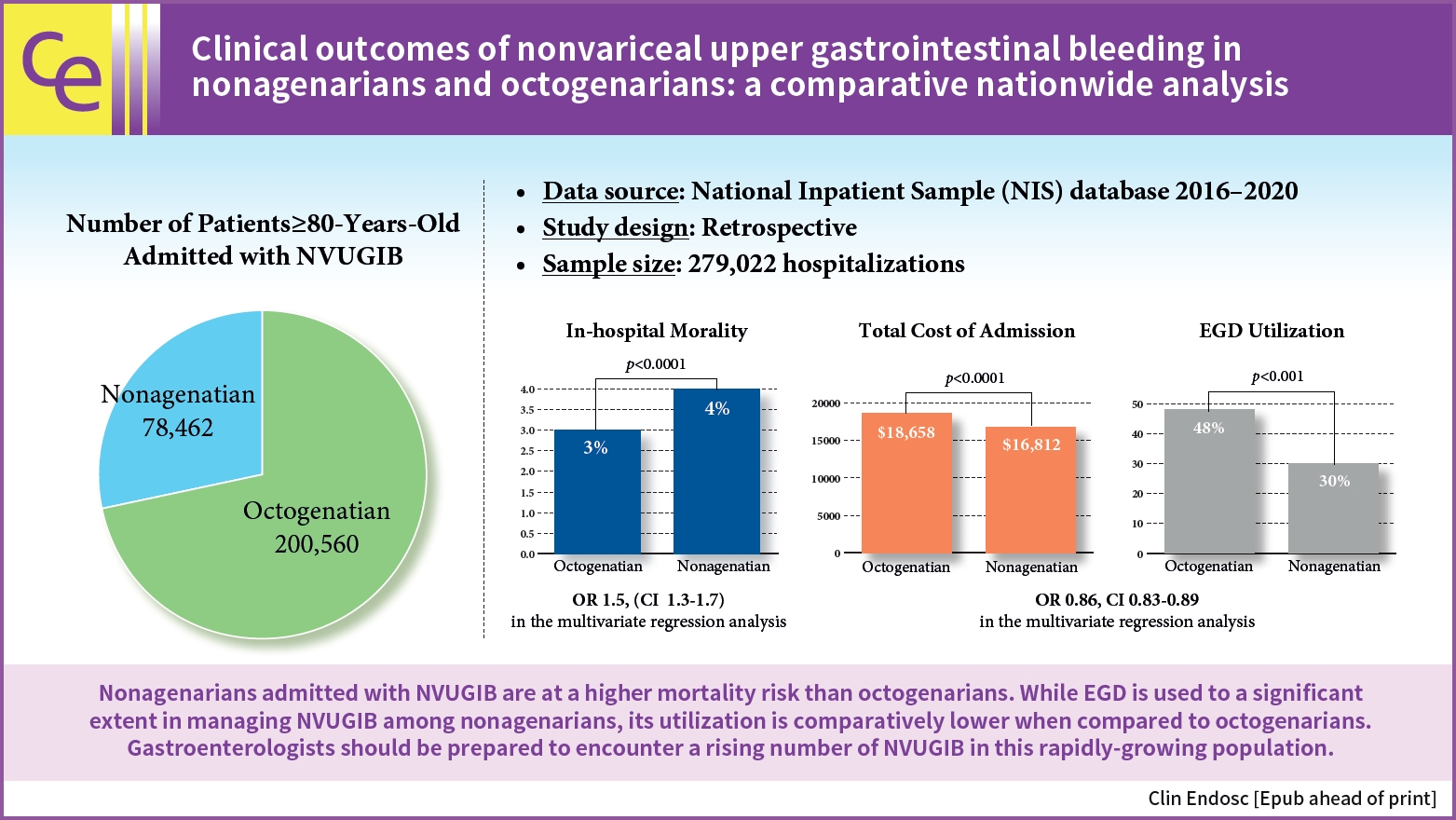
- Clinical outcomes of nonvariceal upper gastrointestinal bleeding in nonagenarians and octogenarians: a comparative nationwide analysis
- Khaled Elfert, James Love, Esraa Elromisy, Fouad Jaber, Suresh Nayudu, Sammy Ho, Michel Kahaleh
- Clin Endosc 2024;57(3):342-349. Published online February 7, 2024
- DOI: https://doi.org/10.5946/ce.2023.130
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub 
- Background
/Aims: Nonagenarians will purportedly account for 10% of the United States population by 2050. However, no studies have assessed the outcomes of nonvariceal upper gastrointestinal bleeding (NVUGIB) in this age group.
Methods
The National Inpatient Sample database between 2016 and 2020 was used to compare the clinical outcomes of NVUGIB in nonagenarians and octogenarians and evaluate predictors of mortality and the use of esophagogastroduodenoscopy (EGD).
Results
Nonagenarians had higher in-hospital mortality than that of octogenarians (4% vs. 3%, p<0.001). EGD utilization (30% vs. 48%, p<0.001) and blood transfusion (27% vs. 40%, p<0.001) was significantly lower in nonagenarians. Multivariate logistic regression analysis revealed that nonagenarians with NVUGIB had higher odds of mortality (odds ratio [OR], 1.5; 95% confidence interval [CI], 1.3–1.7) and lower odds of EGD utilization (OR, 0.86; 95% CI, 0.83–0.89) than those of octogenarians.
Conclusions
Nonagenarians admitted with NVUGIB have a higher mortality risk than that of octogenarians. EGD is used significantly in managing NVUGIB among nonagenarians; however, its utilization is comparatively lower than in octogenarians. More studies are needed to assess predictors of poor outcomes and the indications of EGD in this growing population.
- 2,239 View
- 27 Download

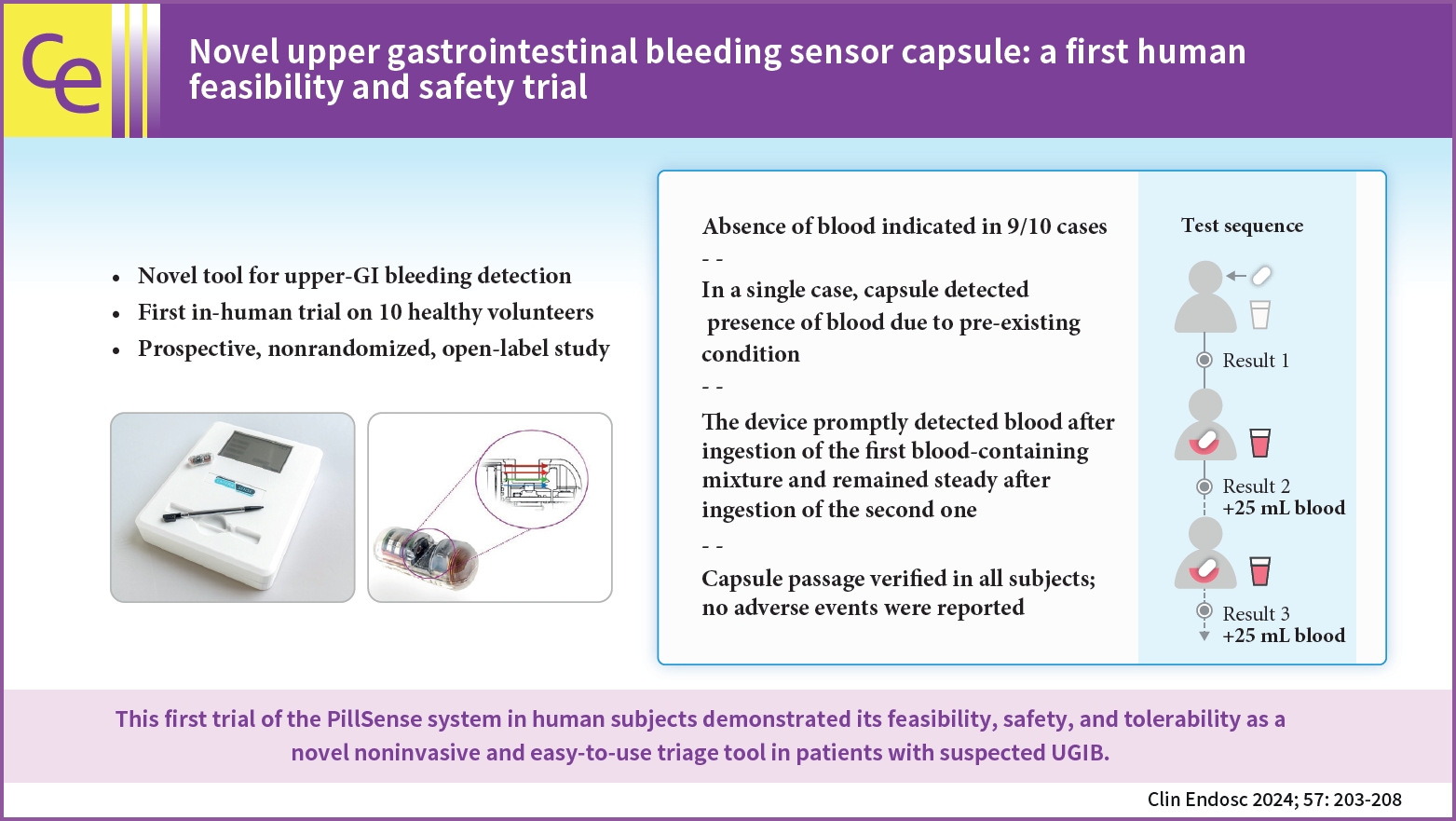
- Novel upper gastrointestinal bleeding sensor capsule: a first human feasibility and safety trial
- Lukas Bajer, Marvin Ryou, Christopher C. Thompson, Pavel Drastich
- Clin Endosc 2024;57(2):203-208. Published online January 17, 2024
- DOI: https://doi.org/10.5946/ce.2023.111
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: Upper gastrointestinal bleeding (UGIB) is the most common GI condition requiring hospitalization, and can be diagnosed by direct visualization. The present study aimed to evaluate the safety and feasibility of using the PillSense system (EnteraSense Ltd.), a novel diagnostic tool designed for the rapid in vivo detection of UGIB, in human volunteers.
Methods
In the present study, 10 volunteers swallowed a PillSense capsule, followed by 2 servings of an autologous blood preparation. Participants were monitored for capsule passage, overall tolerability of the procedure, and adverse events.
Results
The procedure was completed per the protocol established in the present study in 9/10 cases. In 9 of the subjects, after capsule ingestion, the device indicated the absence of blood with sensor output values of 1. After the ingestion of the first blood mixture, the sensor outputs of all devices increased from 2.8 to 4, indicating that each camera detected blood. The sensor output remained within that range after the ingestion of the second mixture; however, in one case, the baseline capsule signal was positive, because of a preexisting condition. The passage of the capsule was verified in all patients, and no adverse events were reported.
Conclusions
The first trial of the PillSense system in human subjects demonstrated the feasibility, safety, and tolerability of utilizing this product as a novel, noninvasive, and easy-to-use triage tool for the diagnosis of patients suspected of having UGIB. -
Citations
Citations to this article as recorded by- Could a bleeding-sensor device be established as a new paradigm for detecting upper gastrointestinal bleeding before performing endoscopy?
Sun Gyo Lim
Clinical Endoscopy.2024; 57(2): 191. CrossRef
- Could a bleeding-sensor device be established as a new paradigm for detecting upper gastrointestinal bleeding before performing endoscopy?
- 2,195 View
- 155 Download
- 1 Crossref

Reviews
- Endoscopic management of postoperative bleeding
- Sung Hyeok Ryou, Ki Bae Bang
- Clin Endosc 2023;56(6):706-715. Published online November 2, 2023
- DOI: https://doi.org/10.5946/ce.2023.028
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Postoperative gastrointestinal bleeding is a rare but serious complication that can lead to prolonged hospitalization and significant morbidity and mortality. It can be managed by reoperation, endoscopy, or radiological intervention. Although reoperation carries risks, particularly in critically ill postoperative patients, minimally invasive interventions, such as endoscopy or radiological intervention, confer advantages. Endoscopy allows localization of the bleeding focus and hemostatic management at the same time. Although there have been concerns regarding the potential risk of creating an anastomotic disruption or perforation during early postoperative endoscopy, endoscopic management has become more popular over time. However, there is currently no consensus on the best endoscopic management for postoperative gastrointestinal bleeding because most practices are based on retrospective case series. Furthermore, there is a wide range of individual complexities in anatomical and clinical settings after surgery. This review focused on the safety and effectiveness of endoscopic management in various surgical settings.
- 2,437 View
- 141 Download

- Management of complications related to colorectal endoscopic submucosal dissection
- Tae-Geun Gweon, Dong-Hoon Yang
- Clin Endosc 2023;56(4):423-432. Published online July 27, 2023
- DOI: https://doi.org/10.5946/ce.2023.104

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Compared to endoscopic mucosal resection (EMR), colonoscopic endoscopic submucosal dissection (C-ESD) has the advantages of higher en bloc resection rates and lower recurrence rates of colorectal neoplasms. Therefore, C-ESD is considered an effective treatment method for laterally spread tumors and early colorectal cancer. However, C-ESD is technically more difficult and requires a longer procedure time than EMR. In addition to therapeutic efficacy and procedural difficulty, safety concerns should always be considered when performing C-ESD in clinical practice. Bleeding and perforation are the main adverse events associated with C-ESD and can occur during C-ESD or after the completion of the procedure. Most bleeding associated with C-ESD can be managed endoscopically, even if it occurs during or after the procedure. More recently, most perforations identified during C-ESD can also be managed endoscopically, unless the mural defect is too large to be sutured with endoscopic devices or the patient is hemodynamically unstable. Delayed perforations are quite rare, but they require surgical treatment more frequently than endoscopically identified intraprocedural perforations or radiologically identified immediate postprocedural perforations. Post-ESD coagulation syndrome is a relatively underestimated adverse event, which can mimic localized peritonitis from perforation. Here, we classify and characterize the complications associated with C-ESD and recommend management options for them.
-
Citations
Citations to this article as recorded by- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
Seung Joo Kang, Chung Hyun Tae, Chang Seok Bang, Cheol Min Shin, Young-Hoon Jeong, Miyoung Choi, Joo Ha Hwang, Yutaka Saito, Philip Wai Yan Chiu, Rungsun Rerknimitr, Christopher Khor, Vu Van Khien, Kee Don Choi, Ki-Nam Shim, Geun Am Song, Oh Young Lee
Clinical Endoscopy.2024; 57(2): 141. CrossRef - Is there a best choice of equipment for colorectal endoscopic submucosal dissection?
Francesco Cocomazzi, Sonia Carparelli, Nunzia Labarile, Antonio Capogreco, Marco Gentile, Roberta Maselli, Jahnvi Dhar, Jayanta Samanta, Alessandro Repici, Cesare Hassan, Francesco Perri, Antonio Facciorusso
Expert Review of Medical Devices.2024; : 1. CrossRef
- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
- 1,728 View
- 130 Download
- 4 Web of Science
- 2 Crossref

Original Article
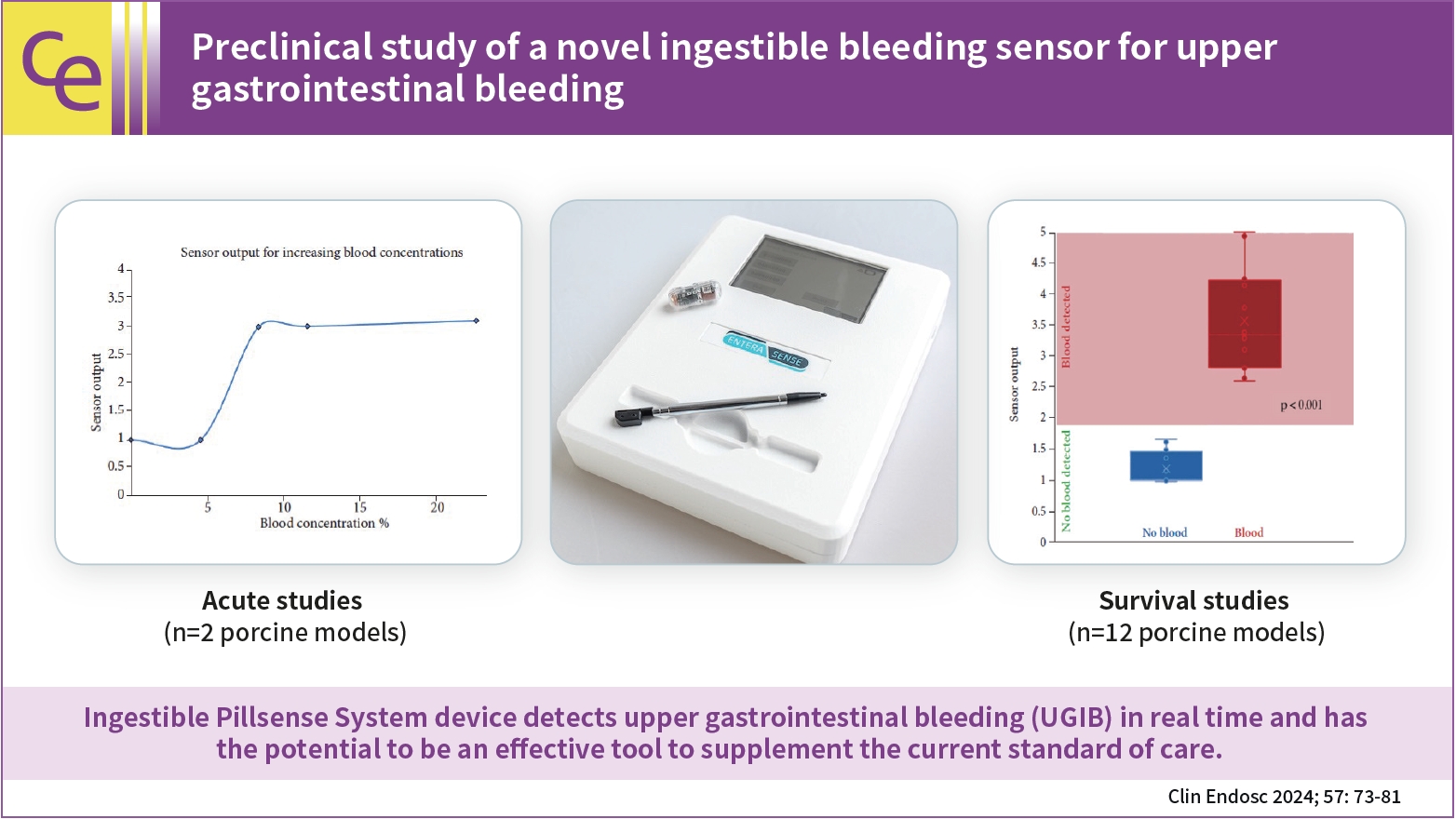
- Preclinical study of a novel ingestible bleeding sensor for upper gastrointestinal bleeding
- Kimberly F. Schuster, Christopher C. Thompson, Marvin Ryou
- Clin Endosc 2024;57(1):73-81. Published online May 31, 2023
- DOI: https://doi.org/10.5946/ce.2022.293
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: Upper gastrointestinal bleeding (UGIB) is a life-threatening condition that necessitates early identification and intervention and is associated with substantial morbidity, mortality, and socioeconomic burden. However, several diagnostic challenges remain regarding risk stratification and the optimal timing of endoscopy. The PillSense System is a noninvasive device developed to detect blood in patients with UGIB in real time. This study aimed to assess the safety and performance characteristics of PillSense using a simulated bleeding model.
Methods
A preclinical study was performed using an in vivo porcine model (14 animals). Fourteen PillSense capsules were endoscopically placed in the stomach and blood was injected into the stomach to simulate bleeding. The safety and sensitivity of blood detection and pill excretion were also investigated.
Results
All the sensors successfully detected the presence or absence of blood. The minimum threshold was 9% blood concentration, with additional detection of increasing concentrations of up to 22.5% blood. All the sensors passed naturally through the gastrointestinal tract.
Conclusions
This study demonstrated the ability of the PillSense System sensor to detect UGIB across a wide range of blood concentrations. This ingestible device detects UGIB in real time and has the potential to be an effective tool to supplement the current standard of care. These favorable results will be further investigated in future clinical studies. -
Citations
Citations to this article as recorded by- Miniaturized Capsule System Toward Real‐Time Electrochemical Detection of H2S in the Gastrointestinal Tract
Justin M. Stine, Katie L. Ruland, Luke A. Beardslee, Joshua A. Levy, Hossein Abianeh, Santiago Botasini, Pankaj J. Pasricha, Reza Ghodssi
Advanced Healthcare Materials.2024;[Epub] CrossRef
- Miniaturized Capsule System Toward Real‐Time Electrochemical Detection of H2S in the Gastrointestinal Tract
- 2,184 View
- 141 Download
- 1 Web of Science
- 1 Crossref

Review
- A practical approach for small bowel bleeding
- Sung Eun Kim, Hyun Jin Kim, Myeongseok Koh, Min Cheol Kim, Joon Sung Kim, Ji Hyung Nam, Young Kwan Cho, A Reum Choe, The Research Group for Capsule Endoscopy and Enteroscopy of the Korean Society of Gastrointestinal Endoscopy
- Clin Endosc 2023;56(3):283-289. Published online May 11, 2023
- DOI: https://doi.org/10.5946/ce.2022.302

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Gastrointestinal (GI) bleeding is one of the most common conditions among patients visiting emergency departments in Korea. GI bleeding is divided into upper and lower GI bleeding, according to the bleeding site. GI bleeding is also divided into overt and occult GI bleeding based on bleeding characteristics. In addition, obscure GI bleeding refers to recurrent or persistent GI bleeding from a source that cannot be identified after esophagogastroduodenoscopy or colonoscopy. The small intestine is the largest part of the alimentary tract. It extends from the pylorus to the cecum. The small intestine is difficult to access owing to its long length. Moreover, it is not fixed to the abdominal cavity. When hemorrhage occurs in the small intestine, the source cannot be found in many cases because of the characteristics of the small intestine. In practice, small-intestinal bleeding accounts for most of the obscure GI bleeding. Therefore, in this review, we introduce and describe systemic approaches and examination methods, including video capsule endoscopy and balloon enteroscopy, that can be performed in patients with suspected small bowel bleeding in clinical practice.
-
Citations
Citations to this article as recorded by- Manejo da hemorragia digestiva baixa na emergência: abordagem cirúrgica
Carla Azevedo Zaibak, Sara Monteiro Barbosa, Nathalia Machado De Lima, Jordane Lula Cruz, Angela Maria Pereira Costa, Maria Eduarda da Silva Borges, Mariana Vasconcellos De Oliveira, Danyelly Rodrigues Machado
Cuadernos de Educación y Desarrollo.2024;[Epub] CrossRef - Case 19: A 65-Year-Old Man With Melena and Hematochezia
Hajin Lee, Younghee Choe, Jung Heo, Gwkang Hui Park, Su Young Lee, Young Wook Cho, Hyo Suk Kim
Journal of Korean Medical Science.2024;[Epub] CrossRef - Aortoduodenal fistula bleeding caused by an aortic stent graft
Seunghyun Hong, Gwang Ha Kim
Clinical Endoscopy.2024; 57(3): 407. CrossRef
- Manejo da hemorragia digestiva baixa na emergência: abordagem cirúrgica
- 2,940 View
- 324 Download
- 3 Web of Science
- 3 Crossref

Original Articles
- Comparison of conventional and new endoscopic band ligation devices for colonic diverticular bleeding
- Ayaka Takasu, Takashi Ikeya, Yasutoshi Shiratori
- Clin Endosc 2022;55(3):408-416. Published online February 18, 2022
- DOI: https://doi.org/10.5946/ce.2021.200

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic band ligation (EBL) is used to treat colonic diverticular bleeding (CDB). An endoscopic variceal ligation device for esophageal varices is used as a conventional EBL device (C-EBL). A new EBL device (N-EBL) was developed by Sumitomo Bakelite Co. in August 2018. We aimed to evaluate the clinical outcomes of N-EBL compared with those of C-EBL.
Methods
Seventy-nine patients who underwent EBL for CDB at St. Luke’s International Hospital, Japan, between 2017 and 2020 were included in this retrospective study. Patients were divided into the C-EBL and N-EBL groups. Their clinical outcomes, including achieving initial hemostasis, early rebleeding, procedure time, and EBL-associated adverse events, were evaluated.
Results
Of the 79 patients, 36 (45.6%) were in the C-EBL group and 43 (54.4%) were in the N-EBL group. The rate of achieving initial hemostasis was 100% in the C-EBL group and 93.0% in the N-EBL group. No significant difference was noted in the early rebleeding rate between the groups (p=0.24). The N-EBL group achieved a shorter median EBL procedure time than the C-EBL group (18.2 minutes vs. 14.2 minutes, p=0.02). No adverse events were observed in either group.
Conclusions
The N-EBL device is safe and useful and may reduce EBL procedure time. -
Citations
Citations to this article as recorded by- Advances in endoscopic management of colonic diverticular bleeding
Yasutoshi Shiratori, Syed Matthew Kodilinye, Ahmed E. Salem
Current Opinion in Gastroenterology.2024;[Epub] CrossRef - Management of Patients With Acute Lower Gastrointestinal Bleeding: An Updated ACG Guideline
Neil Sengupta, Joseph D. Feuerstein, Vipul Jairath, Amandeep K. Shergill, Lisa L. Strate, Robert J. Wong, David Wan
American Journal of Gastroenterology.2023; 118(2): 208. CrossRef - Effective endoscopic band ligation for diverticular perforation with a refractory pelvic abscess
Koichi Soga, Atsushi Majima
Clinical Endoscopy.2023; 56(2): 252. CrossRef - A new band ligation device to treat colonic diverticular bleeding
Yunho Jung
Clinical Endoscopy.2022; 55(3): 367. CrossRef
- Advances in endoscopic management of colonic diverticular bleeding
- 3,847 View
- 237 Download
- 2 Web of Science
- 4 Crossref

- Risk Stratification in Cancer Patients with Acute Upper Gastrointestinal Bleeding: Comparison of Glasgow-Blatchford, Rockall and AIMS65, and Development of a New Scoring System
- Matheus Cavalcante Franco, Sunguk Jang, Bruno da Costa Martins, Tyler Stevens, Vipul Jairath, Rocio Lopez, John J. Vargo, Alan Barkun, Fauze Maluf-Filho
- Clin Endosc 2022;55(2):240-247. Published online January 21, 2022
- DOI: https://doi.org/10.5946/ce.2021.115
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Few studies have measured the accuracy of prognostic scores for upper gastrointestinal bleeding (UGIB) among cancer patients. Thereby, we compared the prognostic scores for predicting major outcomes in cancer patients with UGIB. Secondarily, we developed a new model to detect patients who might require hemostatic care.
Methods
A prospective research was performed in a tertiary hospital by enrolling cancer patients admitted with UGIB. Clinical and endoscopic findings were obtained through a prospective database. Multiple logistic regression analysis was performed to gauge the power of each score.
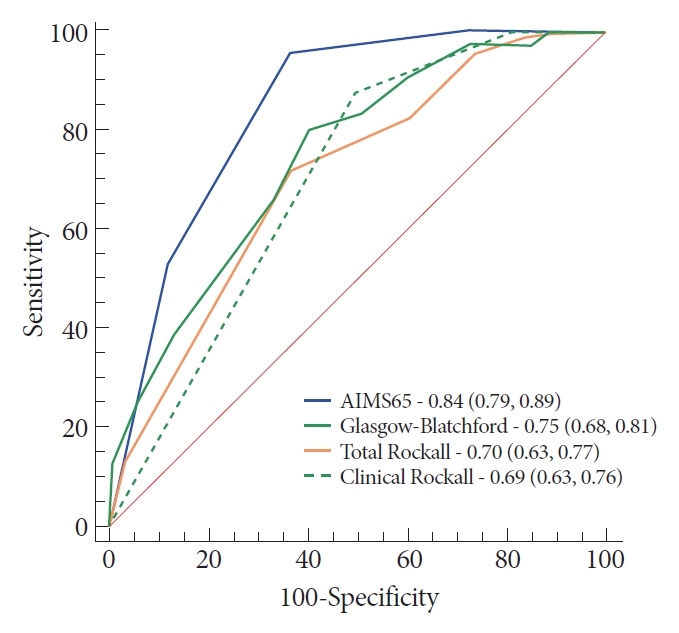
Results
From April 2015 to May 2016, 243 patients met the inclusion criteria. The AIMS65 (area under the curve [AUC] 0.85) best predicted intensive care unit admission, while the Glasgow-Blatchford score best predicted blood transfusion (AUC 0.82) and the low-risk group (AUC 0.92). All scores failed to predict hemostatic therapy and rebleeding. The new score was superior (AUC 0.74) in predicting hemostatic therapy. The AIMS65 (AUC 0.84) best predicted in-hospital mortality.
Conclusions
The scoring systems for prognostication were validated in the group of cancer patients with UGIB. A new score was developed to predict hemostatic therapy. Following this result, future prospective research should be performed to validate the new score. -
Citations
Citations to this article as recorded by- Endoscopic Management of Tumor Bleeding
Frances Dang, Marc Monachese
Gastrointestinal Endoscopy Clinics of North America.2024; 34(1): 155. CrossRef - The Accuracy of Pre-Endoscopic Scores for Mortality Prediction in Patients with Upper GI Bleeding and No Endoscopy Performed
Sergiu Marian Cazacu, Dragoș Ovidiu Alexandru, Răzvan-Cristian Statie, Sevastița Iordache, Bogdan Silviu Ungureanu, Vlad Florin Iovănescu, Petrică Popa, Victor Mihai Sacerdoțianu, Carmen Daniela Neagoe, Mirela Marinela Florescu
Diagnostics.2023; 13(6): 1188. CrossRef - Progress in the Evaluation of Acute Upper Gastrointestinal Bleeding with AIMS65 Scoring System
莉 王
Advances in Clinical Medicine.2023; 13(05): 8163. CrossRef - Interpretations of the Role of Plasma Albumin in Prognostic Indices: A Literature Review
Kim Oren Gradel
Journal of Clinical Medicine.2023; 12(19): 6132. CrossRef
- Endoscopic Management of Tumor Bleeding
- 3,226 View
- 231 Download
- 3 Web of Science
- 4 Crossref

Case Reports
- Management of Biliopancreatic Limb Bleeding after Roux-en-Y Gastric Bypass: A Case Report
- Christophe Riquoir, Luis Antonio Díaz, David Chiliquinga, Roberto Candia, Fernando Pimentel, Alex Arenas
- Clin Endosc 2021;54(5):754-758. Published online May 25, 2021
- DOI: https://doi.org/10.5946/ce.2021.060
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
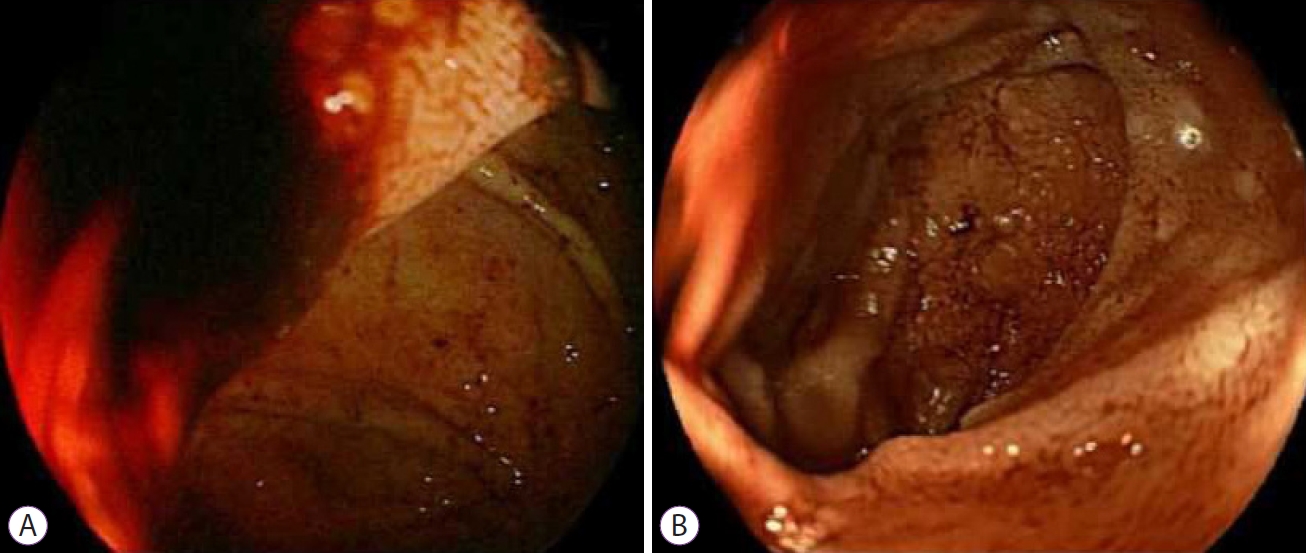
ePub - The Roux-en-Y gastric bypass is one of the most extensive surgical treatments for obesity. The treatment of upper gastrointestinal bleeding after Roux-en-Y gastric bypass is complex due to the difficulty of accessing the excluded gastric antrum and duodenal bulb. There is no consensus regarding the management of this complication. While various techniques have been described to access the biliopancreatic limb, double-balloon enteroscopy is the most commonly used. If double-balloon enteroscopy is unavailable, a pediatric colonoscope may be used as an alternative; however, its use in such cases has not been described. We report the case of a 50-year-old male patient who underwent gastric bypass 13 years ago and was admitted for a second episode of upper gastrointestinal bleeding. The initial approach using upper endoscopy, colonoscopy, and abdominal computed tomography angiography did not reveal the cause of gastrointestinal hemorrhage; therefore, an endoscopic study of the biliopancreatic limb was performed using a pediatric colonoscope. A Forrest Ib ulcer was found in the duodenal bulb, and endoscopic therapy was administered. The evolution was found to be satisfactory.
-
Citations
Citations to this article as recorded by- Endoscopic management of postoperative bleeding
Sung Hyeok Ryou, Ki Bae Bang
Clinical Endoscopy.2023; 56(6): 706. CrossRef
- Endoscopic management of postoperative bleeding
- 3,113 View
- 74 Download
- 1 Web of Science
- 1 Crossref

- Gastrointestinal Bleeding and Endoscopic Outcomes in Patients with SARS-CoV-2
- Faruq Pradhan, Yasmin Alishahi
- Clin Endosc 2021;54(3):428-431. Published online March 3, 2021
- DOI: https://doi.org/10.5946/ce.2020.244
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
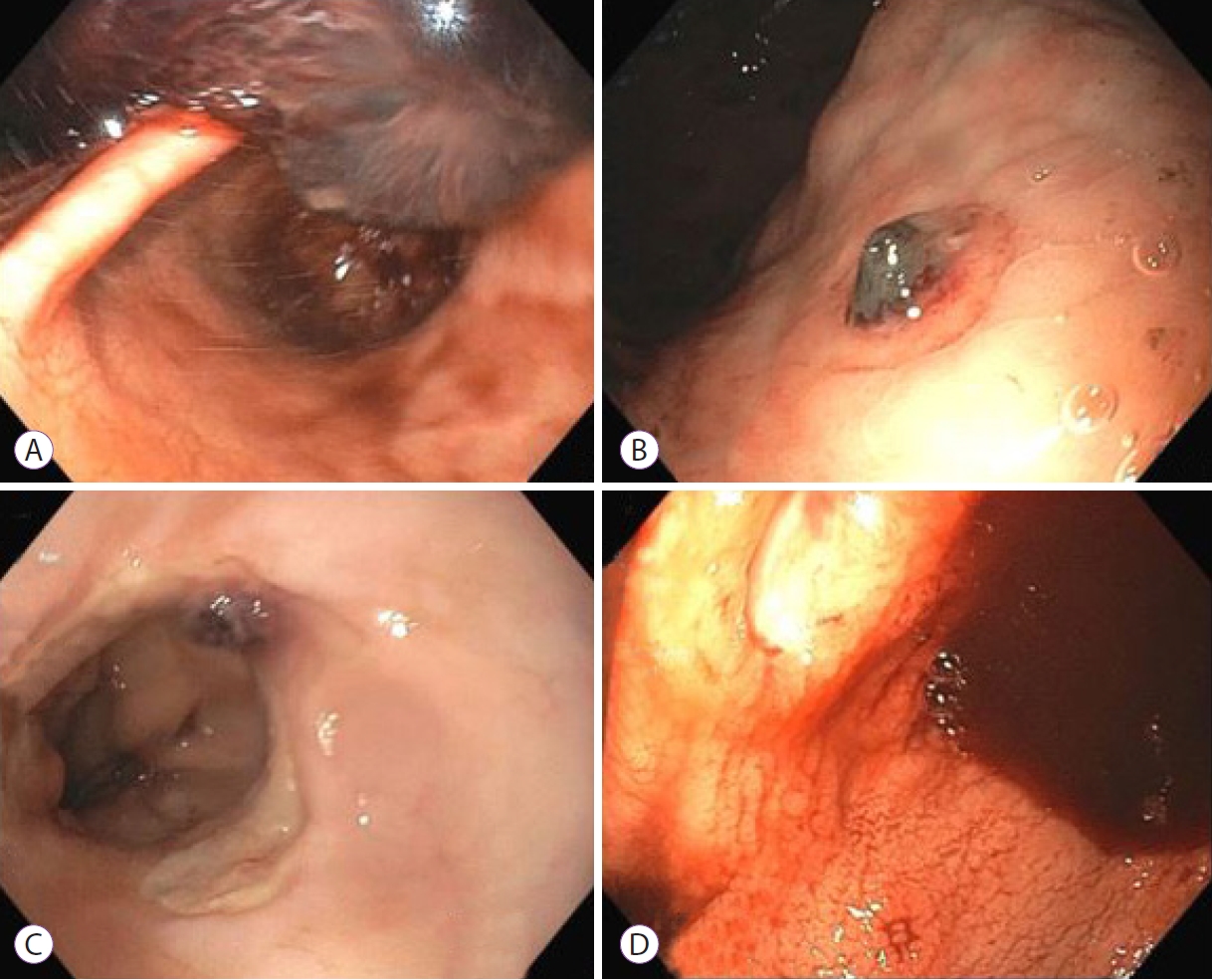
ePub - Over the past year, the novel coronavirus has been a topic of significant research. Multiple gastroenterological symptoms have been associated with this infection, in addition to the well-established pulmonary presentations. Gastrointestinal bleeding can be a complication of infection by severe acute respiratory syndrome coronavirus-2, which can be exacerbated by the anticoagulants used to treat its thrombotic sequelae. We describe the clinical cases of four patients infected with the novel coronavirus, with significant upper gastrointestinal bleeding requiring endoscopic visualization, along with their clinical outcomes.
-
Citations
Citations to this article as recorded by- Prevalence and outcomes of upper gastrointestinal bleeding in COVID‐19: A systematic review and meta‐analysis
Sawai Singh Rathore, Zario Shai Wint, Aman Goyal, Bijay Mukesh Jeswani, Ameer Mustafa Farrukh, María Alejandra Nieto‐Salazar, Thanmai Reddy Thugu, Snigdha Erva, Raafay Mehmood, Adriana Carolina Toro‐velandia, Hamam Aneis, Sunny Ratnani, Ibrahim Marouf Yas
Reviews in Medical Virology.2024;[Epub] CrossRef - Gastrointestinal Bleeding in COVID-19 Infected Patients, and Management Outcomes
Amnah Al Hanaei, Fatima AlKindi, Aysha Alkhemeiri, Satish Nair
International Journal of General Medicine.2024; Volume 17: 1145. CrossRef - Gastrointestinal Bleeding in COVID-19-Infected Patients
Mitchell S. Cappell, David M. Friedel
Gastroenterology Clinics of North America.2023; 52(1): 77. CrossRef - Endoscopic findings are not different in patients with upper gastrointestinal bleeding with COVID-19
Fatma Ebru AKIN, Öykü TAYFUR YÜREKLİ, Mustafa TAHTACI, Osman ERSOY
Akademik Gastroenteroloji Dergisi.2023; 22(1): 20. CrossRef
- Prevalence and outcomes of upper gastrointestinal bleeding in COVID‐19: A systematic review and meta‐analysis
- 4,547 View
- 162 Download
- 3 Web of Science
- 4 Crossref

- Rare and Fatal Gastrointestinal Mucormycosis (Zygomycosis) in a COVID-19 Patient: A Case Report
- Epifanio Silvino do Monte Junior, Marcos Eduardo Lera dos Santos, Igor Braga Ribeiro, Gustavo de Oliveira Luz, Elisa Ryoka Baba, Bruno Salomão Hirsch, Mateus Pereira Funari, Eduardo Guimarães Hourneaux de Moura
- Clin Endosc 2020;53(6):746-749. Published online November 19, 2020
- DOI: https://doi.org/10.5946/ce.2020.180

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - The novel coronavirus disease (COVID-19) quickly spread to all continents. However, data regarding all the signs and symptoms of COVID-19 are insufficient. Patients with COVID-19 might present higher susceptibility to fungal coinfections. Mucormycosis is a rare and often life-threatening fungal disease characterized by vascular invasion by hyphae, resulting in thrombosis and necrosis. This is the first case report of mucormycosis in a COVID-19 patient. An 86-year-old male patient was admitted to the emergency room with acute diarrhea, cough, dyspnea, and fever from 5 days prior. Blood tests revealed a hemoglobin level of 14.3 mg/dL. Five days following the admission, the patient presented with melena and a hemoglobin level of 5.6 mg/dL. A transfusion of three units of red blood cells was required. Esophagogastroduodenoscopy revealed two giant gastric ulcers with necrotic debris and a deep hemorrhagic base without active bleeding. Furthermore, biopsies confirmed mucormycosis. Despite intensive care, the patient died 36 hours after the esophagogastroduodenoscopy.
-
Citations
Citations to this article as recorded by- The potential for rapid antigen testing for mucormycosis in the context of COVID-19
Christopher R. Thornton
Expert Review of Molecular Diagnostics.2024; 24(3): 161. CrossRef - A New Proposed Combined CT and MRI Staging System for Covid-19-Associated Rhino-Orbito-Cerebral Fungal Infection: A Multi-center Study with Pathological Correlation
Noha Yahia Ebaid, Haitham Foda, Doaa Khedr Mohamed Khedr, Ahmed Ebeed, Mahmoud Ahmed Ebada, Rabab Mohamed Abdelhay, Ali Awad, Amany Abd Al Badea, Basma Hamed Ibrahim, Emad Hassan Emara
Academic Radiology.2024; 31(3): 1055. CrossRef - Development of a Machine Learning Model to Predict Risk of Development of COVID-19-Associated Mucormycosis
Rajashri Patil, Sahjid Mukhida, Jyoti Ajagunde, Uzair Khan, Sameena Khan, Nageswari Gandham, Chanda Vyawhare, Nikunja K Das, Shahzad Mirza
Future Microbiology.2024; 19(4): 297. CrossRef - COVID-19 Second Wave with Mucormycosis, a Deadly Combination: A Systemic Review
Neetu Jain, Seema Bhadauria
Biomedical and Biotechnology Research Journal.2024; 8(1): 13. CrossRef - The cross-talk between mucormycosis, steroids and diabetes mellitus amidst the global contagion of COVID-19
Shrey Dwivedi, Princy Choudhary, Ayushi Gupta, Sangeeta Singh
Critical Reviews in Microbiology.2023; 49(3): 318. CrossRef - Magnetic resonance imaging spectrum of COVID-associated rhino-orbital-cerebral mucormycosis and assessment of anatomical severity
Ishan Kumar, Ashish Verma, Jyoti Dangwal, Pramod Kumar Singh, Ram Chandra Shukla, Jaya Chakravarty
The Neuroradiology Journal.2023; 36(4): 404. CrossRef - Mucormycosis and Its Upsurge During COVID-19 Epidemic: An Updated Review
Bharti Sharma, Skarma Nonzom
Current Microbiology.2023;[Epub] CrossRef - Mucormycosis: A hidden mystery of fungal infection, possible diagnosis, treatment and development of new therapeutic agents
Mohd Kamil Hussain, Shaista Ahmed, Andleeb Khan, Arif Jamal Siddiqui, Shahnaaz Khatoon, Sadaf Jahan
European Journal of Medicinal Chemistry.2023; 246: 115010. CrossRef -
cotH
Genes Are Necessary for Normal Spore Formation and Virulence in
Mucor lusitanicus
Csilla Szebenyi, Yiyou Gu, Teclegiorgis Gebremariam, Sándor Kocsubé, Sándor Kiss-Vetráb, Olivér Jáger, Roland Patai, Krisztina Spisák, Rita Sinka, Ulrike Binder, Mónika Homa, Csaba Vágvölgyi, Ashraf S. Ibrahim, Gábor Nagy, Tamás Papp, Anuradha Chowdhary
mBio.2023;[Epub] CrossRef - Post COVID-19: Risk Factors, Prevention, and Management of Black
Fungus
Aimen Salman, Suneela Dhaneshwar, Shaik Shafiulla
Anti-Infective Agents.2023; 21(1): 39. CrossRef - Surge of mucormycosis during the COVID-19 pandemic
Paulami Dam, Marlon H. Cardoso, Sukhendu Mandal, Octávio L. Franco, Pınar Sağıroğlu, Osman Ahmet Polat, Kerem Kokoglu, Rittick Mondal, Amit Kumar Mandal, Ismail Ocsoy
Travel Medicine and Infectious Disease.2023; 52: 102557. CrossRef - COVID-19 and Mucormycosis of Orofacial Region: A Scoping Review
Abhishek Banerjee, Moumalini Das, Pooja Verma, Abhishek Chatterjee, Karthikeyan Ramalingam, Kumar Chandan Srivastava
Cureus.2023;[Epub] CrossRef - Case Reports on Black Fungus of the Gastrointestinal Tract: A New Complication in COVID-19 Patients
Sachin Arora, Ashish Singh, Pallavi Prasad, Rahul, Rajneesh Singh
The Korean Journal of Gastroenterology.2023; 81(5): 221. CrossRef - Galangin for COVID-19 and Mucormycosis co-infection: a potential therapeutic strategy of targeting critical host signal pathways triggered by SARS-CoV-2 and Mucormycosis
Md. Imran Hasan, Md. Arju Hossain, Md Habibur Rahman, Md Sohel, Asif Ahsan, Md. Sadat Hossain Soikot, Md. Nazrul Islam, Mohammad Ruhul Amin, Deepak Kumar Jain
Network Modeling Analysis in Health Informatics and Bioinformatics.2023;[Epub] CrossRef - Opportunistic Fungal Invasion in COVID-19 Pandemic: A Critical Review in Diagnosis and Management
Abhishek Sharma, Gulnaz Bano, Abdul Malik, Yuman Rasool, Samrina Manzar, Tarun Singh, Manish Maity
Avicenna Journal of Medicine.2023; 13(03): 131. CrossRef - Effect of antifungal drugs against mucormycosis and impact on human health
Shivangi Giri, Sujata Sharma, Kumud Kant Awasthi, Lata Shahani
Materials Today: Proceedings.2023; 95: 43. CrossRef - Epidemiology, Risk Factors, Diagnosis and Treatment of Mucormycosis
(Black Fungus): A Review
Pragati Upadhayay, Keshav Bansal, Ahsas Goyal
Current Pharmaceutical Biotechnology.2023; 24(13): 1645. CrossRef - View of mucormycosis during the era of COVID-19 infection: A cross-sectional study
Ossama M. Zakaria, Dana W. Alkuwaity
Journal of Family Medicine and Primary Care.2023; 12(11): 2608. CrossRef - An Update on COVID‐19 Associated Mucormycosis Characteristics, Risk Factors, and Outcomes: a Systematic Review and Meta-Analysis
Kazem Khiabani, Mohammad Hosein Amirzade-Iranaq, Hanie Ahmadi
Current Fungal Infection Reports.2023; 17(4): 282. CrossRef - CT Findings of COVID-19–associated Pulmonary Mucormycosis: A Case Series and Literature Review
Mandeep Garg, Nidhi Prabhakar, Valliappan Muthu, Shameema Farookh, Harsimran Kaur, Vikas Suri, Ritesh Agarwal
Radiology.2022; 302(1): 214. CrossRef - Mucormycosis (black fungus) ensuing COVID-19 and comorbidity meets - Magnifying global pandemic grieve and catastrophe begins
Karthika Pushparaj, Haripriya Kuchi Bhotla, Vijaya Anand Arumugam, Manikantan Pappusamy, Murugesh Easwaran, Wen-Chao Liu, Utthapon Issara, Kannan R.R. Rengasamy, Arun Meyyazhagan, Balamuralikrishnan Balasubramanian
Science of The Total Environment.2022; 805: 150355. CrossRef - Mucormycosis: A Case Series of Patients Admitted in Non-COVID-19 Intensive Care Unit of a Tertiary Care Center during the Second Wave
Nikhil Kothari, Amit Goyal, Ankur Sharma, Shilpa Goyal, Pradeep K Bhatia, Sangam Yadav
Indian Journal of Critical Care Medicine.2022; 25(10): 1193. CrossRef - A systematic review on SARS‐CoV‐2‐associated fungal coinfections
Shringika Soni, Ramesh Namdeo Pudake, Utkarsh Jain, Nidhi Chauhan
Journal of Medical Virology.2022; 94(1): 99. CrossRef - Rhino-orbito-cerebral mucormycosis during the COVID-19 third wave in 2021: an Egyptian preliminary report from a single tertiary hospital
Taha K. Alloush, Osama Mansour, Adel T. Alloush, Tamer Roushdy, Eman Hamid, Mahmoud El-Shamy, Hossam M. Shokri
Neurological Sciences.2022; 43(2): 799. CrossRef - Cumulative Mortality and Factors Associated With Outcomes of Mucormycosis After COVID-19 at a Multispecialty Tertiary Care Center in India
Twinkle Choksi, Anamika Agrawal, Purva Date, Darshana Rathod, Anuja Gharat, Avinash Ingole, Bhushan Chaudhari, Nitin Pawar
JAMA Ophthalmology.2022; 140(1): 66. CrossRef - Using artificial intelligence-based models to predict the risk of mucormycosis among COVID-19 survivors: An experience from a public hospital in India
Shabbir Syed-Abdul, A. Shoban Babu, Raja Shekhar Bellamkonda, Ramaiah Itumalla, GVRK Acharyulu, Surya Krishnamurthy, Y. Venkat Santosh Ramana, Naresh Mogilicharla, Shwetambara Malwade, Yu-Chuan Li
Journal of Infection.2022; 84(3): 351. CrossRef - Bilateral Renal Mucor Mycosis Presenting as Anuria in a Covid 19 Recovered Patient: A Case Report and Review of Literature
Surya Prakash Vaddi, Seshu Mohan Khetavath, Dilip M. Babu, Nagarjuna Maturu, Bhulaxmi, Swathi, Krithika Mohan, Datta Prasad M, Jawahar B, Rajesh Reddy KRV
Urology.2022; 161: 12. CrossRef - Fungal Infections Other Than Invasive Aspergillosis in COVID-19 Patients
Kerri Basile, Catriona Halliday, Jen Kok, Sharon C-A. Chen
Journal of Fungi.2022; 8(1): 58. CrossRef - Gastrointestinal mucormycosis: A periodic systematic review of case reports from 2015 to 2021
Mojtaba Didehdar, Zahra chegini, Alireza Moradabadi, Ali Arash Anoushirvani, Seidamir Pasha Tabaeian, Milad Yousefimashouf, Aref Shariati
Microbial Pathogenesis.2022; 163: 105388. CrossRef - Gastrointestinal Mucormycosis: A Challenge during COVID-19 Pandemic
Jagdish Chander
Journal of Gastrointestinal Infections.2022; 11(1): 30. CrossRef - COVID-19 associated mucormycosis – An emerging threat
Chien-Ming Chao, Chih-Cheng Lai, Wen-Liang Yu
Journal of Microbiology, Immunology and Infection.2022; 55(2): 183. CrossRef - The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries
Martin Hoenigl, Danila Seidel, Agostinho Carvalho, Shivaprakash M Rudramurthy, Amir Arastehfar, Jean-Pierre Gangneux, Nosheen Nasir, Alexandro Bonifaz, Javier Araiza, Nikolai Klimko, Alexandra Serris, Katrien Lagrou, Jacques F Meis, Oliver A Cornely, John
The Lancet Microbe.2022; 3(7): e543. CrossRef - Fatal allograft mucormycosis complicating severe COVID‐19 infection and bacterial pyelonephritis
Abhilash Chandra, Namrata Rao S., Kiran Preet Malhotra
Transplant Infectious Disease.2022;[Epub] CrossRef - Mucormycosis in COVID-19 pandemic: study at tertiary hospital in India
Reshma P. Chavan, Shivraj M. Ingole, Hamna Abdul Nazir, Wilson V. Desai, Gajanan S. Kanchewad
European Archives of Oto-Rhino-Laryngology.2022; 279(6): 3201. CrossRef - Mucormycosis and COVID-19 pandemic: Clinical and diagnostic approach
Asim Azhar, Wajihul Hasan Khan, Parvez Anwar Khan, Khaled Alhosaini, Mohammad Owais, Aijaz Ahmad
Journal of Infection and Public Health.2022; 15(4): 466. CrossRef - COVID19 associated mucormycosis: A review
PriyadharsiniR Palanisamy, Dhivya Elango
Journal of Family Medicine and Primary Care.2022; 11(2): 418. CrossRef - Mucormycosis: risk factors, diagnosis, treatments, and challenges during COVID-19 pandemic
Ayushi Sharma, Anjana Goel
Folia Microbiologica.2022; 67(3): 363. CrossRef - Retracted: Mucormycosis infection in patients with COVID‐19: A systematic review
SeyedAhmad SeyedAlinaghi, Amirali Karimi, Alireza Barzegary, Zahra Pashaei, Amir Masoud Afsahi, Sanam Alilou, Nazanin Janfaza, Alireza Shojaei, Fatemeh Afroughi, Parsa Mohammadi, Yasna Soleimani, Newsha Nazarian, Ava Amiri, Marcarious M. Tantuoyir, Shahra
Health Science Reports.2022;[Epub] CrossRef - Epidemiology, clinical presentation and management of COVID‐19 associated mucormycosis: A single centre experience from Pune, Western India
Ameet Dravid, Reema Kashiva, Zafer Khan, Balasaheb Bande, Danish Memon, Aparna Kodre, Milind Mane, Vishal Pawar, Dattatraya Patil, Suraj Kalyani, Prathamesh Raut, Madhura Bapte, Charlotte Saldanha, Dinesh Chandak, Teerthagouda Patil, Sateesh Reddy, Krushn
Mycoses.2022; 65(5): 526. CrossRef - Rhino-Orbital-Cerebral Mucormycosis in a Post-COVID-19 Patient from Peru
Linda Ponce-Rosas, Jose Gonzales-Zamora, Nelson Diaz-Reyes, Oliver Alarco-Cadillo, Jorge Alave-Rosas, Mohd Adnan
Case Reports in Infectious Diseases.2022; 2022: 1. CrossRef - Mucormycosis: A Lethal Disease
Pawan N. Karwa, Jyoti K. Soundarmal, Pallavi S. Shinde, Swapna R. Jalde
Asian Journal of Pharmacy and Technology.2022; : 41. CrossRef - An overview of COVID-19 related to fungal infections: what do we know after the first year of pandemic?
R. G. Vitale, J. Afeltra, S. Seyedmousavi, S. L. Giudicessi, S. M. Romero
Brazilian Journal of Microbiology.2022; 53(2): 759. CrossRef - Mucormycosis: A new threat to Coronavirus disease 2019 with special emphasis on India
Deganta Ghosh, Sagardeep Dey, Himanko Chakraborty, Sneha Mukherjee, Ankita Halder, Akash Sarkar, Pallab Chakraborty, Rajdeep Ghosh, Joy Sarkar
Clinical Epidemiology and Global Health.2022; 15: 101013. CrossRef - First Reported Cases of COVID-19-Associated Mucormycosis in Tunisia
Rim Khemakhem, Ichrak Bougharriou, Nesrine Kallel, Anis Bafoun, Feten Mahmoudi, Samy Kammoun
Electronic Journal of Medical and Dental Studies.2022; 12(1): em0097. CrossRef - Iranian patients co-infected with COVID-19 and mucormycosis: the most common predisposing factor, clinical outcomes, laboratory markers and diagnosis, and drug therapies
Hamideh Molaei, Ehsan Shojaeefar, Eghlim Nemati, Leila Khedmat, Sayed Yousef Mojtahedi, Nematollah Jonaidi Jafari, Morteza Izadi, Behzad Einollahi
Infectious Diseases.2022; 54(8): 600. CrossRef - COVID-19 and Plethora of Fungal Infections
Reetu Kundu, Nidhi Singla
Current Fungal Infection Reports.2022; 16(2): 47. CrossRef - A Comprehensive Review on the Management of COVID-19-Associated Mucormycosis (CAM): The New Basics
Divyam Girdhar, Ekta Manocha
BioMed.2022; 2(2): 181. CrossRef - Mucormycosis and COVID-19-Associated Mucormycosis: Insights of a Deadly but Neglected Mycosis
Laura C. García-Carnero, Héctor M. Mora-Montes
Journal of Fungi.2022; 8(5): 445. CrossRef - COVID-19-Associated Fungal Infections: An Urgent Need for Alternative Therapeutic Approach?
Marianna Domán, Krisztián Bányai
Frontiers in Microbiology.2022;[Epub] CrossRef - Current Treatment Options for COVID-19 Associated Mucormycosis: Present Status and Future Perspectives
Yasasve Madhavan, Kadambari Vijay Sai, Dilip Kumar Shanmugam, Aashabharathi Manimaran, Karthigadevi Guruviah, Yugal Kishore Mohanta, Divyambika Catakapatri Venugopal, Tapan Kumar Mohanta, Nanaocha Sharma, Saravanan Muthupandian
Journal of Clinical Medicine.2022; 11(13): 3620. CrossRef - Fungal Infection in Co-infected Patients With COVID-19: An Overview of Case Reports/Case Series and Systematic Review
Sima Sadat Seyedjavadi, Parmida Bagheri, Mohammad Javad Nasiri, Mehdi Razzaghi-Abyaneh, Mehdi Goudarzi
Frontiers in Microbiology.2022;[Epub] CrossRef - COVID-19-associated fungal infections in Iran: A systematic review
Tina Nazari, Fatemeh Sadeghi, Alireza Izadi, Setayesh Sameni, Shahram Mahmoudi, Felix Bongomin
PLOS ONE.2022; 17(7): e0271333. CrossRef - Effect of Indoor Bioaerosols (Fungal) Exposure on the Health of Post-COVID-19 Patients and Possible Mitigation Strategies
Yogesh Kumar Vishwakarma, Amrita Shahi, Ram Sharan Singh
COVID.2022; 2(7): 940. CrossRef - Mucormycosis in patients with COVID-19 in Russia: the results of a prospective multi-center study
S. N. Khostelidi, V. A. Zaytsev, S. A. Vartanyan, N. A. Nikitin, G. N. Evtukh, M. N. Gilalov, G. V. Portnov, A. A. Zubareva, I. B. Baranova, T. S. Bogomolova, Yu. L. Avdeenko, O. V. Shadrivova, E. A. Desyatik, E. V. Shagdileeva, Yu. V. Borzova, Yu. A. Kri
Journal Infectology.2022; 14(2): 116. CrossRef - Development of a Monoclonal Antibody and a Serodiagnostic Lateral-Flow Device Specific to Rhizopus arrhizus (Syn. R. oryzae), the Principal Global Agent of Mucormycosis in Humans
Genna E. Davies, Christopher R. Thornton
Journal of Fungi.2022; 8(7): 756. CrossRef - Mucormycosis co-infection in COVID-19 patients: An update
Abdullah S. Alkhamiss, Ahmed A. Ahmed, Zafar Rasheed, Ruqaih Alghsham, Ali Shariq, Thamir Alsaeed, Sami A. Althwab, Suliman Alsagaby, Abdullah S. M. Aljohani, Fahad A. Alhumaydhi, Sharifa K. Alduraibi, Alaa K. Alduraibi, Homaidan T. Alhomaidan, Khaled S.
Open Life Sciences.2022; 17(1): 917. CrossRef - COVID-19-Associated Mucormycosis: A Matter of Concern Amid the SARS-CoV-2 Pandemic
Pankaj Chandley, Priyanka Subba, Soma Rohatgi
Vaccines.2022; 10(8): 1266. CrossRef - Antifungal therapy in the management of fungal secondary infections in COVID-19 patients: A systematic review and meta-analysis
Sujit Kumar Sah, Atiqulla Shariff, Niharika Pathakamuri, Subramanian Ramaswamy, Madhan Ramesh, Krishna Undela, Malavalli Siddalingegowda Srikanth, Teggina Math Pramod Kumar, Joy Sturtevant
PLOS ONE.2022; 17(7): e0271795. CrossRef - The role of SARS-CoV-2 immunosuppression and the therapy used to manage COVID-19 disease in the emergence of opportunistic fungal infections: A review
Nahid Akhtar, Atif Khurshid Wani, Surya Kant Tripathi, Ajit Prakash, M. Amin-ul Mannan
Current Research in Biotechnology.2022; 4: 337. CrossRef - Mortality-Related Risk Factors for Coronavirus Disease (COVID-19)-Associated Mucormycosis: a systematic review and meta-analysis
Vahid Reza Ostovan, Reza Tabrizi, Hanieh Bazrafshan, Zahra Bahrami, Hajar Khazraei, Samaneh Khazraei, Afshin Borhani-Haghighi, Mohsen Moghadami, Matthew Grant
Current Fungal Infection Reports.2022; 16(4): 143. CrossRef - Comparative risk assessment of COVID‐19 associated mucormycosis and aspergillosis: A systematic review
Prodip Kumar Baral, Md. Abdul Aziz, Mohammad Safiqul Islam
Health Science Reports.2022;[Epub] CrossRef - COVID-19 and Fungal infections: a double debacle
Sara Mina, Hajar Yaakoub, Cédric Annweiler, Vincent Dubée, Nicolas Papon
Microbes and Infection.2022; 24(8): 105039. CrossRef - Wave of Invasive Fungal Disease on the Shores of COVID-19: A Case Series of COVID-19 Associated Rhino-Orbital Fungal Rhinosinusitis and Literature Review
Sandeep Trehan, Neena Chaudhary, Ashwin Bhasarkar
Indian Journal of Otolaryngology and Head & Neck Surgery.2022; 74(S2): 3359. CrossRef - A study of rhino-orbito-cerebral mucormycosis with COVID-19: A new challenge in North West of Rajasthan
Surendra Kumar, Harish Kumar, Manoj Mali, BabuLal Meena
Annals of African Medicine.2022; 21(4): 383. CrossRef - Is the production of reactive oxygen and nitrogen species by macrophages associated with better infectious control in female mice with experimentally disseminated and pulmonary mucormycosis?
Amanda Ribeiro dos Santos, Thais Fernanda Fraga-Silva, Débora de Fátima Almeida-Donanzam, Angela Carolina Finatto, Camila Marchetti, Maria Izilda Andrade, Olavo Speranza de Arruda, Maria Sueli Parreira de Arruda, James Venturini, Michal A Olszewski
PLOS ONE.2022; 17(12): e0270071. CrossRef - Mucormycosis, COVID-19 Pandemic and the Lessons Learnt: A Review
Anila Varghese, Anita Upadhyay, RoyA Daniel, Twinkle Sharma, MShyam Mohan, Balaji Susindran, Priyanka Singh, Chandrakant Lahariya
Journal of Medical Evidence.2022; 3(3): 256. CrossRef - Gastritis enfisematosa secundaria a mucormicosis gástrica en paciente con COVID-19. Reporte de un caso
Martín Islas Torres, Ana Laura Castillo Luna, José Juan Rodríguez Moreno, Valeria Priscilla Rendón Muñoz, José Gerardo Zamora Inzuna, Albert Antonio Ibarra Trejo
Cirujano General.2022; 44(2): 87. CrossRef - A Fatal Case of Rhizopus azygosporus Pneumonia Following COVID-19
Anubhav Kanwar, Alex Jordan, Scott Olewiler, Kurt Wehberg, Michael Cortes, Brendan R. Jackson
Journal of Fungi.2021; 7(3): 174. CrossRef - The double‐edged sword of systemic corticosteroid therapy in viral pneumonia: A case report and comparative review of influenza‐associated mucormycosis versus COVID‐19 associated mucormycosis
Kazem Ahmadikia, Seyed Jamal Hashemi, Sadegh Khodavaisy, Muhammad Ibrahim Getso, Neda Alijani, Hamid Badali, Hossein Mirhendi, Mohammadreza Salehi, Azin Tabari, Mojtaba Mohammadi Ardehali, Mohammad Kord, Emmanuel Roilides, Sassan Rezaie
Mycoses.2021; 64(8): 798. CrossRef - Antibacterials/hydrocortisone/oseltamivir
Reactions Weekly.2021; 1845(1): 43. CrossRef - Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient – Case report and review of literature
Akshay Khatri, Kai-Ming Chang, Ilan Berlinrut, Frances Wallach
Journal of Medical Mycology.2021; 31(2): 101125. CrossRef - Research and Management of Rare Diseases in the COVID-19 Pandemic Era: Challenges and Countermeasures
Sanjana Fatema Chowdhury, Syed Muktadir Al Sium, Saeed Anwar
Frontiers in Public Health.2021;[Epub] CrossRef - Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India
Awadhesh Kumar Singh, Ritu Singh, Shashank R. Joshi, Anoop Misra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2021; 15(4): 102146. CrossRef - Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: Case series from the north-western part of India
Hardeva Ram Nehara, Inder Puri, Vipin Singhal, Sunil IH, Bhagirath Ram Bishnoi, Pramendra Sirohi
Indian Journal of Medical Microbiology.2021; 39(3): 380. CrossRef - COVID 19 infection and mucormycosis—a dangerously increasing combination
Satvinder Singh Bakshi, Vinoth Kumar Kalidoss
The Egyptian Journal of Otolaryngology.2021;[Epub] CrossRef - Autoptic identification of disseminated mucormycosis in a young male presenting with cerebrovascular event, multi-organ dysfunction and COVID-19 infection
Vidya Krishna, Jaymin Morjaria, Rona Jalandari, Fatima Omar, Sundeep Kaul
IDCases.2021; 25: e01172. CrossRef - Does COVID 19 generate a milieu for propagation of mucormycosis?
Deepak Pandiar, N. Siva Kumar, Rahul Anand, Mala Kamboj, Anjali Narwal, P.M. Shameena
Medical Hypotheses.2021; 152: 110613. CrossRef - Mucormycosis associated with COVID‐19 in two kidney transplant patients
Carolt Arana, Rafael E. Cuevas Ramírez, Marc Xipell, Joaquim Casals, Asunción Moreno, Sabina Herrera, Marta Bodro, Frederic Cofan, Fritz Diekmann, Núria Esforzado
Transplant Infectious Disease.2021;[Epub] CrossRef - COVID-19 associated mucormycosis: the urgent need to reconsider the indiscriminate use of immunosuppressive drugs
Alfonso J. Rodriguez-Morales, Ranjit Sah, Jose Millan-Oñate, Angel Gonzalez, Juan J. Montenegro-Idrogo, Sias Scherger, Carlos Franco-Paredes, Andrés F. Henao-Martínez
Therapeutic Advances in Infectious Disease.2021; 8: 204993612110270. CrossRef - COVID‐19‐associated mucormycosis: An updated systematic review of literature
Rimesh Pal, Birgurman Singh, Sanjay Kumar Bhadada, Mainak Banerjee, Ranjitpal Singh Bhogal, Neemu Hage, Ashok Kumar
Mycoses.2021; 64(12): 1452. CrossRef - Rare case of gastrointestinal mucormycosis with colonic perforation in an immunocompetent patient with COVID-19
Ravinder Pal Singh, Nishkarsh Gupta, Tanudeep Kaur, Anju Gupta
BMJ Case Reports.2021; 14(7): e244096. CrossRef - The double trouble: COVID-19 associated mucormycosis a focused review and future perspectives
Arun Kumar Agnihotri, Monika Vij, Okezie I. Aruoma, Vipul D Yagnik, Theeshan Bahorun, Maria Elena Villamil, Godfred A. Menezes, Vineet Gupta
Global Journal of Medical, Pharmaceutical, and Biomedical Update.2021; 16: 4. CrossRef - Mucormycosis: An opportunistic pathogen during COVID-19
Iyer Mahalaxmi, Kaavya Jayaramayya, Dhivya Venkatesan, Mohana Devi Subramaniam, Kaviyarasi Renu, Padmavathi Vijayakumar, Arul Narayanasamy, Abilash Valsala Gopalakrishnan, Nachimuthu Senthil Kumar, Palanisamy Sivaprakash, Krothapalli R.S. Sambasiva Rao, B
Environmental Research.2021; 201: 111643. CrossRef - Epidemiology of Systemic Mycoses in the COVID-19 Pandemic
María Guadalupe Frías-De-León, Rodolfo Pinto-Almazán, Rigoberto Hernández-Castro, Eduardo García-Salazar, Patricia Meza-Meneses, Carmen Rodríguez-Cerdeira, Roberto Arenas, Esther Conde-Cuevas, Gustavo Acosta-Altamirano, Erick Martínez-Herrera
Journal of Fungi.2021; 7(7): 556. CrossRef - EFFICACY OF ENDOSCOPIC TOPICAL MITOMYCIN C APPLICATION IN CAUSTIC ESOPHAGEAL STRICTURES IN THE PEDIATRIC POPULATION: A SYSTEMATIC REVIEW AND META-ANALYSIS OF RANDOMIZED CONTROLLED TRIALS
Marcelo Mochate FLOR, Igor Braga RIBEIRO, Diogo Turiani Hourneaux DE MOURA, Sérgio Barbosa MARQUES, Wanderley Marques BERNARDO, Eduardo Guimarães Hourneaux DE MOURA
Arquivos de Gastroenterologia.2021; 58(2): 253. CrossRef - Coronavirus Disease-Associated Mucormycosis from a Tertiary Care Hospital in India: A Case Series
Yudhyavir Singh, Venkata Ganesh, Shailendra Kumar, Nishant Patel, Richa Aggarwala, Kapil Dev Soni, Anjan Trikha
Cureus.2021;[Epub] CrossRef - Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature
Deepak Garg, Valliappan Muthu, Inderpaul Singh Sehgal, Raja Ramachandran, Harsimran Kaur, Ashish Bhalla, Goverdhan D. Puri, Arunaloke Chakrabarti, Ritesh Agarwal
Mycopathologia.2021; 186(2): 289. CrossRef - Mucormycosis and COVID‐19: An epidemic within a pandemic in India
Lav Selarka, Suktara Sharma, Dinesh Saini, Sanjay Sharma, Amit Batra, Vishal T. Waghmare, Pratibha Dileep, Sanket Patel, Monarch Shah, Tejas Parikh, Prakash Darji, Amit Patel, Gaurav Goswami, Anand Shah, Sandeep Shah, Harsh Lathiya, Moksha Shah, Pranita S
Mycoses.2021; 64(10): 1253. CrossRef - COVID-19 and mucormycosis superinfection: the perfect storm
Jaffar A. Al-Tawfiq, Saad Alhumaid, Abeer N. Alshukairi, Mohamad-Hani Temsah, Mazin Barry, Abbas Al Mutair, Ali A. Rabaan, Awadh Al-Omari, Raghavendra Tirupathi, Manaf AlQahtani, Salma AlBahrani, Kuldeep Dhama
Infection.2021; 49(5): 833. CrossRef - COVID-19 Associated Rhino-Orbital Mucormycosis Complicated by Gangrenous and Bone Necrosis—A Case Report from Honduras
Elsa Yolanda Palou, María Auxiliadora Ramos, Emec Cherenfant, Adoni Duarte, Itzel Carolina Fuentes-Barahona, Lysien I. Zambrano, Fausto Muñoz-Lara, Sandra Aracely Montoya-Ramirez, Alex Francisco Cardona-Ortiz, Jorge Alberto Valle-Reconco, Juan J. Monteneg
Vaccines.2021; 9(8): 826. CrossRef - A rare case of knee joint mucormycosis with pathological fracture after COVID-19 infection
Sergiu Andrei Iordache, Adrian Cursaru, Bogdan Şerban, Mihnea Ioan Gabriel Popa
Romanian Journal of Orthopaedic Surgery and Traumatology.2021; 4(1): 9. CrossRef - Epidemiology and Pathophysiology of COVID-19-Associated Mucormycosis: India Versus the Rest of the World
Valliappan Muthu, Shivaprakash M. Rudramurthy, Arunaloke Chakrabarti, Ritesh Agarwal
Mycopathologia.2021; 186(6): 739. CrossRef - COVID-19-associated mucormycosis: Case report and systematic review
Ahmet Dilek, Resat Ozaras, Sevket Ozkaya, Mustafa Sunbul, Elif Itir Sen, Hakan Leblebicioglu
Travel Medicine and Infectious Disease.2021; 44: 102148. CrossRef - COVID-19 and mucormycosis in Latin America – An emerging concern
Alfonso J. Rodriguez-Morales, Carlos S. Mamani-García, Janeth N. Nuñez-Lupaca, Darwin A. León-Figueroa, Mely Olarte-Durand, Robinson A. Yrene-Cubas, Diana M. Ticona, Sebastian Abanto-Urbano
Travel Medicine and Infectious Disease.2021; 44: 102156. CrossRef - Overview on the Prevalence of Fungal Infections, Immune Response, and Microbiome Role in COVID-19 Patients
Maryam Roudbary, Sunil Kumar, Awanish Kumar, Lucia Černáková, Fatemeh Nikoomanesh, Célia F. Rodrigues
Journal of Fungi.2021; 7(9): 720. CrossRef - A case report of rhino-facial mucormycosis in a non-diabetic patient with COVID-19: a systematic review of literature and current update
Faezeh Mohammadi, Milad Badri, Shapoor Safari, Nima Hemmat
BMC Infectious Diseases.2021;[Epub] CrossRef - Mucormycosis: A manifestation in COVID-19 infection
Abhishek Sharma, Gulnaz Bano, Abdul Malik
Indian Journal of Pharmacy and Pharmacology.2021; 8(3): 189. CrossRef - A Review of Coronavirus Disease Covid-19
Swapnali Zore
International Journal of Advanced Research in Science, Communication and Technology.2021; : 104. CrossRef - COVID-19 Associated Mucormycosis: A Systematic Review from Diagnostic Challenges to Management
Farah Yasmin, Hala Najeeb, Aisha Naeem, Kartik Dapke, Rachana Phadke, Muhammad Sohaib Asghar, Syed Muhammad Ismail Shah, Domenico De Berardis, Irfan Ullah
Diseases.2021; 9(4): 65. CrossRef - COVID-19-Associated Mucormycosis (CAM): An Updated Evidence Mapping
Salman Hussain, Harveen Baxi, Abanoub Riad, Jitka Klugarová, Andrea Pokorná, Simona Slezáková, Radim Líčeník, Abul Kalam Najmi, Miloslav Klugar
International Journal of Environmental Research and Public Health.2021; 18(19): 10340. CrossRef - Rhino-orbital Mucormycosis as a complication of severe COVID-19 pneumonia
Mohammed A. Alamin, Mohammed Abdulgayoom, Sushil Niraula, Elabbass Abdelmahmuod, Ashraf O. Ahmed, Mohammed I. Danjuma
IDCases.2021; 26: e01293. CrossRef - COVID-19-Associated Mucormycosis (CAM): Case-Series and Global Analysis of Mortality Risk Factors
Abanoub Riad, Alshaimaa Ahmed Shabaan, Julien Issa, Sally Ibrahim, Hatem Amer, Yossef Mansy, Islam Kassem, Amira Bisher Kassem, Hans-Peter Howaldt, Miloslav Klugar, Sameh Attia
Journal of Fungi.2021; 7(10): 837. CrossRef - Sinoorbital Mucormycosis Associated with Corticosteroid Therapy in COVID-19 Infection
Zeinab Mehrabi, Maryam Salimi, Kianoush Niknam, Farzaneh Mohammadi, Hesan Jelodari Mamaghani, Mohammad Reza Sasani, Mohammad Javad Ashraf, Amirhossein Salimi, Mohammad Hassan Zahedroozegar, Zohreh Erfani, Huban Atilla
Case Reports in Ophthalmological Medicine.2021; 2021: 1. CrossRef - Mucormycosis infection in severe COVID‐19 patient with multiple underlying health conditions
Zahra Heydarifard, Moslem Safaei, Sevrin Zadheidar, Soroush Ehsan, Nazanin Zahra Shafiei‐Jandaghi
Clinical Case Reports.2021;[Epub] CrossRef - Invasive Fungal Infections Complicating COVID-19: A Narrative Review
Giacomo Casalini, Andrea Giacomelli, Annalisa Ridolfo, Cristina Gervasoni, Spinello Antinori
Journal of Fungi.2021; 7(11): 921. CrossRef - Coronavirus Disease 2019–Associated Invasive Fungal Infection
John W Baddley, George R Thompson, Sharon C -A Chen, P Lewis White, Melissa D Johnson, M Hong Nguyen, Ilan S Schwartz, Andrej Spec, Luis Ostrosky-Zeichner, Brendan R Jackson, Thomas F Patterson, Peter G Pappas
Open Forum Infectious Diseases.2021;[Epub] CrossRef - Manifestations and risk factors of COVID-19 and mucormycosis: A mini-review
Jugal Sutradhar, BapiRay Sarkar
Journal of Acute Disease.2021; 10(6): 221. CrossRef - Invasive Mucormycosis – An Enigma
Anil Prasad, Minakshi Mishra, Kaushik Saha
Cureus.2021;[Epub] CrossRef - Salix spp. Bark Hot Water Extracts Show Antiviral, Antibacterial, and Antioxidant Activities—The Bioactive Properties of 16 Clones
Jenni Tienaho, Dhanik Reshamwala, Tytti Sarjala, Petri Kilpeläinen, Jaana Liimatainen, Jinze Dou, Anneli Viherä-Aarnio, Riikka Linnakoski, Varpu Marjomäki, Tuula Jyske
Frontiers in Bioengineering and Biotechnology.2021;[Epub] CrossRef - Mucormycosis – resurgence of a deadly opportunist during COVID-19 pandemic: Four case reports
Shalini Upadhyay, Tanisha Bharara, Manisha Khandait, Ankit Chawdhry, Bharat Bhushan Sharma
World Journal of Clinical Cases.2021; 9(36): 11338. CrossRef - Mucormycosis following COVID19: clinical case and literature review
Sofya N. Khostelidi, V.A. Zaytsev, E.V. Pelikh, E.V. Yashina, O.N. Rodionova, T.S. Bogomolova, Yu.L. Avdeenko, Nikolay N. Klimko
Clinical Microbiology and Antimicrobial Chemotherapy.2021; 23(3): 255. CrossRef
- The potential for rapid antigen testing for mucormycosis in the context of COVID-19
- 7,750 View
- 256 Download
- 107 Web of Science
- 111 Crossref

Original Articles
-
Utility of the Gel Immersion Method for Treating Massive Colonic Diverticular Bleeding

- Kazuki Yamamoto, Yasutoshi Shiratori, Takashi Ikeya
- Clin Endosc 2021;54(2):256-260. Published online August 11, 2020
- DOI: https://doi.org/10.5946/ce.2020.081
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
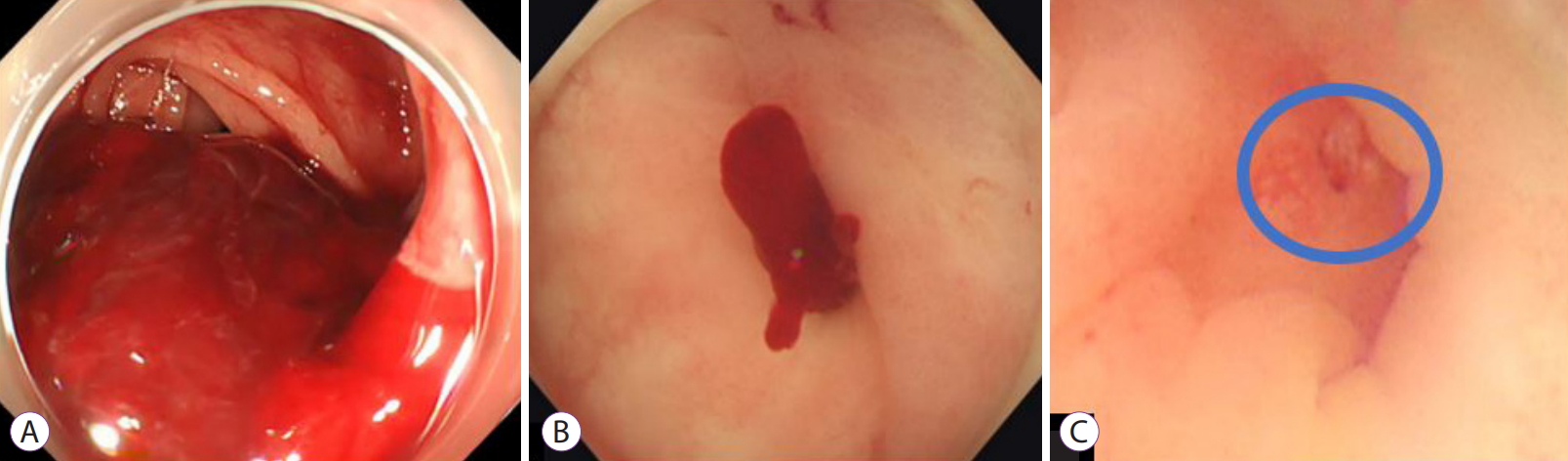
/Aims: In Asia, right-sided diverticular bleeding is more common than that of the left side. It often causes massive bleeding and difficulties in identifying the stigmata of recent hemorrhage (SRH) of colonic diverticular bleeding (CDB). This case series demonstrates the efficacy of the gel immersion method using OS-1 Jelly (Otsuka Pharmaceuticals Factory, Tokushima, Japan) in patients with CDB.
Methods
This retrospective case series analyzed data of patients with CDB who underwent the gel immersion method from April 2016 to February 2020 at St. Luke’s International Hospital, Japan. All patients diagnosed with CDB who underwent the gel immersion method were included. We collected data on the site of bleeding, identification of SRH, and efficacy of the method from the electronic medical records.
Results
A total of 9 patients (including 7 with right-sided CDB) underwent gel immersion method and were included in this study. SRH were successfully found in 66.7% (6/9) of patients. Moreover, effective hemostasis was achieved in 85.7% (6/7) of patients with right-sided CDB. There were no adverse events.
Conclusions
The gel immersion method was found to be effective, especially for massive right-sided CDB. -
Citations
Citations to this article as recorded by- Advances in endoscopic management of colonic diverticular bleeding
Yasutoshi Shiratori, Syed Matthew Kodilinye, Ahmed E. Salem
Current Opinion in Gastroenterology.2024;[Epub] CrossRef - Successful direct clipping of the bleeding source of a colonic diverticular hemorrhage using the “long-hood gel-filling” method
Satoshi Abiko, Koji Hirata, Kazuharu Suzuki, Kenji Kinoshita, Kazuteru Hatanaka, Yoshiya Yamamoto, Hirohito Naruse
Endoscopy.2023; 55(S 01): E606. CrossRef - Utility of under-gel endoscopic mucosal resection with partial submucosal injection for a laterally spreading tumor
Kazuki Yamamoto, Naoki Kanomata, Takashi Ikeya
Endoscopy.2022; 54(03): E88. CrossRef - Localizing spontaneously hemostatic colonic diverticular bleeding using VISCOCLEAR gel: A case report
Daisuke Suto, Masashi Yoshida, Takaaki Otake, Eiichiro Ichiishi, Kiichi Sato, Yosuke Osawa, Hirotoshi Ebinuma, Hironori Odaira, Yutaka Suzuki, Yutaka Kohgo
Annals of Medicine & Surgery.2022;[Epub] CrossRef - Gel Immersion Endoscopic Mucosal Resection (EMR) for Superficial Nonampullary Duodenal Epithelial Tumors May Reduce Procedure Time Compared with Underwater EMR (with Video)
Takeshi Yamashina, Masaaki Shimatani, Yu Takahashi, Masahiro Takeo, Natsuko Saito, Hironao Matsumoto, Takeshi Kasai, Masataka Kano, Kimi Sumimoto, Toshiyuki Mitsuyama, Hiroyuki Marusawa, Akiyoshi Nishio, Takafumi Yuba, Toshihito Seki, Makoto Naganuma, Tat
Gastroenterology Research and Practice.2022; 2022: 1. CrossRef - Digital compression for hemostasis in acute hemorrhagic rectal ulcer: a report of 4 cases and review of the literature
Takeshi Okamoto, Ayaka Takasu, Takaaki Yoshimoto, Kazuki Yamamoto, Yasutoshi Shiratori, Takashi Ikeya, Katsuyuki Fukuda
Clinical Journal of Gastroenterology.2021; 14(3): 796. CrossRef - Efficiency of a novel gel product for duodenal ulcer bleeding
Shuichi Miyamoto, Kazuharu Suzuki, Kenji Kinoshita
Digestive Endoscopy.2021;[Epub] CrossRef - Development of a gel dedicated to gel immersion endoscopy
Tomonori Yano, Atsushi Ohata, Yuji Hiraki, Makoto Tanaka, Satoshi Shinozaki, Alan Kawarai Lefor, Hironori Yamamoto
Endoscopy International Open.2021; 09(06): E918. CrossRef - Gel immersion endoscopy: Innovation in securing the visual field – Clinical experience with 265 consecutive procedures
Tomonori Yano, Takahito Takezawa, Kousei Hashimoto, Ayako Ohmori, Satoshi Shinozaki, Manabu Nagayama, Hirotsugu Sakamoto, Yoshimasa Miura, Yoshikazu Hayashi, Keijiro Sunada, Alan Kawarai Lefor, Hironori Yamamoto
Endoscopy International Open.2021; 09(07): E1123. CrossRef
- Advances in endoscopic management of colonic diverticular bleeding
- 5,241 View
- 176 Download
- 10 Web of Science
- 9 Crossref

-
Efficacy of the Envelope Method in Applying Polyglycolic Acid Sheets to Post-Endoscopic Submucosal Dissection Ulcers in Living Pigs

- Hiroya Sakaguchi, Toshitatsu Takao, Yoshitaka Takegawa, Yuki Koga, Kazunori Yamanaka, Masataka Sagata, Shinwa Tanaka, Yoshinori Morita, Takashi Toyonaga, Yuzo Kodama
- Clin Endosc 2021;54(1):64-72. Published online July 16, 2020
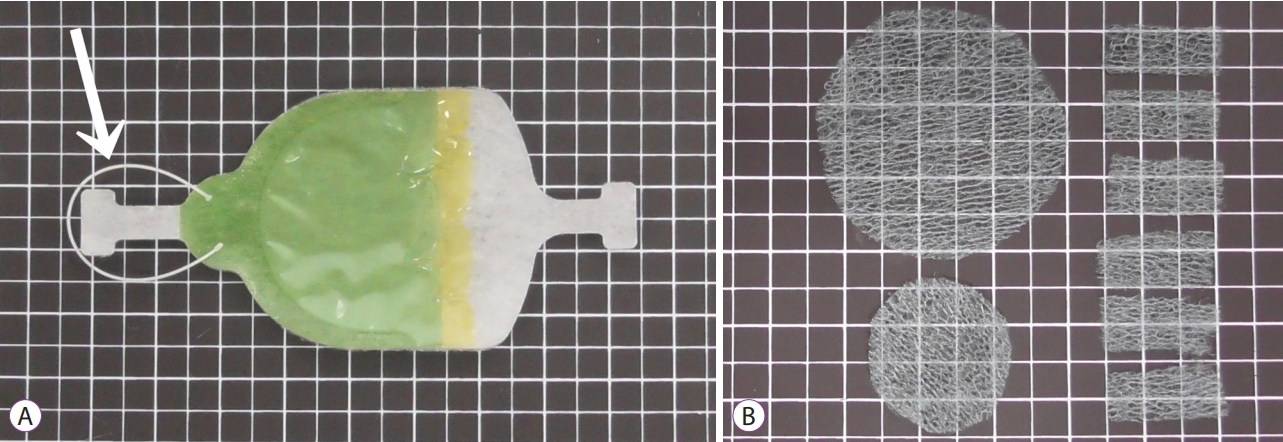
- DOI: https://doi.org/10.5946/ce.2020.014
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Application of polyglycolic acid (PGA) sheets using fibrin glue in post-endoscopic submucosal dissection (ESD) ulcers to prevent bleeding has been reported to be difficult with the conventional delivery method because of gravity. This study assessed the usefulness of the envelope-based delivery system with and against gravity in living pigs.
Methods
PGA sheets were applied on post-ESD ulcers with and against gravity six times each using the conventional and envelope methods, respectively. The PGA sheet delivery time and the endoscopic and histological findings of the treated ulcer floors were compared.
Results
With gravity, the median PGA sheet application time was 1.00 (0.68–1.30) min/cm2 and 0.32 (0.18–0.52) min/cm2 with the conventional and envelope techniques (p=0.002), respectively, and against gravity, it was 1.20 (1.13–1.63) min/cm2 and 0.50 (0.39–0.58) min/cm2 (p=0.002), respectively. Against gravity, the endoscopic and histological findings revealed that the conventional group had insufficient fixation of the PGA sheets, but the envelope groups had sufficient fixation. The results with gravity were similar between the groups.
Conclusions
The envelope method makes it possible to deliver PGA sheets to the stomach quickly and cover ulcers appropriately both with and against gravity in living pigs. -
Citations
Citations to this article as recorded by- Endoscopic sealing hemostasis with polyglycolic acid sheet and fibrin glue as a novel endoscopic hemostatic technique: a report of three cases
Kai Korekawa, Atsushi Kunimitsu
Clinical Journal of Gastroenterology.2024;[Epub] CrossRef - Clinical Impact of Different Reconstruction Methods on Remnant Gastric Cancer at the Anastomotic Site after Distal Gastrectomy
Kei Matsumoto, Shinwa Tanaka, Takashi Toyonaga, Nobuaki Ikezawa, Mari Nishio, Masanao Uraoka, Tomoatsu Yoshihara, Hiroya Sakaguchi, Hirofumi Abe, Tetsuya Yoshizaki, Madoka Takao, Toshitatsu Takao, Yoshinori Morita, Hiroshi Yokozaki, Yuzo Kodama
Clinical Endoscopy.2022; 55(1): 86. CrossRef - The importance of pH adjustment for preventing fibrin glue dissolution in the stomach: an in vitro study
Yoshitaka Takegawa, Toshitatsu Takao, Hiroya Sakaguchi, Tatsuya Nakai, Kazuhiro Takeo, Yoshinori Morita, Takashi Toyonaga, Yuzo Kodama
Scientific Reports.2022;[Epub] CrossRef - A Novel Self-Assembled Gel for Gastric Endoscopic Submucosal Dissection-Induced Ulcer: A Preclinical Study in a Porcine Model
Meng Li, Haifeng Jin, Changpei Shi, Bin Lyu, Xiao Ying, Yuan Shi
Frontiers in Pharmacology.2021;[Epub] CrossRef
- Endoscopic sealing hemostasis with polyglycolic acid sheet and fibrin glue as a novel endoscopic hemostatic technique: a report of three cases
- 4,907 View
- 118 Download
- 5 Web of Science
- 4 Crossref

- Endoscopic Findings in Patients Under the Age of 40 Years with Hematochezia in Singapore
- Man Hon Tang, Fung Joon Foo, Chee Yung Ng
- Clin Endosc 2020;53(4):466-470. Published online June 18, 2020
- DOI: https://doi.org/10.5946/ce.2019.029
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Sigmoidoscopy is performed in most medical centers to evaluate the distal colons of young adults presenting with hematochezia who are at risk of developing proximal lesions. Colonoscopies offer more complete evaluations but are associated with a higher incidence of complications and possible low yield.
Methods
An analysis was conducted on colonoscopies performed in our center on patients 40 years of age or younger. The study population was sub-divided into 2 age groups for analysis: <30 years of age and 30–39 years of age.
Results
We recruited 453 patients for the study. Patients were 115 and 338 individuals that were <30 and 30–39 years of age, respectively. Hemorrhoids was identified as the cause of bleeding in the majority of cases. The overall incidence of polyps was 6.5%; this was significantly higher in the 30–39 age group (7.4% vs. 1.7%, p=0.026). There were two cases of advanced/malignant polyps. While the majority of the polyps were in the distal colon, 28% of the polyps in the older age group were found in the proximal colon. There was one case of colonic perforation.
Conclusions
Colonic polyps are more prevalent in patients aged 30–39. Colonoscopies should be considered for patients over the age of 30 with rectal bleeding. -
Citations
Citations to this article as recorded by- Comparing efficacy and factors of postoperative bleeding in endoscopic mucosal resection vs coagulation for intestinal polyps
Zhiang Li, Fei Yu, Chaoqian Wang, Zhang Du
Medicine.2023; 102(37): e34941. CrossRef - The role of colonoscopy in young patients with rectal bleeding: a systematic review and meta-analysis
Tuane Colles, Patrícia K. Ziegelmann, Daniel C. Damin
International Journal of Colorectal Disease.2023;[Epub] CrossRef - Usefulness of Colonoscopy in Patients with Hematochezia Aged under 40 Years
Hee Chan Yang, Sang Wook Kim
Clinical Endoscopy.2020; 53(4): 385. CrossRef
- Comparing efficacy and factors of postoperative bleeding in endoscopic mucosal resection vs coagulation for intestinal polyps
- 4,136 View
- 82 Download
- 2 Web of Science
- 3 Crossref

Case Report
- Endoscopic Ultrasound-Guided Vascular Therapy for Portoduodenal Fistula
- Tanyaporn Chantarojanasiri, Apichet Sirinawasatien, Chalermrat Bunchorntavakul, Aroon Siripun, Sa-ard Treepongkaruna, Thawee Ratanachu-ek
- Clin Endosc 2020;53(6):750-753. Published online February 13, 2020
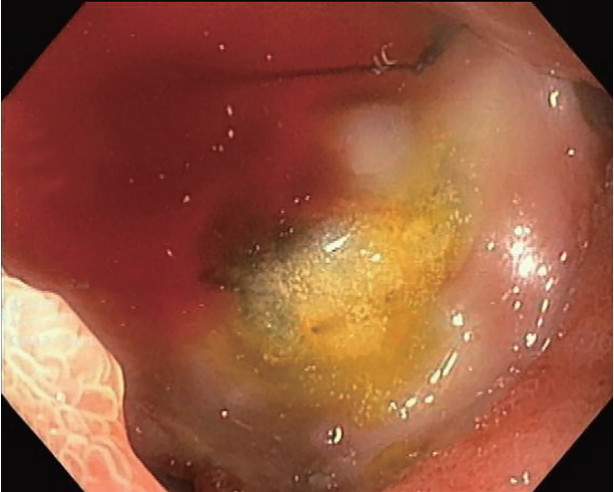
- DOI: https://doi.org/10.5946/ce.2019.167
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Portoenteric fistula is a rare cause of massive upper gastrointestinal bleeding. Most cases can be treated with radiointervention or surgery, but portoenteric fistula is associated with a high mortality. We reported a case of intermittent massive upper gastrointestinal bleeding in a 33-year-old man with cholangiocarcinoma who underwent surgical resection followed by chemoradiation. A portoduodenal fistula due to chronic duodenal ulceration was identified. The bleeding was successfully controlled by endoscopic ultrasound-guided coil placement through the duodenal bulb using the anchoring technique. Follow-up endoscopy and computed tomography scan showed multiple coil placements between a part of the portal vein and the duodenal bulb without any evidence of portal vein thrombosis. There were no complications, and bleeding did not recur during the 8-month follow-up period.
-
Citations
Citations to this article as recorded by- Management of non-variceal upper gastrointestinal bleeding: role of endoscopic ultrasound-guided treatments
Chaoqun Han, Xin Ling, Jun Liu, Rong Lin, Zhen Ding
Therapeutic Advances in Gastroenterology.2022; 15: 175628482110561. CrossRef - A Case of an Internal Pancreatic Stent Penetrating the Portal Vein after Pancreaticoduodenectomy for Ampullary Carcinoma
Masanobu Taniguchi, Atsushi Mitsunaka, Yumi Zen, Takayuki Higashiguchi, Masaru Nagato, Yasuhisa Tango, Ichiro Nakamura, Tomoaki Nakamura, Hisanori Shiomi
The Japanese Journal of Gastroenterological Surgery.2022; 55(2): 99. CrossRef - Endoscopic ultrasound-guided portal vein coiling: troubleshooting interventional endoscopic ultrasonography
Shin Haba, Kazuo Hara, Nobumasa Mizuno, Takamichi Kuwahara, Nozomi Okuno, Akira Miyano, Daiki Fumihara, Moaz Elshair
Clinical Endoscopy.2022; 55(3): 458. CrossRef
- Management of non-variceal upper gastrointestinal bleeding: role of endoscopic ultrasound-guided treatments
- 4,101 View
- 85 Download
- 2 Web of Science
- 3 Crossref

Original Article
- Endoscopic Management with a Novel Over-The-Scope Padlock Clip System
- Mahesh Kumar Goenka, Gajanan Ashokrao Rodge, Indrajeet Kumar Tiwary
- Clin Endosc 2019;52(6):574-580. Published online November 26, 2019
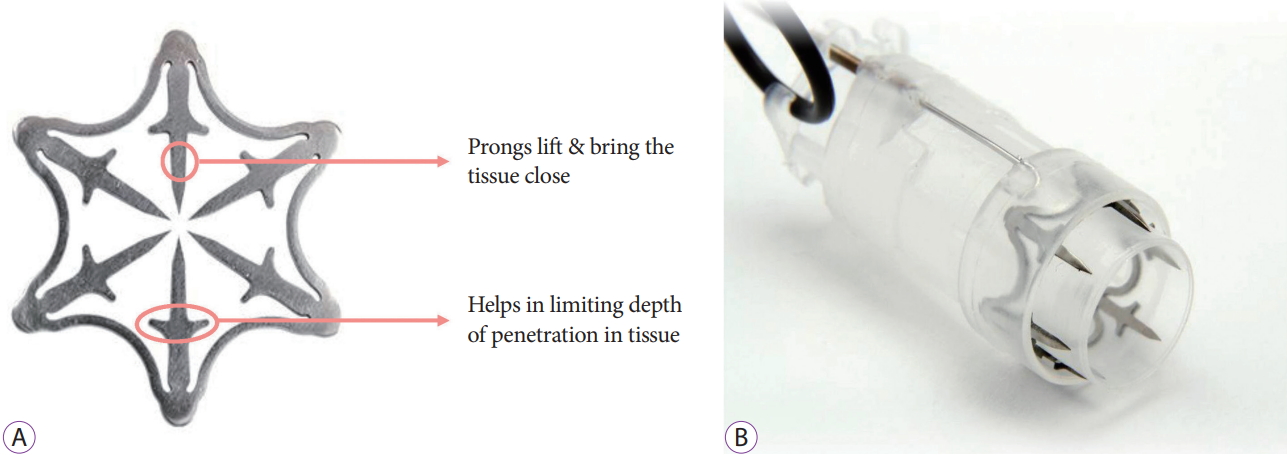
- DOI: https://doi.org/10.5946/ce.2019.122
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The Padlock clip is a recently introduced over-the-scope clip (OTSC) that requires the use of an alternate technique and has a different design from previous OTSCs. However, data regarding its clinical use are limited. The aim of this study is to present our clinical experience using this novel Padlock clip system.
Methods
Between September 2018 and June 2019, 7 consecutive patients underwent Padlock clip application at our center by an experienced endoscopist. A Padlock clip was used for achieving hemostasis in 4 patients presenting with gastrointestinal (GI) bleeding, as well as for endoscopic full-thickness resection in the remaining 3 patients.
Results
All 7 patients achieved technical as well as clinical success, with absence of complications or rebleeding, during a follow-up of a minimum of 3 weeks. All patients were hospitalized post procedure for a minimum of 48 hours, and an absence of adverse events was noted in our patient population throughout the procedure and post-procedure period. Antiplatelet therapy was reinstated shortly after the application of the Padlock clip, with no GI bleeding observed.
Conclusions
The Padlock clip is a novel OTSC, with benefits that include safe, simple, and rapid deployment. Antiplatelet therapy may be reinstated for patients, when necessary, shortly after applying the Padlock clip due to full-thickness closure of the tissue. -
Citations
Citations to this article as recorded by- Updates on the Prevention and Management of Post-Polypectomy Bleeding in the Colon
Hisham Wehbe, Aditya Gutta, Mark A. Gromski
Gastrointestinal Endoscopy Clinics of North America.2024; 34(2): 363. CrossRef - Analysis of Reported Adverse Events Related to Over-the-Scope Clips: A MAUDE Database Analysis
Daniyal Abbas, Mohamed Abdallah, Khalid Ahmed, Abubaker O. Abdalla, Nicholas McDonald, Shifa Umar, Brian J. Hanson, Mohammad Bilal
Techniques and Innovations in Gastrointestinal Endoscopy.2023; 25(2): 106. CrossRef - Colonic diverticular bleeding: An update on pathogenesis and management
Sneha Annie Sebastian, Edzel Lorraine Co, Venkatesh Panthangi, Radha Bansal, Vaishnavi Narayanan, Shachi Paudel, Rabab Raja, Inderbir Padda, Babu P Mohan
Disease-a-Month.2023; 69(11): 101543. CrossRef - Endoscopic Recognition and Resection of Malignant Colorectal Polyps
Natalie Wilson, Moamen Gabr, Mohammad Bilal
Techniques and Innovations in Gastrointestinal Endoscopy.2023; 25(4): 385. CrossRef - Endoscopic Salvage of Gastrointestinal Anastomosis Leaks—Past, Present, and Future—A Narrated Review
Alexandra Menni, George Stavrou, Georgios Tzikos, Anne D. Shrewsbury, Katerina Kotzampassi
Gastrointestinal Disorders.2023; 5(3): 383. CrossRef - Boerhaave's syndrome: Better late than never – Delayed management using endoscopic over-the-scope clip
Arulprakash Sarangapani, TarunJ George, S Malathi
Gastroenterology, Hepatology and Endoscopy Practice.2023; 3(4): 167. CrossRef - Tratamiento endoscópico de la perforación mediante Padlock Clip®, a propósito de 2 casos
M. Reyes Busta Nistal, Lourdes del Olmo Martínez, Benito Velayos Jimenez, Luis Fernández Salazar, Miguel Durà Gil
Gastroenterología y Hepatología.2022; 45: 99. CrossRef - The application of endoscopic loop ligation in defect repair following endoscopic full-thickness resection of gastric submucosal tumors originating from the muscularis propria layer
Guoxiang Wang, Yanli Xiang, Yangde Miao, Honggang Wang, Meidong Xu, Guang Yu
Scandinavian Journal of Gastroenterology.2022; 57(1): 119. CrossRef - OTSC (Padlock Clip) as a Rescue Endoscopic Method for a Severe Post-Bariatric Complication
Luiza L. Ramos, Ravi C. Marques, Hugo G. Guedes
Obesity Surgery.2022; 32(5): 1761. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Mucosectomy device‐assisted endoscopic resection of gastric subepithelial lesions
Lian Yong Li, Bai Wen Li, Parit Mekaroonkamol, Hui Min Chen, Shan Shan Shen, Hui Luo, Sunil Dacha, Yue Xue, Sarah Cristofaro, Steven Keilin, Field Willingham, Qiang Cai
Journal of Digestive Diseases.2020; 21(4): 215. CrossRef - Another Use for Padlock Clip
Awf Mouchli, Vikas Chitnavis
Cureus.2020;[Epub] CrossRef - Successful Endoscopic Removal of Toothpick Perforating Gastric Antrum With Over-the-Scope Padlock Clip Closure
Darshan Suthar, Elisabeth H Kramer, Harshit S Khara
Cureus.2020;[Epub] CrossRef
- Updates on the Prevention and Management of Post-Polypectomy Bleeding in the Colon
- 6,128 View
- 187 Download
- 13 Web of Science
- 13 Crossref

Focused Review Series: Endoscopic Hemostasis: An Overview of Principles and Recent Applications
- Endoscopic Therapy and Radiologic Intervention of Acute Gastroesophageal Variceal Bleeding
- Jeong Eun Song, Byung Seok Kim
- Clin Endosc 2019;52(5):407-415. Published online September 30, 2019
- DOI: https://doi.org/10.5946/ce.2019.178

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Acute gastroesophageal variceal hemorrhage is a dreaded complication in patients with liver cirrhosis. Endoscopic therapy and radiologic intervention for gastroesophageal bleeding have rapidly developed in the recent decades. Endoscopic treatment is initially performed to stop variceal hemorrhage. For the treatment of esophageal variceal bleeding, endoscopic variceal ligation (EVL) is considered the endoscopic treatment of choice. In cases of gastric variceal hemorrhage, the type of gastric varices (GVs) is important in deciding the strategy of endoscopic treatment. Endoscopic variceal obturation (EVO) is recommended for fundal variceal bleeding. For the management of gastroesophageal varix type 1 bleeding, both EVO and EVL are available treatment options; however, EVO is preferred over EVL. If endoscopic management fails to control variceal hemorrhage, radiologic interventional modalities could be considered. Transjugular intrahepatic portosystemic shunt is a good option for rescue treatment in refractory variceal bleeding. In cases of refractory hemorrhage of GVs in patients with a gastrorenal shunt, balloon-occluded retrograde transvenous obliteration could be considered as a salvage treatment.
-
Citations
Citations to this article as recorded by- Transjugular intrahepatic portosystemic shunt for esophagojejunal variceal bleeding after total gastrectomy: A case report
Sang Un Kim, Jihoon Hong
Radiology Case Reports.2024; 19(8): 3231. CrossRef - Efficacy of early re-ligation after endoscopic gastric glue injection combined with endoscopic variceal ligation in preventing rebleeding of esophagogastric varices in patients with cirrhosis
Hui-Min Liu, Zhi-Bin Gong
World Chinese Journal of Digestology.2022; 30(17): 748. CrossRef - The Role of Interventional Radiology in Esophageal Varices and Hematemesis: Review Article
Qaed Salem Alhammami, Maisa Hamad Freaj Alanazi, Shahad Khalid A Bedaiwi, Ghazir Aneed N Alruwili, Shouq Fayed Khalaf Alanazi
Archives of Pharmacy Practice.2022; 13(4): 7. CrossRef - Comprehensive treatment of patients with gastric variceal bleeding
S.M. Chooklin, S.S. Chuklin
EMERGENCY MEDICINE.2022; 18(8): 14. CrossRef
- Transjugular intrahepatic portosystemic shunt for esophagojejunal variceal bleeding after total gastrectomy: A case report
- 8,252 View
- 320 Download
- 1 Web of Science
- 4 Crossref

Review
- Atraumatic Splenic Hemorrhage as a Rare Complication of Pancreatitis: Case Report and Literature Review
- Deepanshu Jain, Byeori Lee, Michael Rajala
- Clin Endosc 2020;53(3):311-320. Published online July 24, 2019
- DOI: https://doi.org/10.5946/ce.2019.087
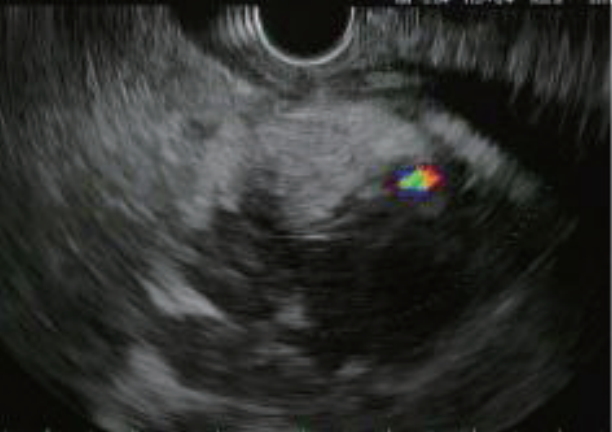
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Splenic hemorrhage (hematoma and rupture) is a rare complication of pancreatitis. In this article, we present a rare case of spontaneous splenic rupture as a complication of acute pancreatitis. A literature review was also completed to describe the patient characteristics, associated pancreatitis etiology, clinical presentations, risk factors, diagnostic and treatment modalities, and outcomes.
-
Citations
Citations to this article as recorded by- Akute nekrotisierende Pankreatitis mit hämorrhagischem Schock bei sekundärer Milzruptur: Ein Fallbericht und Literaturübersicht
Leon Kaiser, Golo Petzold, Ali Seif Amir Hosseini, Volker Ellenrieder, Albrecht Neesse, Christoph Ammer-Herrmenau
Zeitschrift für Gastroenterologie.2023; 61(11): 1494. CrossRef - Association of Atraumatic Splenic Rupture and Acute Pancreatitis: Case Report with Literature Review
Lidija Ljubicic, Vibor Sesa, Silvija Cukovic-Cavka, Ivan Romic, Igor Petrovic, Neil Donald Merrett
Case Reports in Surgery.2022; 2022: 1. CrossRef - Acute pancreatitis with necrosis of the transverse colon and the great gastric curvature
Pietro CUMBO, Gabriella CAVALOT, Annalisa ROMANO, Marco ALLASIA, Carlo PALENZONA, Francesco POTENTE, Mariangela AZZELLINO, Luca B. LO PICCOLO
Chirurgia.2022;[Epub] CrossRef - Chronic lymphocytic leukemia, a rare cause of spontaneous rupture of the spleen
Madani Ayoub, Mohamed Yassine Mabrouk, Hajar Abdelouahab, Imane Kamaoui, Miry Achraf, Siham Hamaz, Khalid Serraj, Jabi Rachid, Bouziane Mohamed
International Journal of Surgery Case Reports.2022; 96: 107315. CrossRef - Complications of chronic pancreatitis prior to and following surgical treatment: A proposal for classification
Marko Murruste, Ülle Kirsimägi, Karri Kase, Tatjana Veršinina, Peep Talving, Urmas Lepner
World Journal of Clinical Cases.2022; 10(22): 7808. CrossRef - Case report of a spontaneous splenic rupture in a patient with chronic lymphocytic leukaemia treated by arterial splenic embolization
Héloïse Tessely, Stéphane Journe, Raphaël Katz, Jean Lemaitre
International Journal of Surgery Case Reports.2021; 80: 105607. CrossRef - Atraumatic splenic rupture in patient with acute pancreatitis
Roshini Nadaraja, Zarif Yahya, Krinal Mori, Ahmad Aly
BMJ Case Reports.2021; 14(3): e238559. CrossRef - Splenic injury following endoscopic drainage of a large pancreatic pseudocyst: a case report
Krittin J. Supapannachart, Christopher R. Funk, Lauren M. Gensler, Matthew P. Butters
Journal of Medical Case Reports.2021;[Epub] CrossRef - A Rare Case of Atraumatic Splenic Rupture Due to Chronic Pancreatitis
Rita Martelo, João C Morais, Angeles Rábago, Inês C Borges, Francisco Rodrigues
Cureus.2021;[Epub] CrossRef - Splenic rupture caused by pancreatic pseudocyst successfully treated by endoscopic ultrasound-guided drainage
Naoyuki Hasegawa, Yoshimi Ito, Masamichi Yamaura, Masato Endo, Kazunori Ishige, Kuniaki Fukuda, Ichinosuke Hyodo, Yuji Mizokami
Clinical Journal of Gastroenterology.2020; 13(5): 981. CrossRef - Splenic Subcapsular Hematoma Complicating a Case of Pancreatitis
Aveek Mukherjee, Raisa Ghosh, Sugirdhana Velpari
Cureus.2020;[Epub] CrossRef
- Akute nekrotisierende Pankreatitis mit hämorrhagischem Schock bei sekundärer Milzruptur: Ein Fallbericht und Literaturübersicht
- 7,805 View
- 176 Download
- 11 Web of Science
- 11 Crossref

Focused Review Series: Endoscopic Hemostasis: An Overview of Principles and Recent Applications
- Endoscopic Hemostasis for Non-Variceal Upper Gastrointestinal Bleeding: New Frontiers
- Adam Kichler, Sunguk Jang
- Clin Endosc 2019;52(5):401-406. Published online July 16, 2019
- DOI: https://doi.org/10.5946/ce.2018.103
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Non-variceal upper gastrointestinal bleeding (NVUGIB) refers to blood loss from the gastrointestinal tract proximal to the ligament of Treitz due to lesions that are non-variceal in origin. The distinction of the bleeding source as non-variceal is important in numerous aspects, but none more so than endoscopic approaches for successful hemostasis. When a patient presents with acute overt blood loss, NVUGIB is a medical emergency, which requires immediate intervention. There have been major strides in pharmacologic and endoscopic interventions for successful induction and remission of hemostasis in the last two decades. Despite achieving tangible improvements, the burden of the disease and the consequent mortality remain high. To address endoscopic outcomes better, several new technologies have emerged and have been subsequently incorporated to the armamentarium of hemostatic tools. This study aims to provide a succinct review on novel technologies for endoscopic hemostasis.
-
Citations
Citations to this article as recorded by- Clinical characteristics of acute non-varicose upper gastrointestinal bleeding and the effect of endoscopic hemostasis
Xiao-Juan Wang, Yu-Peng Shi, Li Wang, Ya-Ni Li, Li-Juan Xu, Yue Zhang, Shuang Han
World Journal of Clinical Cases.2024; 12(9): 1597. CrossRef - Short Peptide Nanofiber Biomaterials Ameliorate Local Hemostatic Capacity of Surgical Materials and Intraoperative Hemostatic Applications in Clinics
Zehong Yang, Lihong Chen, Ji Liu, Hua Zhuang, Wei Lin, Changlong Li, Xiaojun Zhao
Advanced Materials.2023;[Epub] CrossRef - Accessory Splenic Artery Causing Massive Gastrointestinal Bleed
Priyesh Patel, Pravallika Chadalavada, Amandeep Singh, Ram Kishore Gurajala, Jean-Paul Achkar
ACG Case Reports Journal.2021; 8(3): e00550. CrossRef - Diode Laser—Can It Replace the Electrical Current Used in Endoscopic Submucosal Dissection? (with Video)
Yunho Jung, Gwang Ho Baik, Weon Jin Ko, Bong Min Ko, Seong Hwan Kim, Jin Seok Jang, Jae-Young Jang, Wan-Sik Lee, Young Kwan Cho, Sun Gyo Lim, Hee Seok Moon, In Kyung Yoo, Joo Young Cho
Clinical Endoscopy.2021; 54(4): 555. CrossRef -
In Vivo Investigation of Noncontact Rapid Photothermal Hemostasis on Venous and Arterial Bleeding
Myeongjin Kim, Van Gia Truong, Sungwon Kim, Hyejin Kim, Thomas Hasenberg, Hyun Wook Kang
IEEE Transactions on Biomedical Engineering.2021; 68(9): 2689. CrossRef - Comparison of high and low-dose epinephrine & endoclip application in peptic ulcer bleeding
Tamer Akay, Metin Leblebici
Medicine.2021; 100(52): e28480. CrossRef - Endoscopic Ultrasound-Guided Treatments for Non-Variceal Upper GI Bleeding: A Review of the Literature
Claudio Giovanni De Angelis, Pablo Cortegoso Valdivia, Stefano Rizza, Ludovica Venezia, Felice Rizzi, Marcantonio Gesualdo, Giorgio Maria Saracco, Rinaldo Pellicano
Journal of Clinical Medicine.2020; 9(3): 866. CrossRef - Preparation and Study of Hemostatic Materials Based on Chitosan and Chitin Nanofibrils
E. N. Maevskaia, E. N. Dresvyanina, A. S. Shabunin, I. P. Dobrovol’skaya, M. B. Paneyah, A. M. Fediuk, P. L. Sushchinskii, G. P. Smirnov, V. E. Yudin, E. V. Zinoviev
Nanotechnologies in Russia.2020; 15(7-8): 466. CrossRef
- Clinical characteristics of acute non-varicose upper gastrointestinal bleeding and the effect of endoscopic hemostasis
- 9,184 View
- 482 Download
- 9 Web of Science
- 8 Crossref

Case Reports
- Acquired Hemophilia A with Gastrointestinal Bleeding
- Narae Park, Jin Seok Jang, Jae Hwang Cha
- Clin Endosc 2020;53(1):90-93. Published online July 8, 2019
- DOI: https://doi.org/10.5946/ce.2019.036

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Peptic ulcer disease is the most common cause of acute gastrointestinal bleeding, followed by variceal bleeding, Mallory–Weiss syndrome, and malignancy. On the contrary, acquired hemophilia A is a very rare hemorrhagic disease, which usually manifests with musculocutaneous bleeding, caused by autoantibodies against coagulation factor VIII.
A 78-year-old man presented to the Emergency Department with melena. Dieulafoy’s lesions were observed on esophagogastroduodenoscopy, and endoscopic cauterization was performed. However, the patient complained of back pain and symptoms indicative of upper gastrointestinal bleeding. Abdominopelvic computed tomography was performed, and hematoma in the psoas muscle was detected. Antibodies against coagulation factor VIII were confirmed with a blood test, and the diagnosis of acquired hemophilia A was made. Here, we report a case of acquired hemophilia A presenting with upper gastrointestinal bleeding symptoms and present a brief review of literature. -
Citations
Citations to this article as recorded by- Variceal hemorrhage in a patient with cirrhosis and congenital hemophilia A: A therapeutic challenge
Rafael Gregorio Peña Amaya, María del Carmen Figueredo Peña
SAGE Open Medical Case Reports.2024;[Epub] CrossRef - A case of refractory bleeding from duodenal angioectasia with acquired hemophilia A
Hiroko Abe, Masahiro Saito, Kaname Uno, Tomoyuki Koike, Satoshi Ichikawa, Masashi Saito, Takeshi Kanno, Waku Hatta, Naoki Asano, Atsushi Masamune
Clinical Journal of Gastroenterology.2023; 16(3): 355. CrossRef - C-Reactive Protein: Pathophysiology, Diagnosis, False Test Results and a Novel Diagnostic Algorithm for Clinicians
Dimitra S. Mouliou
Diseases.2023; 11(4): 132. CrossRef - THE RESTRICTIVE EFFECTS OF THE COVID-19 PANDEMIC ON THE MANAGEMENT OF PLASTRON APPENDICITIS IN A KNOWN HEMOPHILIA A PATIENT
Mert Yurtsever, İrfan Arda Aykut, Beste Girgin, Berkay Aldemir, Oğuzhan Alp Öztürk, Zeliha Türkyılmaz
TURKISH MEDICAL STUDENT JOURNAL.2022; 9(3): 84. CrossRef - A rare cause of lower gastrointestinal bleeding: acquired hemophilia A
Pilar Del Pino Bellido, María Fernanda Guerra Veloz, Reyes Aparcero López
Revista Española de Enfermedades Digestivas.2021;[Epub] CrossRef - Undiagnosed Acquired Hemophilia A: Presenting as Recurrent Gastrointestinal Bleeding
Arya Mariam Roy, Aisha Siddiqui, Anand Venkata
Cureus.2020;[Epub] CrossRef
- Variceal hemorrhage in a patient with cirrhosis and congenital hemophilia A: A therapeutic challenge
- 4,962 View
- 151 Download
- 5 Web of Science
- 6 Crossref

- Massive Duodenal Bleeding after the Migration of Endovascular Coils into the Small Bowel
- Chung-Jo Choi, Hyun Lim, Dong-Suk Kim, Yong-Seol Jeong, Sang-Young Park, Jeong-Eun Kim
- Clin Endosc 2019;52(6):612-615. Published online May 20, 2019
- DOI: https://doi.org/10.5946/ce.2019.020
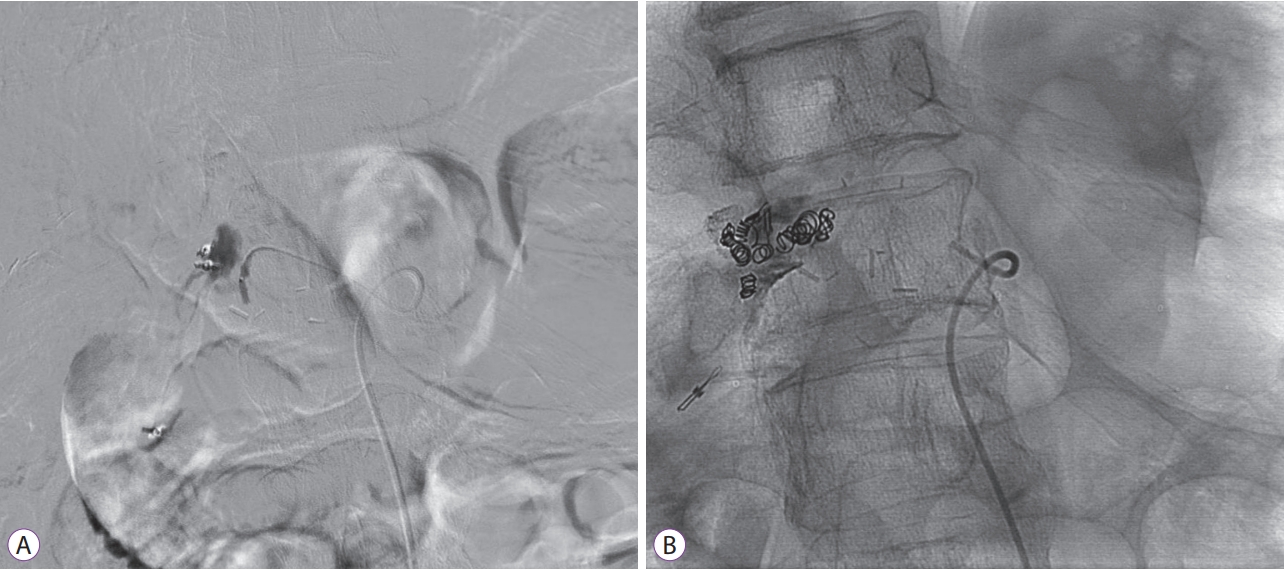
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Among gastrointestinal emergencies, acute upper gastrointestinal bleeding remains a challenging clinical problem owing to significant patient morbidity and costs involved in management. Endoscopic hemostatic therapy is the mainstay of treatment and decreases the incidence of re-bleeding, the need for surgery, morbidity, and mortality. However, in 8%–15% of patients with upper gastrointestinal bleeding, endoscopic hemostatic therapy does not successfully control bleeding. Trans-arterial coil embolization is an effective alternative treatment for endoscopic hemostatic failure; however, this procedure can induce adverse outcomes, such as non-target vessel occlusion, vessel dissection and perforation, and coil migration. Coil migration is rare but causes severe complications, such as re-bleeding and bowel ischemia. However, in most cases, coil migration is local and involves spontaneous healing without serious complications. Here, we report the case of a patient who underwent trans-arterial coil embolization of the gastroduodenal artery with the purpose of controlling massive duodenal bleeding, resulting in a fatal outcome caused by coil migration.
-
Citations
Citations to this article as recorded by- Pulsation of visible vessel or adherent clot in duodenal ulcer may indicate pseudoaneurysm: Case series
Jiayu Ju, Ziyao Cheng, Qingliang Zhu, Mingming Deng, Hailong Zhang
Medicine.2023; 102(5): e32819. CrossRef - Rare but critical: Aberrant vascular communication leading to multiorgan ischemia after prophylactic gastroduodenal artery embolization for refractory upper gastrointestinal bleeding
Muhammad Ibrahim Saeed, Amna Subhan Butt, Jahanzeb Shahid, Junaid Iqbal
Radiology Case Reports.2023; 18(11): 3926. CrossRef - Gastric Bleeding Caused by Migrated Coil: A Rare Complication of Splenic Artery Coil Embolization
Tian Li, Bayan Alsuleiman, Manuel Martinez
Gastro Hep Advances.2022; 1(1): 67. CrossRef - Intraluminal Endovascular Coil Migration: A Rare Complication Post-Embolization of the Gastroduodenal Artery for a Previously Bleeding Duodenal Ulcer
Yassin Naga, Mahendran Jayaraj, Yousif Elmofti, Annie Hong, Gordon Ohning
Cureus.2021;[Epub] CrossRef - Management of Gastroduodenal Artery Pseudoaneurysm Rupture With Duodenal Ulcer Complicated by Coil Migration
Dennis Chang, Purvi Patel, Seth Persky, Joseph Ng, Alan Kaell
ACG Case Reports Journal.2020; 7(4): e00347. CrossRef - Persisting bleeding from the duodenal ulcer in patients with occlusion of the celiac trunk: a case report
Andrzej Żyluk, Samir Zeair, Janusz Kordowski, Ewa Gabrysz-Trybek
Polish Journal of Surgery.2020; 93(SUPLEMENT): 54. CrossRef
- Pulsation of visible vessel or adherent clot in duodenal ulcer may indicate pseudoaneurysm: Case series
- 6,295 View
- 113 Download
- 5 Web of Science
- 6 Crossref

Original Article
- Endoscopy Timing in Patients with Acute Upper Gastrointestinal Bleeding
- Gonçalo Alexandrino, Tiago Dias Domingues, Rita Carvalho, Mariana Nuno Costa, Luís Carvalho Lourenço, Jorge Reis
- Clin Endosc 2019;52(1):47-52. Published online October 5, 2018
- DOI: https://doi.org/10.5946/ce.2018.093
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The role of very early (≤12 hours) endoscopy in nonvariceal upper gastrointestinal bleeding is controversial. We aimed to compare results of very early and early (12–24 hours) endoscopy in patients with upper gastrointestinal bleeding demonstrating low-risk versus high-risk features and nonvariceal versus variceal bleeding.
Methods
This retrospective study included patients with nonvariceal and variceal upper gastrointestinal bleeding. The primary outcome was a composite of inpatient death, rebleeding, or need for surgery or intensive care unit admission. Endoscopy timing was defined as very early and early. We performed the analysis in two subgroups: (1) high-risk vs. low-risk patients and (2) variceal vs. nonvariceal bleeding.
Results
A total of 102 patients were included, of whom 59.8% underwent urgent endoscopy. Patients who underwent very early endoscopy received endoscopic therapy more frequently (p=0.001), but there was no improvement in other clinical outcomes. Furthermore, patients at low risk and with nonvariceal bleeding who underwent very early endoscopy had a higher risk of the composite outcome.
Conclusions
Very early endoscopy does not seem to be associated with improved clinical outcomes and may lead to poorer outcomes in specific populations with upper gastrointestinal bleeding. The actual benefit of very early endoscopy remains controversial and should be further clarified. -
Citations
Citations to this article as recorded by- Acute Nonvariceal Upper Gastrointestinal Bleeding in Patients Using Anticoagulants: Does the Timing of Endoscopy Affect Outcomes?
Tiago Lima Capela, Vítor Macedo Silva, Marta Freitas, Tiago Cúrdia Gonçalves, José Cotter
Digestive Diseases and Sciences.2024; 69(2): 570. CrossRef - Endoscopia urgente frente a endoscopia precoz: ¿tiene algún papel la endoscopia urgente en la hemorragia digestiva alta aguda no varicosa?
Javier Lucas Ramos, Jorge Yebra Carmona, Irene Andaluz García, Marta Cuadros Martínez, Patricia Mayor Delgado, Maria Ángeles Ruiz Ramírez, Joaquín Poza Cordón, Cristina Suárez Ferrer, Ana Delgado Suárez, Nerea Gonzalo Bada, Consuelo Froilán Torres
Gastroenterología y Hepatología.2023; 46(8): 612. CrossRef - Urgent endoscopy versus early endoscopy: Does urgent endoscopy play a role in acute non-variceal upper gastrointestinal bleeding?
Javier Lucas Ramos, Jorge Yebra Carmona, Irene Andaluz García, Marta Cuadros Martínez, Patricia Mayor Delgado, Maria Ángeles Ruiz Ramírez, Joaquín Poza Cordón, Cristina Suárez Ferrer, Ana Delgado Suárez, Nerea Gonzalo Bada, Consuelo Froilán Torres
Gastroenterología y Hepatología (English Edition).2023; 46(8): 612. CrossRef - Clinical outcome of early endoscopy in patients with acute upper gastrointestinal bleeding in Alexandria emergency department
Mina Montasser, Wael Nabil Abdel Salam, Amany Elbanna, Dina Magdy, Ahmed A. Sabry
Egyptian Journal of Anaesthesia.2023; 39(1): 840. CrossRef - Az akut gastroduodenalis fekélyvérzés gyógyszeres és endoszkópos kezelésének újabb szempontjai
István Rácz
Orvosi Hetilap.2023; 164(23): 883. CrossRef - How Can Patient’s Risk Dictate the Timing of Endoscopy in Upper Gastrointestinal Bleeding?
Marta Freitas, Vítor Macedo Silva, Tiago Cúrdia Gonçalves, Carla Marinho, José Cotter
GE - Portuguese Journal of Gastroenterology.2022; 29(2): 96. CrossRef - Impacto del tiempo a la endoscopia digestiva en pacientes con hemorragia de tubo digestivo alto no variceal: una revisión sistemática y metaanálisis
H.G. Bilder, C. Soccini, J.S. Lasa, I. Zubiaurre
Revista de Gastroenterología de México.2022; 87(3): 320. CrossRef - Impact of time to esophagogastroduodenoscopy in patients with nonvariceal upper gastrointestinal bleeding: A systematic review and meta-analysis
H.G. Bilder, C. Soccini, J.S. Lasa, I. Zubiaurre
Revista de Gastroenterología de México (English Edition).2022; 87(3): 320. CrossRef - Urgent Endoscopy in Nonvariceal Upper Gastrointestinal Hemorrhage: A Retrospective Analysis
Jia-lun Guan, Ying-ying Han, Dan Fang, Mu-ru Wang, Ge Wang, De-an Tian, Pei-yuan Li
Current Medical Science.2022; 42(4): 856. CrossRef - Acute upper gastrointestinal bleeding: a clinical review
Katherine Haggan, Gerri Mortimore
Gastrointestinal Nursing.2022; 20(5): 20. CrossRef - Endoscopic management of acute oesophageal variceal bleeding within 12 hours of admission is superior to 12–24 hours
N Mousa, A Abdel-Razik, T Sheta, A G Deiab, A Habib, M Diasty, A Eldesoky, A Taha, E Mousa, A Yassen, A Fathy, A Elgamal
British Journal of Biomedical Science.2021; 78(3): 130. CrossRef - Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline – Update 2021
Ian M. Gralnek, Adrian J. Stanley, A. John Morris, Marine Camus, James Lau, Angel Lanas, Stig B. Laursen, Franco Radaelli, Ioannis S. Papanikolaou, Tiago Cúrdia Gonçalves, Mario Dinis-Ribeiro, Halim Awadie, Georg Braun, Nicolette de Groot, Marianne Udd, A
Endoscopy.2021; 53(03): 300. CrossRef - Optimal timing of endoscopy for acute upper gastrointestinal bleeding: a systematic review and meta-analysis
Elettra Merola, Andrea Michielan, Giovanni de Pretis
Internal and Emergency Medicine.2021; 16(5): 1331. CrossRef - Which scoring system should be used for non‐variceal upper gastrointestinal bleeding? Old or new?
Hong Jae Jeon, Hee Seok Moon, In Sun Kwon, Sun Hyung Kang, Jae Kyu Sung, Hyun Yong Jeong
Journal of Gastroenterology and Hepatology.2021; 36(10): 2819. CrossRef - Biopsy in emergency gastroscopy does not increase the risk of rebleeding in patients with Forrest I acute nonvariceal upper gastrointestinal bleeding combined with suspected malignant gastric ulcer: a multicenter retrospective cohort study
Quchuan Zhao, Tianyu Chi
BMC Gastroenterology.2021;[Epub] CrossRef - The mortality rate among patients with acute upper GI bleeding (with/without EGD) at Aleppo University Hospital: A retrospective study
Ziad Aljarad, Bashir Badawi Mobayed
Annals of Medicine and Surgery.2021; 71: 102958. CrossRef - Validation of a new risk score system for non-variceal upper gastrointestinal bleeding
Min Seong Kim, Hee Seok Moon, In Sun Kwon, Jae Ho Park, Ju Seok Kim, Sun Hyung Kang, Jae Kyu Sung, Eaum Seok Lee, Seok Hyun Kim, Byung Seok Lee, Hyun Yong Jeong
BMC Gastroenterology.2020;[Epub] CrossRef - Interventional Algorithm in Gastrointestinal Bleeding—An Expert Consensus Multimodal Approach Based on a Multidisciplinary Team
Anabela Rodrigues, Alexandre Carrilho, Nuno Almeida, Cilénia Baldaia, Ângela Alves, Manuela Gomes, Luciana Gonçalves, António Robalo Nunes, Carla Leal Pereira, Mário Jorge Silva, José Aguiar, Rosário Orfão, Pedro Duarte, Rui Tato Marinho
Clinical and Applied Thrombosis/Hemostasis.2020; 26: 107602962093194. CrossRef - Photoacoustic endoscopy: A progress review
Heng Guo, Ying Li, Weizhi Qi, Lei Xi
Journal of Biophotonics.2020;[Epub] CrossRef - Upper gastrointestinal bleeding: Is only an injection of epinephrine sufficient? Success rates by Forrest classification
Ahmet Surek, Eyup Gemici, Abdussamet Bozkurt, Mehmet Karabulut
Sanamed.2020; 15(3): 309. CrossRef - When Should We Perform Endoscopy for Patients with Upper Gastrointestinal Bleeding?
Kyoungwon Jung, Moo In Park
Clinical Endoscopy.2019; 52(1): 1. CrossRef - Current Controversies Concerning Capsule Endoscopy
David R. Cave, Shahrad Hakimian, Krunal Patel
Digestive Diseases and Sciences.2019; 64(11): 3040. CrossRef
- Acute Nonvariceal Upper Gastrointestinal Bleeding in Patients Using Anticoagulants: Does the Timing of Endoscopy Affect Outcomes?
- 7,754 View
- 467 Download
- 19 Web of Science
- 22 Crossref

Case Reports
- Gastric Ulceration and Bleeding with Hemodynamic Instability Caused by an Intragastric Balloon for Weight Loss
- Larrite Reed, Hawa Edriss, Kenneth Nugent
- Clin Endosc 2018;51(6):584-586. Published online June 1, 2018
- DOI: https://doi.org/10.5946/ce.2018.038
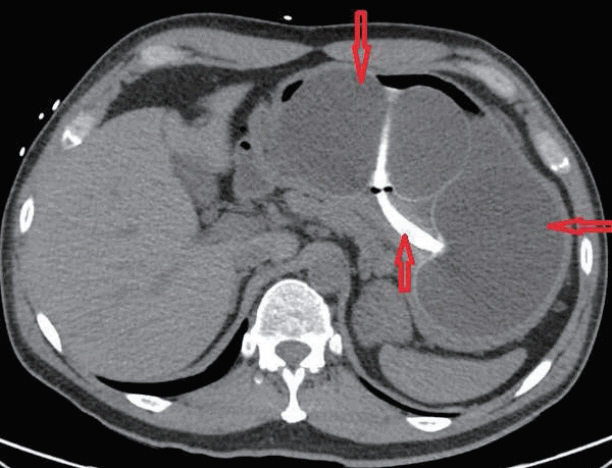
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Obesity in the United States is a medical crisis with many people attempting to lose weight with caloric restriction. Some patients choose minimally invasive weight loss solutions, such as intragastric balloon systems. These balloon systems were approved by the Federal Drug Administration (FDA) in 2015–2016 and have been considered safe, with minimal side effects. We report a patient with a two-day history of melena, abdominal pain, hypotension, and syncope which developed five months after placement of an intragastric balloon. Esophagogastroduodenoscopy with balloon removal revealed a small 8-mm gastric ulcer in the incisura. This gastric ulcer probably developed secondary to mechanical compression of the stomach mucosa by the gastric balloon which contained 900 mL of saline. The FDA is now investigating five deaths since 2016 associated with these second-generation balloons. Clinicians should be aware of these complications when evaluating patients with gastrointestinal complications, such as bleeding.
-
Citations
Citations to this article as recorded by- Prediction Factors of Early Postoperative Bleeding after Bariatric Surgery
Mahdieh Golzarand, Karamollah Toolabi, Reza Parsaei
Obesity Surgery.2022; 32(7): 1. CrossRef - Clinical follow-up on weight loss, glycemic control, and safety aspects of 24 months of duodenal-jejunal bypass liner implantation
B. Betzel, M. I. Cooiman, E. O. Aarts, I. M. C. Janssen, P. J. Wahab, M. J. M. Groenen, J. P. H. Drenth, F. J. Berends
Surgical Endoscopy.2020; 34(1): 209. CrossRef - Hidden dangers and updated labels on gastric balloons
Sindhura Kolli, Andrew Ofosu, Harini Gurram, Simcha Weissman, Paul Khoi Dang‐Ho, Tej I. Mehta, Hailie Gill, Krishna C. Gurram
Clinical Case Reports.2020; 8(11): 2116. CrossRef
- Prediction Factors of Early Postoperative Bleeding after Bariatric Surgery
- 4,520 View
- 99 Download
- 3 Web of Science
- 3 Crossref

- Tranexamic Acid-Induced Acute Renal Cortical Necrosis in Post-Endoscopic Papillectomy Bleeding
- Doo Hyun Ko, Tae Hyung Kim, Jong Wook Kim, Ja Joong Gu, Baek Hyun Yoon, Ji Hong Oh, Seung Goun Hong
- Clin Endosc 2017;50(6):609-613. Published online August 9, 2017
- DOI: https://doi.org/10.5946/ce.2017.021
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Acute renal failure can be the result of acute renal cortical necrosis (RCN), which commonly occurs from complications occurring during pregnancy. RCN is rarely caused by medications, although tranexamic acid, which is used in patients with acute bleeding for its antifibrinolytic effects, reportedly causes acute RCN in rare cases. An 82-year-old woman experienced gastrointestinal bleeding after endoscopic papillectomy of an ampullary adenoma. The bleeding was controlled with tranexamic acid administration; however, 4 days later, her urine volume decreased and she developed pulmonary edema and dyspnea. Serum creatinine levels increased from 0.8 to 3.9 mg/dL and dialysis was performed. Abdominal pelvic computed tomography with contrast enhancement revealed bilateral RCN with no renal cortex enhancement. Renal dysfunction and oliguria persisted and hemodialysis was continued. Clinicians must be aware that acute RCN can occur after tranexamic acid administration to control bleeding.
-
Citations
Citations to this article as recorded by- The safety of tranexamic acid administration in total knee arthroplasty: a population‐based study from Taiwan
L.‐I. Hsu, H.‐W. Hsu, J.‐W. Chen, S.‐T. Wei, S.‐M. Hou
Anaesthesia.2023; 78(3): 303. CrossRef - Tranexamic acid in total knee arthroplasty
C. R. Bailey
Anaesthesia.2023; 78(3): 275. CrossRef - Intraoperative use of tranexamic acid to reduce blood loss during cytoreductive surgery for advanced ovarian cancer: A randomized controlled clinical trial
Xijun Yang, Mao Chai, Lingfang Xia, Zhiyong He, Xiaohua Wu, Jun Zhang
Acta Obstetricia et Gynecologica Scandinavica.2023; 102(7): 950. CrossRef - Safe and Effective Blood Preservation Through Acute Normovolemic Hemodilution and Low-Dose Tranexamic Acid in Open Partial Hepatectomy
Jian Yang, Jing Zhang, Jiayan Luo, Jie Ouyang, Qicai Qu, Qitao Wang, Yongyu Si
Journal of Pain Research.2023; Volume 16: 3905. CrossRef - Systematic review of hematuria and acute renal failure with tranexamic acid
Stephanie G. Lee, John Fralick, Christopher J. D. Wallis, Monica Boctor, Michelle Sholzberg, Michael Fralick
European Journal of Haematology.2022; 108(6): 510. CrossRef - Safety of Tranexamic Acid in Hip and Knee Arthroplasty in High-risk Patients
Jashvant Poeran, Jimmy J. Chan, Nicole Zubizarreta, Madhu Mazumdar, Leesa M. Galatz, Calin S. Moucha
Anesthesiology.2021; 135(1): 57. CrossRef - N-methylamphetamine (“Crystal Meth”)−Associated Acute Renal Cortical Necrosis
Anu Gupta, Michael Kuperman, Silvi Shah
Kidney International Reports.2018; 3(6): 1473. CrossRef
- The safety of tranexamic acid administration in total knee arthroplasty: a population‐based study from Taiwan
- 6,999 View
- 186 Download
- 8 Web of Science
- 7 Crossref

-
Congenital Jejunal Diverticular Bleeding in a Young Adult

- Ji-Yung Lee, Jae-Young Jang, Min-Je Kim, Tae-In Lee, Jung-Wook Kim, Young-Woon Chang
- Clin Endosc 2017;50(5):495-499. Published online June 14, 2017
- DOI: https://doi.org/10.5946/ce.2016.154
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Diverticular bleeding of the small bowel is rare and occurs primarily in adults aged more than 60 years. In younger adults, Meckel’s diverticulum, a true diverticulum that congenitally occurs in the distal ileum, is the most common cause of diverticular bleeding of the small bowel. Unlike Meckel’s diverticula, other kinds of small bowel diverticula are not congenital and their incidence is related to age. Furthermore, congenital true diverticular bleeding of the jejunum in adults is very rare. We report the case of a 24-year-old man with subepithelial tumor-like lesion accompanied with obscure overt gastrointestinal bleeding (OOGIB). This lesion was initially suspected to be a subepithelial tumor based on radiologic tests and capsule endoscopy. He was finally diagnosed with a congenital true diverticulum in the jejunum with the appearance of a Meckel’s diverticulum after surgical resection.
-
Citations
Citations to this article as recorded by- Diverticular hemorrhage of terminal ileum successfully treated by ultra-selective transcatheter arterial embolization using triaxial system: a case report
Yuki Yaginuma, Kenichi Utano, Yuka Utano, Daiki Nemoto, Masato Aizawa, Hajime Matsuida, Noriyuki Isohata, Shungo Endo, Kazutomo Togashi
Clinical Journal of Gastroenterology.2021; 14(2): 517. CrossRef - Small bowel bleeding
Stefania Chetcuti Zammit, Reena Sidhu
Current Opinion in Gastroenterology.2018; 34(3): 165. CrossRef - Diagnosis of Meckel’s Diverticulum Using Colon Capsule Endoscopy for Small Bowel Investigation
Lidia Ciobanu, Oliviu Pascu, Marcel Tanțău
Clinical Endoscopy.2018; 51(4): 395. CrossRef
- Diverticular hemorrhage of terminal ileum successfully treated by ultra-selective transcatheter arterial embolization using triaxial system: a case report
- 6,705 View
- 121 Download
- 4 Web of Science
- 3 Crossref

- Combined Endoscopic and Surgical Treatment of Severe Gastrointestinal Bleeding in a Patient with Heart Assist Device under Therapeutic Anticoagulation
- Edris Wedi, Mohamed Bounnah, Riccardo Memeo, Carlo Jung
- Clin Endosc 2017;50(6):598-601. Published online June 1, 2017
- DOI: https://doi.org/10.5946/ce.2017.024
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Gastrointestinal (GI) bleeding is a common complication after heart assist device placement. Reasons for bleeding are multifactorial. Endoscopic therapy is the treatment of choice, whereas invasive procedures are avoided in these critically ill patients. We present the case of a 65-year-old male patient experiencing severe GI bleeding after left ventricular assist device (LVAD) and right ventricular assist device (RVAD) placement with therapeutic anticoagulation. Endoscopically, multiple gastric bleeding sources were found but could not be treated effectively due to a large blood clot. A combined endoscopic and surgical treatment was initiated, including gastrotomy for blood clot removal, surgical transgastric suturing, endoscopic over-the-scope clip (OTSC) placement and hemospray application. Postoperative endoscopic visualization showed effective bleeding control. The patient unfortunately died due to causes unrelated to the treatment. This case shows that a minimal invasive combination of endoscopic and surgical techniques can be an alternative treatment for severe upper GI bleeding in critically ill and anticoagulated patients.
- 5,927 View
- 167 Download

Original Articles
- Colonic Postpolypectomy Bleeding Is Related to Polyp Size and Heparin Use
- Flavia Pigò, Helga Bertani, Mauro Manno, Vincenzo Giorgio Mirante, Angelo Caruso, Santi Mangiafico, Raffaele Manta, Anna Maria Rebecchi, Rita Luisa Conigliaro
- Clin Endosc 2017;50(3):287-292. Published online February 9, 2017
- DOI: https://doi.org/10.5946/ce.2016.126
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: We studied factors influencing colon postpolypectomy bleeding (PPB), with a focus on antithrombotic and anticoagulation therapy.
Methods
We conducted a retrospective case-control study of all patients who underwent polypectomy at our tertiary referral center in Italy between 2007 and 2014. Polyp characteristics (number of polyps removed per patient, size, morphology, location, resection technique, prophylactic hemostasis methods) and patient characteristics (age, sex, comorbidities, medication) were analyzed.
Results
The case and control groups included 118 and 539 patients, respectively. The two groups differed in the frequency of comorbidities (69% vs. 40%, p=0.001), polyps removed (27% vs. 18%, p=0.02), and use of heparin therapy (23% vs. 1%, p<0.001). A total of 279 polyps in the case group and 966 in the control group were nonpedunculated (69% vs. 81%, p=0.01) and measured ≥10 mm (78% vs. 32%, p=0.001). Multivariate analysis showed that polyps ≥10 mm (odds ratio [OR], 6.1; 95% confidence interval [CI], 2.3–15.5), administration of heparin (OR, 16.5; 95% CI, 6.2–44), comorbidity (OR, 2.3; 95% CI, 1.4–3.9), and presence of ≥2 risk factors (OR, 3.2; 95% CI, 1.7–6.0) were associated with PPB.
Conclusions
The incidence of PPB increases with polyp size ≥10 mm, heparin use, comorbidity, and presence of ≥2 risk factors. -
Citations
Citations to this article as recorded by- Risk factors for post-polypectomy bleeding in patients with end-stage renal disease undergoing colonoscopic polypectomy
Jung Hyun Ji, Hyun Woo Kim, Jihye Park, Soo Jung Park, Jae Hee Cheon, Tae Il Kim, Jae Jun Park
Surgical Endoscopy.2023;[Epub] CrossRef - Polypectomy for Diminutive and Small Colorectal Polyps
Melissa Zarandi-Nowroozi, Roupen Djinbachian, Daniel von Renteln
Gastrointestinal Endoscopy Clinics of North America.2022; 32(2): 241. CrossRef - Effects of antithrombotic agents on post-operative bleeding after endoscopic resection of gastrointestinal neoplasms and polyps: A systematic review and meta-analysis
Bing-Jie Xiang, Yu-Hong Huang, Min Jiang, Cong Dai
World Journal of Meta-Analysis.2020; 8(5): 410. CrossRef - Effects of antithrombotic agents on post-operative bleeding after endoscopic resection of gastrointestinal neoplasms and polyps: A systematic review and meta-analysis
Bing-Jie Xiang, Yu-Hong Huang, Min Jiang, Cong Dai
World Journal of Meta-Analysis.2020; 8(5): 411. CrossRef - Post-polypectomy Visible Vessel
Matthew Woo, Robert Bechara
Journal of the Canadian Association of Gastroenterology.2018; 1(2): 51. CrossRef - Colorectal cancer screening program using FIT: quality of colonoscopy varies according to hospital type
Isabel Portillo, Isabel Idigoras, Isabel Bilbao, Eunate Arana-Arri, María José Fernández-Landa, Jose Luis Hurtado, Cristina Sarasaqueta, Luis Bujanda
Endoscopy International Open.2018; 06(09): E1149. CrossRef - Prediction and Prevention of Postpolypectomy Bleeding: Necessity of a Different Approach for Patients Using Antithrombotic Agents
Duk Hwan Kim
Clinical Endoscopy.2017; 50(3): 217. CrossRef
- Risk factors for post-polypectomy bleeding in patients with end-stage renal disease undergoing colonoscopic polypectomy
- 7,414 View
- 202 Download
- 5 Web of Science
- 7 Crossref

- Double-Balloon Endoscopy in Overt and Occult Small Bowel Bleeding: Results, Complications, and Correlation with Prior Videocapsule Endoscopy in a Tertiary Referral Center
- Carlijn Hermans, Arnold Stronkhorst, Annemarie Tjhie-Wensing, Jan Kamphuis, Bas van Balkom, Rob Dahlmans, Lennard Gilissen
- Clin Endosc 2017;50(1):69-75. Published online January 12, 2017
- DOI: https://doi.org/10.5946/ce.2016.079
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Videocapsule endoscopy (VCE) and double-balloon endoscopy (DBE) allow deep exploration in patients with suspected small bowel pathology. VCE is often performed as an initial small bowel examination to explore whether an intervention by DBE is indicated and to determine insertion route. The study aim was to evaluate the correlation between DBE and VCE in patients with obscure or overt bleeding or anemia, as well as intervention frequency, and complications.
Methods
Retrospective observational study.
Results
DBE procedures (n=205) showed small bowel lesions in 64% cases. Antegrade DBE showed positive results in 79% cases, mostly angiodysplasias (63%). Retrograde DBE showed positive results in 22% cases. An intervention was performed in 64% of DBE procedures. The major complication rate was 0.5%, which was one case of perforation. Pancreatitis did not occur. The overall diagnostic agreement was 66% among the 134 DBEs with preceded VCE.
Conclusions
In cases of overt or occult bleeding or anemia, DBE was positive in 64%, with only a few complications. Positive correlation was 66% among initially performed VCEs and DBEs. Owing to the time-consuming and invasive character of DBE, performing VCE before DBE might still be clinically relevant. -
Citations
Citations to this article as recorded by- Early double-balloon enteroscopy was not related to better clinical outcomes in patients with suspected overt small bowel bleeding
Yong-Cheng Ye, Kuan-Yi Sung, Tien-En Chang, Pei-Shan Wu, Yen-Po Wang, Jiing-Chyuan Luo, Ming-Chih Hou, Ching-Liang Lu
Journal of the Chinese Medical Association.2024; 87(4): 377. CrossRef - Video capsule endoscopy versus computed tomography enterography in assessing suspected small bowel bleeding: a systematic review and diagnostic test accuracy meta-analysis
Mohammad Yaghoobi, Julie Tan, Yousef Th. A. Th. A. Alshammari, Katie Scandrett, Khashayar Mofrad, Yemisi Takwoingi
European Journal of Gastroenterology & Hepatology.2023; 35(11): 1253. CrossRef - Clinicopathological Features of Small Bowel Tumors Diagnosed by Video Capsule Endoscopy and Balloon-Assisted Enteroscopy: A Single Center Experience
Ah Young Yoo, Beom Jae Lee, Won Shik Kim, Seong Min Kim, Seung Han Kim, Moon Kyung Joo, Hyo Jung Kim, Jong-Jae Park
Clinical Endoscopy.2021; 54(1): 85. CrossRef - Revising the European Society of Gastrointestinal Endoscopy (ESGE) research priorities: a research progress update
Pradeep Bhandari, Gaius Longcroft-Wheaton, Diogo Libanio, Pedro Pimentel-Nunes, Eduardo Albeniz, Mathieu Pioche, Reena Sidhu, Cristiano Spada, Andrea Anderloni, Alessandro Repici, Rehan Haidry, Marc Barthet, Helmut Neumann, Giulio Antonelli, Alberto Testo
Endoscopy.2021; 53(05): 535. CrossRef - The impact of reader fatigue on the accuracy of capsule endoscopy interpretation
Sabina Beg, Tim Card, Reena Sidhu, Ewa Wronska, Krish Ragunath, Hey-Long Ching, Anastasios Koulaouzidis, Diana Yung, Simon Panter, Mark Mcalindon, Matthew Johnson, Arun Kurup, Anthony Shonde, Miliedis San-Juan Acosta, Stefano Sansone, Ebby Simmon, Victori
Digestive and Liver Disease.2021; 53(8): 1028. CrossRef - Small bowel enteroscopy � A joint clinical guideline by the Spanish and Portuguese small-bowel study groups
Enrique Pérez-Cuadrado Robles, Rolando Pinho, Begoña González-Suárez, Susana M�o-de-Ferro, Cristina Chagas, Pilar Esteban Delgado, Cristina Carretero, Pedro Figueiredo, Bruno Rosa, Javier García-Lledó, Óscar Nogales, Ana Ponte, Patrícia Andrade, José Fran
Revista Española de Enfermedades Digestivas.2020;[Epub] CrossRef - Predictors for finding lesions in the small bowel by enteroscopy after a positive capsule endoscopy
Juan Manuel Blancas-Valencia, Gerardo Blanco Velasco, Luis Fernando García Contreras, Omar Michel Solórzano-Pineda, Óscar Víctor Hernández-Mondragón
Revista Española de Enfermedades Digestivas.2020;[Epub] CrossRef - Small Bowel Enteroscopy – A Joint Clinical Guideline from the Spanish and Portuguese Small Bowel Study Groups
Enrique Pérez-Cuadrado-Robles, Rolando Pinho, Begoña Gonzalez, Susana Mão de Ferro, Cristina Chagas, Pilar Esteban Delgado, Cristina Carretero, Pedro Figueiredo, Bruno Rosa, Javier García Lledó, Óscar Nogales, Ana Ponte, Patrícia Andrade, Jose Francisco J
GE - Portuguese Journal of Gastroenterology.2020; 27(5): 324. CrossRef - Endoscopic ultrasonography and small‐bowel endoscopy: Present and future
Gian Eugenio Tontini, Guido Manfredi, Stefania Orlando, Helmut Neumann, Maurizio Vecchi, Elisabetta Buscarini, Luca Elli
Digestive Endoscopy.2019; 31(6): 627. CrossRef - Video capsule endoscopy vs double-balloon enteroscopy in the diagnosis of small bowel bleeding: A systematic review and meta-analysis
Hélcio Pedrosa Brito, Igor Braga Ribeiro, Diogo Turiani Hourneaux de Moura, Wanderley Marques Bernardo, Dalton Marques Chaves, Rogério Kuga, Ethan Dwane Maahs, Robson Kiyoshi Ishida, Eduardo Turiani Hourneaux de Moura, Eduardo Guimarães Hourneaux de Moura
World Journal of Gastrointestinal Endoscopy.2018; 10(12): 400. CrossRef - What is the Role of Double-Balloon Endoscopy in Patients Presenting with Obscure Gastrointestinal Bleeding?
Jung Ho Kim, Kwang An Kwon
Clinical Endoscopy.2017; 50(1): 8. CrossRef
- Early double-balloon enteroscopy was not related to better clinical outcomes in patients with suspected overt small bowel bleeding
- 7,150 View
- 127 Download
- 11 Web of Science
- 11 Crossref

- Endoscopic Management of Gastrointestinal Leaks and Bleeding with the Over-the-Scope Clip: A Prospective Study
- Mahesh Kumar Goenka, Vijay Kumar Rai, Usha Goenka, Indrajit Kumar Tiwary
- Clin Endosc 2017;50(1):58-63. Published online October 31, 2016
- DOI: https://doi.org/10.5946/ce.2016.028
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The over-the-scope clip (OTSC) is a device used for endoscopic closure of perforations, leaks and fistulas, and for endoscopic hemostasis. To evaluate the clinical effectiveness and safety of OTSC.
Methods
Between October 2013 and November 2015, 12 patients underwent OTSC placement by an experienced endoscopist. OTSC was used for the closure of gastrointestinal (GI) leaks and fistula in six patients, three of which were iatrogenic (esophageal, gastric, and duodenal) and three of which were inflammatory. In six patients, OTSC was used for hemostasis of non-variceal upper GI bleeding. Endoscopic tattooing using India ink was used to assist the accurate placement of the clip.
Results
All subjects except one with a colonic defect experienced immediate technical success as well as long-term clinical success, during a mean follow-up of 6 weeks. Only one clip was required to close each of the GI defects and to achieve hemostasis in all patients. There were no misfirings or complications of clips. The procedure was well tolerated, and patients were hospitalized for an average of 8 days (range, 3 to 10). Antiplatelet therapy was continued in patients with GI bleeding.
Conclusions
In our experience, OTSC was safe and effective for the closure of GI defect and to achieve hemostasis of non-variceal GI bleeding. -
Citations
Citations to this article as recorded by- Endoscopic self-expandable metal stent versus endoscopy vacuum therapy for traumatic esophageal perforations: a retrospective cohort study
Alessandrino Terceiro de Oliveira, Márcio Alencar Barreira, José Wilson da Cunha Parente Júnior, José Ruver Lima Herculano Junior, Jeany Borges e Silva Ribeiro, Orleancio Gomes Ripardo de Azevedo, Paulo Roberto Cavalcante de Vasconcelos
Surgical Endoscopy.2024; 38(4): 2142. CrossRef - Meta‐analysis: Over‐the‐scope clips in patients at high risk of re‐bleeding following upper gastrointestinal tract bleeding
Kun He, Ke Pang, Lujing Ying, Daiyu Yang, Kai Song, Yangjin Ciren, Xiaxiao Yan, Ziqi Guo, Chengzhen Lyu, Qiang Wang, Dong Wu
Alimentary Pharmacology & Therapeutics.2024;[Epub] CrossRef - Use of over-the-scope clip (OTSC) versus standard therapy for the prevention of rebleeding in large peptic ulcers (size ≥1.5 cm): an open-labelled, multicentre international randomised controlled trial
Shannon Chan, Rapat Pittayanon, Hsiu-Po Wang, Jiann-Hwa Chen, Anthony YB Teoh, Yu Ting Kuo, Raymond SY Tang, Hon Chi Yip, Stephen Ka Kei Ng, Sunny Wong, Joyce Wing Yan Mak, Heyson Chan, Louis Lau, Rashid N Lui, Marc Wong, Rungsun Rerknimitr, Enders K Ng,
Gut.2023; 72(4): 638. CrossRef - Current status in endoscopic management of upper gastrointestinal perforations, leaks and fistulas
Shannon Melissa Chan, Kitty Kit Ying Auyeung, Siu Fung Lam, Philip Wai Yan Chiu, Anthony Yuen Bun Teoh
Digestive Endoscopy.2022; 34(1): 43. CrossRef - The application of endoscopic loop ligation in defect repair following endoscopic full-thickness resection of gastric submucosal tumors originating from the muscularis propria layer
Guoxiang Wang, Yanli Xiang, Yangde Miao, Honggang Wang, Meidong Xu, Guang Yu
Scandinavian Journal of Gastroenterology.2022; 57(1): 119. CrossRef - Gastrointestinal Emergencies and the Role of Endoscopy
Vinod Kumar Dixit, Manoj Kumar Sahu, Vybhav Venkatesh, Varanasi Yugandhar Bhargav, Vinod Kumar, Mayank Bhushan Pateriya, Jayanthi Venkataraman
Journal of Digestive Endoscopy.2022; 13(03): 179. CrossRef - Closure of Gastrointestinal Fistulas and Leaks with the Over-the-Scope Clip: Case-Series Analysis
Abdullah Senlikci, Tahsin Dalgic, Ahmet Alyanak, Erdal Birol Bostanci
Indian Journal of Surgery.2021; 83(6): 1413. CrossRef - Combination Endoscopic Therapy is Effective for Treatment of Nonbariatric Postoperative Gastroenteric Leaks
Nikhil R. Thiruvengadam, Christopher Hamerski, Andrew Nett, Yasser Bhat, Janak Shah, Jona Bernabe, Steven Kane, Kenneth Binmoeller, Rabindra R. Watson
Techniques and Innovations in Gastrointestinal Endoscopy.2021; 23(2): 122. CrossRef - Efficacy of the OTSC System in the treatment of GI bleeding and wall defects: a PMCF meta-analysis
Timo Weiland, Sabrina Rohrer, Arthur Schmidt, Edris Wedi, Peter Bauerfeind, Karel Caca, Mouen A. Khashab, Juergen Hochberger, Franziska Baur, Thomas Gottwald, Marc O. Schurr
Minimally Invasive Therapy & Allied Technologies.2020; 29(3): 121. CrossRef - Long-term assessment of over-the-scope clip (OTSC) behavior after gastric application
Jinlong Hu, Yang Yang, Nan Ge, Sheng Wang, Jintao Guo, Xiang Liu, Guoxin Wang, Siyu Sun
Minimally Invasive Therapy & Allied Technologies.2020; 29(2): 86. CrossRef - Use of the Over the Scope Clip to Close Perforations and Fistulas
Panida Piyachaturawat, Parit Mekaroonkamol, Rungsun Rerknimitr
Gastrointestinal Endoscopy Clinics of North America.2020; 30(1): 25. CrossRef - Clinical efficacy of the over-the-scope clip device: A systematic review
Nicholas Bartell, Krystle Bittner, Vivek Kaul, Truptesh H Kothari, Shivangi Kothari
World Journal of Gastroenterology.2020; 26(24): 3495. CrossRef - Life on a knife edge: the optimal approach to the management of perforations during endoscopic submucosal dissection (ESD)
Shria Kumar, Young Hoon Youn, Jeffrey H. Lee
Expert Review of Gastroenterology & Hepatology.2020; 14(10): 965. CrossRef - Gastrointestinal tract injuries after thermal ablative therapies for hepatocellular carcinoma: A case report and review of the literature
Teresa Marzia Rogger, Andrea Michielan, Sandro Sferrazza, Cecilia Pravadelli, Luisa Moser, Flora Agugiaro, Giovanni Vettori, Sonia Seligmann, Elettra Merola, Marcello Maida, Francesco Antonio Ciarleglio, Alberto Brolese, Giovanni de Pretis
World Journal of Gastroenterology.2020; 26(35): 5375. CrossRef - Fístula broncoesofágica secundaria a aspergilosis pulmonar
Antonio María Caballero-Mateos, Mercedes López de Hierro Ruíz, Eduardo Redondo Cerezo
Revista Colombiana de Gastroenterología.2020; 35(4): 558. CrossRef - Over-the-Scope Clip to the Rescue! A Novel Tool for Refractory Acute Nonvariceal Upper Gastrointestinal Hemorrhage
Shivantha Amarnath, Jobin Philipose, Jeffrey Abergel, Hafiz Khan
Case Reports in Gastroenterology.2020; 14(2): 261. CrossRef - Over‐the‐scope clip system: A review of 1517 cases over 9 years
Hideki Kobara, Hirohito Mori, Noriko Nishiyama, Shintaro Fujihara, Keiichi Okano, Yasuyuki Suzuki, Tsutomu Masaki
Journal of Gastroenterology and Hepatology.2019; 34(1): 22. CrossRef - Over-the-scope-clips as primary and rescue therapy for non-variceal gastrointestinal bleeding: a systematic review and meta-analysis
Andrew Ofosu, Daryl Ramai, Febin John, Mohammed Barakat, Tagore Sunkara, Santosh Sharma, Vinaya Gaduputi, Douglas G. Adler, Madhavi Reddy
Minerva Gastroenterologica e Dietologica.2019;[Epub] CrossRef - Cierre endoscópico de perforaciones y fístulas del tracto digestivo mediante el sistema «Over-the scope clip» (Ovesco), en un centro terciario
G. Mosquera-Klinger, R. Torres-Rincón, J. Jaime-Carvajal
Revista de Gastroenterología de México.2019; 84(2): 263. CrossRef - Endoscopic closure of gastrointestinal perforations and fistulas using the Ovesco Over-The-Scope Clip system at a tertiary care hospital center
G. Mosquera-Klinger, R. Torres-Rincón, J. Jaime-Carvajal
Revista de Gastroenterología de México (English Edition).2019; 84(2): 263. CrossRef - Management of Bleeding from Unresectable Gastric Cancer
Hideaki Kawabata, Misuzu Hitomi, Shigehiro Motoi
Biomedicines.2019; 7(3): 54. CrossRef - Over‐the‐scope clip for acute esophageal variceal bleeding
Carolina Mangas‐Sanjuan, Belén Martínez-Moreno, Maryana Bozhychko, Luis Compañy, Juan Martinez, Francisco Ruiz, Juan Antonio Casellas, José Ramón Aparicio
Digestive Endoscopy.2019; 31(6): 712. CrossRef - Clinical outcomes of over-the-scope-clip system for the treatment of acute upper non-variceal gastrointestinal bleeding: a systematic review and meta-analysis
Chunyu Zhong, Shali Tan, Yutang Ren, Muhan Lü, Yan Peng, Xiangsheng Fu, Xiaowei Tang
BMC Gastroenterology.2019;[Epub] CrossRef - Endoscopic Management with a Novel Over-The-Scope Padlock Clip System
Mahesh Kumar Goenka, Gajanan Ashokrao Rodge, Indrajeet Kumar Tiwary
Clinical Endoscopy.2019; 52(6): 574. CrossRef - Over the Scope Clips for Treatment of Acute Nonvariceal Gastrointestinal Bleeding in Children Are Safe and Effective
Paul Tran, Joshua Carroll, Bradley A. Barth, Nandini Channabasappa, David M. Troendle
Journal of Pediatric Gastroenterology and Nutrition.2018; 67(4): 458. CrossRef - Endoscopic titanium clip closure of gastric fistula after splenectomy: A case report
Jing Yu, Cheng-Ji Zhou, Pan Wang, Shou-Jiang Wei, Jin-Song He, Jin Tang
World Journal of Clinical Cases.2018; 6(15): 1047. CrossRef - New endoscopic techniques in treating gastrointestinal bleeding
Young Sin Cho
International Journal of Gastrointestinal Intervention.2018; 7(3): 131. CrossRef - Over-the-Scope Clip in the Management of Gastrointestinal Defect and Intractable Non-Variceal Bleeding
Hyungkil Kim
Clinical Endoscopy.2017; 50(1): 3. CrossRef - Hémorragies digestives : qui ? quand ? place des nouveaux traitements ?
D. Heresbach, A. Laquière
Acta Endoscopica.2017; 47(5): 281. CrossRef - Over-the-scope clip
Bhanwar Singh Dhandhu, Kumar Shwetanshu Narayan, Surinder Sultania, Sandeep Nijhawan
Journal of Digestive Endoscopy.2016; 07(02): 047. CrossRef
- Endoscopic self-expandable metal stent versus endoscopy vacuum therapy for traumatic esophageal perforations: a retrospective cohort study
- 9,603 View
- 308 Download
- 31 Web of Science
- 30 Crossref


 KSGE
KSGE


 First
First Prev
Prev



