Previous issues
- Page Path
- HOME > Browse Articles > Previous issues
Commentarys
- Safety and Competency are the Main Priorities in Pediatric Endoscopy
- Byung-Ho Choe
- Clin Endosc 2020;53(4):379-380. Published online July 15, 2020
- DOI: https://doi.org/10.5946/ce.2020.124
- 2,971 View
- 84 Download

- Safeness of Endoscopic Resection in Patients with End-Stage Renal Disease on Dialysis
- Sun-Jin Boo
- Clin Endosc 2020;53(4):381-382. Published online July 30, 2020
- DOI: https://doi.org/10.5946/ce.2020.179
- 3,576 View
- 85 Download

- Endoscopic Treatment for Gastrointestinal Stromal Tumors in the Upper Gastrointestinal Tract
- In Kyung Yoo, Joo Young Cho
- Clin Endosc 2020;53(4):383-384. Published online July 3, 2020
- DOI: https://doi.org/10.5946/ce.2020.122
-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- Exploration of a new method for Photoshop-assisted endoscopic ultrasound to distinguish gastrointestinal stromal tumor and leiomyoma
Ying Zhao, Zeyu Wang, Jiageng Tian, Yadi Ren, Man Li
Scandinavian Journal of Gastroenterology.2023; 58(3): 291. CrossRef - On the Track of New Endoscopic Alternatives for the Treatment of Selected Gastric GISTs—A Pilot Study
Artur Raiter, Katarzyna M. Pawlak, Katarzyna Kozłowska-Petriczko, Jan Petriczko, Joanna Szełemej, Anna Wiechowska-Kozłowska
Medicina.2021; 57(6): 625. CrossRef
- Exploration of a new method for Photoshop-assisted endoscopic ultrasound to distinguish gastrointestinal stromal tumor and leiomyoma
- 3,546 View
- 88 Download
- 3 Web of Science
- 2 Crossref

- Usefulness of Colonoscopy in Patients with Hematochezia Aged under 40 Years
- Hee Chan Yang, Sang Wook Kim
- Clin Endosc 2020;53(4):385-386. Published online July 30, 2020
- DOI: https://doi.org/10.5946/ce.2020.153
- 3,531 View
- 85 Download

Focused Review Series: Present and Future of Diagnosis and Managements of Small Bowel Diseases Exploiting Artificial Intelligence and Advanced Endoscopy
- The Future of Capsule Endoscopy: The Role of Artificial Intelligence and Other Technical Advancements
- Young Joo Yang
- Clin Endosc 2020;53(4):387-394. Published online July 16, 2020
- DOI: https://doi.org/10.5946/ce.2020.133
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Capsule endoscopy has revolutionized the management of small-bowel diseases owing to its convenience and noninvasiveness. Capsule endoscopy is a common method for the evaluation of obscure gastrointestinal bleeding, Crohn’s disease, small-bowel tumors, and polyposis syndrome. However, the laborious reading process, oversight of small-bowel lesions, and lack of locomotion are major obstacles to expanding its application. Along with recent advances in artificial intelligence, several studies have reported the promising performance of convolutional neural network systems for the diagnosis of various small-bowel lesions including erosion/ulcers, angioectasias, polyps, and bleeding lesions, which have reduced the time needed for capsule endoscopy interpretation. Furthermore, colon capsule endoscopy and capsule endoscopy locomotion driven by magnetic force have been investigated for clinical application, and various capsule endoscopy prototypes for active locomotion, biopsy, or therapeutic approaches have been introduced. In this review, we will discuss the recent advancements in artificial intelligence in the field of capsule endoscopy, as well as studies on other technological improvements in capsule endoscopy.
-
Citations
Citations to this article as recorded by- Toward Automatic Stomach Screening Using a Wireless Magnetically Actuated Capsule Endoscope
Heng Zhang, Yehui Li, Xinfa Shi, Yichong Sun, Jixiu Li, Yisen Huang, Wing Yin Ng, Chengxiang Liu, Philip Wai Yan Chiu, Chi-Kwan Lee, Zheng Li
IEEE Transactions on Medical Robotics and Bionics.2024; 6(2): 512. CrossRef - Machine learning based small bowel video capsule endoscopy analysis: Challenges and opportunities
Haroon Wahab, Irfan Mehmood, Hassan Ugail, Arun Kumar Sangaiah, Khan Muhammad
Future Generation Computer Systems.2023; 143: 191. CrossRef - Artificial Intelligence in Inflammatory Bowel Disease Endoscopy: Implications for Clinical Trials
Harris A Ahmad, James E East, Remo Panaccione, Simon Travis, James B Canavan, Keith Usiskin, Michael F Byrne
Journal of Crohn's and Colitis.2023; 17(8): 1342. CrossRef - A Wireless Power Transfer System Based on a Hybrid Transmitting Coil for Targeted Therapy Microrobots in the Intestine
Ding Han, Guozheng Yan, Zhiwu Wang, Pingping Jiang, Lin Yan
International Journal of Precision Engineering and Manufacturing.2023; 24(6): 977. CrossRef - Self-supervised out-of-distribution detection in wireless capsule endoscopy images
Arnau Quindós, Pablo Laiz, Jordi Vitrià, Santi Seguí
Artificial Intelligence in Medicine.2023; 143: 102606. CrossRef - Design, actuation, and functionalization of untethered soft magnetic robots with life-like motions: A review
Jiaqi Miao, Siqi Sun
Journal of Magnetism and Magnetic Materials.2023; 586: 171160. CrossRef - A Three-Step Calibration Method of Sensors’ Pose for Magnetic Localization System
Weijun Lin, Shijie Ding, Zheng Li, Ning Yang
IEEE Transactions on Instrumentation and Measurement.2023; 72: 1. CrossRef - Wireless Capsule Endoscopy Infected Images Detection and Classification Using MobileNetV2-BiLSTM Model
P. Padmavathi, J. Harikiran
International Journal of Image and Graphics.2023;[Epub] CrossRef - Real-Time Lightweight Convolutional Neural Network for Polyp Detection in Endoscope Images
Bingqi Si, Chenxi Pang, Zhiwu Wang, Pingping Jiang, Guozheng Yan
Journal of Shanghai Jiaotong University (Science).2023;[Epub] CrossRef - AI-Driven Colon Cleansing Evaluation in Capsule Endoscopy: A Deep Learning Approach
Miguel José Mascarenhas Saraiva, João Afonso, Tiago Ribeiro, Pedro Cardoso, Francisco Mendes, Miguel Martins, Ana Patrícia Andrade, Hélder Cardoso, Miguel Mascarenhas Saraiva, João Ferreira, Guilherme Macedo
Diagnostics.2023; 13(23): 3494. CrossRef - Computer vision-based solutions to overcome the limitations of wireless capsule endoscopy
Ana Horovistiz, Marina Oliveira, Helder Araújo
Journal of Medical Engineering & Technology.2023; 47(4): 242. CrossRef - A Hybrid RF and Vision Aware Fusion Scheme for Multi-Sensor Wireless Capsule Endoscopic Localization
P. Narmatha, Venkatesan Thangavel, D. Sri Vidhya
Wireless Personal Communications.2022; 123(2): 1593. CrossRef - A survey of small bowel modelling and its applications for capsule endoscopy
Yang Liu, Jiyuan Tian, Luigi Manfredi, Benjamin S. Terry, Shyam Prasad, Imdadur Rahman, Wojciech Marlicz, Anastasios Koulaouzidis
Mechatronics.2022; 83: 102748. CrossRef - Robot-Assisted Medical Imaging: A Review
Septimiu E. Salcudean, Hamid Moradi, David G. Black, Nassir Navab
Proceedings of the IEEE.2022; 110(7): 951. CrossRef - A Three-Dimensional Orthogonal Receiving Coil for In Vivo Microrobot Wireless Power Transmission Systems
Ding Han, Guozheng Yan, Pingping Jiang, Zhiwu Wang, Wei Wang
Energies.2022; 15(17): 6321. CrossRef - Artificial intelligence to improve polyp detection and screening time in colon capsule endoscopy
Pere Gilabert, Jordi Vitrià, Pablo Laiz, Carolina Malagelada, Angus Watson, Hagen Wenzek, Santi Segui
Frontiers in Medicine.2022;[Epub] CrossRef - Key research questions for implementation of artificial intelligence in capsule endoscopy
Romain Leenhardt, Anastasios Koulaouzidis, Aymeric Histace, Gunnar Baatrup, Sabina Beg, Arnaud Bourreille, Thomas de Lange, Rami Eliakim, Dimitris Iakovidis, Michael Dam Jensen, Martin Keuchel, Reuma Margalit Yehuda, Deirdre McNamara, Miguel Mascarenhas,
Therapeutic Advances in Gastroenterology.2022; 15: 175628482211326. CrossRef - The optimal use of colon capsule endoscopes in clinical practice
Thomas Bjørsum-Meyer, Anastasios Koulaouzidis, Gunnar Baatrup
Therapeutic Advances in Chronic Disease.2022; 13: 204062232211375. CrossRef - Application of capsule endoscopy in patients with chronic and recurrent abdominal pain
Wei Yang, Zheng Li, Rui Liu, Xudong Tong, Wei Wang, Dongqiang Xu, Shan Gao
Medical Engineering & Physics.2022; 110: 103901. CrossRef - The scientific progress and prospects of artificial intelligence in digestive endoscopy: A comprehensive bibliometric analysis
Pei-Ling Gan, Shu Huang, Xiao Pan, Hui-Fang Xia, Mu-Han Lü, Xian Zhou, Xiao-Wei Tang
Medicine.2022; 101(47): e31931. CrossRef - Artificial intelligence for cancer detection of the upper gastrointestinal tract
Hideo Suzuki, Tokai Yoshitaka, Toshiyuki Yoshio, Tomohiro Tada
Digestive Endoscopy.2021; 33(2): 254. CrossRef - Colon Capsule Endoscopy: An Alternative for Conventional Colonoscopy?
Britt B.S.L. Houwen, Evelien Dekker
Clinical Endoscopy.2021; 54(1): 4. CrossRef - How should we do colon capsule endoscopy reading: a practical guide
Anastasios Koulaouzidis, Konstantinos Dabos, Michael Philipper, Ervin Toth, Martin Keuchel
Therapeutic Advances in Gastrointestinal Endoscopy.2021; 14: 263177452110019. CrossRef - Kvasir-Capsule, a video capsule endoscopy dataset
Pia H. Smedsrud, Vajira Thambawita, Steven A. Hicks, Henrik Gjestang, Oda Olsen Nedrejord, Espen Næss, Hanna Borgli, Debesh Jha, Tor Jan Derek Berstad, Sigrun L. Eskeland, Mathias Lux, Håvard Espeland, Andreas Petlund, Duc Tien Dang Nguyen, Enrique Garcia
Scientific Data.2021;[Epub] CrossRef - Role of Artificial Intelligence in Video Capsule Endoscopy
Ioannis Tziortziotis, Faidon-Marios Laskaratos, Sergio Coda
Diagnostics.2021; 11(7): 1192. CrossRef - Artificial intelligence in gastrointestinal diseases
Shihori Tanabe, Edward J Perkins, Ryuichi Ono, Hiroki Sasaki
Artificial Intelligence in Gastroenterology.2021; 2(3): 69. CrossRef - Artificial intelligence assisted assessment of endoscopic disease activity in inflammatory bowel disease
Bobby Lo, Johan Burisch
Artificial Intelligence in Gastrointestinal Endoscopy.2021; 2(4): 95. CrossRef - Capsule Endoscopy: Pitfalls and Approaches to Overcome
Seung Han Kim, Hoon Jai Chun
Diagnostics.2021; 11(10): 1765. CrossRef - Follow-up outcomes in patients with negative initial colon capsule endoscopy findings
Konosuke Nakaji, Mitsutaka Kumamoto, Mikiko Yodozawa, Kazuki Okahara, Shigeo Suzumura, Yukinori Nakae
World Journal of Gastrointestinal Endoscopy.2021; 13(10): 502. CrossRef - Artificial intelligence in gastroenterology: A state-of-the-art review
Paul T Kröner, Megan ML Engels, Benjamin S Glicksberg, Kipp W Johnson, Obaie Mzaik, Jeanin E van Hooft, Michael B Wallace, Hashem B El-Serag, Chayakrit Krittanawong
World Journal of Gastroenterology.2021; 27(40): 6794. CrossRef - WCE polyp detection with triplet based embeddings
Pablo Laiz, Jordi Vitrià, Hagen Wenzek, Carolina Malagelada, Fernando Azpiroz, Santi Seguí
Computerized Medical Imaging and Graphics.2020; 86: 101794. CrossRef
- Toward Automatic Stomach Screening Using a Wireless Magnetically Actuated Capsule Endoscope
- 7,921 View
- 354 Download
- 29 Web of Science
- 31 Crossref

-
A New Active Locomotion Capsule Endoscopy under Magnetic Control and Automated Reading Program

- Dong Jun Oh, Kwang Seop Kim, Yun Jeong Lim
- Clin Endosc 2020;53(4):395-401. Published online July 30, 2020
- DOI: https://doi.org/10.5946/ce.2020.127

-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Capsule endoscopy (CE) is the first-line diagnostic modality for detecting small bowel lesions. CE is non-invasive and does not require sedation, but its movements cannot be controlled, it requires a long time for interpretation, and it has lower image quality compared to wired endoscopy. With the rapid advancement of technology, several methods to solve these problems have been developed. This article describes the ongoing developments regarding external CE locomotion using magnetic force, artificial intelligence-based interpretation, and image-enhancing technologies with the CE system.
-
Citations
Citations to this article as recorded by- Robotic Pills as Innovative Personalized Medicine Tools: A Mini Review
Komal Rane, Garima Kukreja, Siddhi Deshmukh, Urmisha Kakad, Pranali Jadhav, Vinita Patole
Recent Advances in Drug Delivery and Formulation.2024; 18(1): 2. CrossRef - A composite electro-permanent magnetic actuator for microrobot manipulation
Kim Tien Nguyen, Han-Sol Lee, Jayoung Kim, Eunpyo Choi, Jong-Oh Park, Chang-Sei Kim
International Journal of Mechanical Sciences.2022; 229: 107516. CrossRef - Endoscopic methods for the detection and treatment of gastric cancer
Negar Niknam, Steven Obanor, Linda A. Lee
Current Opinion in Gastroenterology.2022; 38(5): 436. CrossRef - Preparation of image databases for artificial intelligence algorithm development in gastrointestinal endoscopy
Chang Bong Yang, Sang Hoon Kim, Yun Jeong Lim
Clinical Endoscopy.2022; 55(5): 594. CrossRef - Automatic lumen detection and magnetic alignment control for magnetic-assisted capsule colonoscope system optimization
Sheng-Yang Yen, Hao-En Huang, Gi-Shih Lien, Chih-Wen Liu, Chia-Feng Chu, Wei-Ming Huang, Fat-Moon Suk
Scientific Reports.2021;[Epub] CrossRef - Feasibility of Upper Gastrointestinal Examination in Home Care Setting with a Magnetically Assisted Capsule Endoscopy System: A Retrospective Study
Yang-Chao Lin, Ching-Lin Chen, Yi-Wei Kao, Chi-Yang Chang, Ming-Chih Chen, Chih-Kuang Liu
Healthcare.2021; 9(5): 577. CrossRef - Artificial intelligence in small intestinal diseases: Application and prospects
Yu Yang, Yu-Xuan Li, Ren-Qi Yao, Xiao-Hui Du, Chao Ren
World Journal of Gastroenterology.2021; 27(25): 3734. CrossRef - A Current and Newly Proposed Artificial Intelligence Algorithm for Reading Small Bowel Capsule Endoscopy
Dong Jun Oh, Youngbae Hwang, Yun Jeong Lim
Diagnostics.2021; 11(7): 1183. CrossRef - Role of Artificial Intelligence in Video Capsule Endoscopy
Ioannis Tziortziotis, Faidon-Marios Laskaratos, Sergio Coda
Diagnostics.2021; 11(7): 1192. CrossRef - Study on 13.56‐MHz out‐to‐in body channel and its coexistence with human body communication for capsule endoscope
Jaehyo Jung, Meina Li, Youn Tae Kim
Microwave and Optical Technology Letters.2021; 63(11): 2819. CrossRef - Examination of Entire Gastrointestinal Tract: A Perspective of Mouth to Anus (M2A) Capsule Endoscopy
Ji Hyung Nam, Kwang Hoon Lee, Yun Jeong Lim
Diagnostics.2021; 11(8): 1367. CrossRef - Swimming characteristics of a petal-shaped capsule robot with fluid resistance torsion moment-weaken effect
Minglu Chi, Huadong Zheng, Rongsheng Liu, Cheng Chang, Yuanli Wang, Xiaoyan Qian, Ruihua Ren
Journal of the Brazilian Society of Mechanical Sciences and Engineering.2021;[Epub] CrossRef
- Robotic Pills as Innovative Personalized Medicine Tools: A Mini Review
- 5,071 View
- 129 Download
- 11 Web of Science
- 12 Crossref

- Roles of Capsule Endoscopy and Balloon-Assisted Enteroscopy in the Optimal Management of Small Bowel Bleeding
- Hani Abutalib, Tomonori Yano, Satoshi Shinozaki, Alan Kawarai Lefor, Hironori Yamamoto
- Clin Endosc 2020;53(4):402-409. Published online July 30, 2020
- DOI: https://doi.org/10.5946/ce.2020.143
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - The small bowel had long been considered a dark unapproachable tunnel until the invention of capsule endoscopy and doubleballoon enteroscopy in the 21st century, which revolutionized the diagnosis and management of small bowel diseases, including bleeding. Various imaging modalities such as computed tomographic enterography, angiography, capsule endoscopy, and balloonassisted enteroscopy play vital roles in the diagnosis and management of small bowel bleeding. The choice of modality to use and timing of application differ according to the availability of the modalities, patient’s history, and physician’s experience. Small bowel bleeding is managed using different strategies as exemplified by medical treatment, interventional radiology, endoscopic therapy, or surgical intervention. Balloon-assisted enteroscopy enables endoscopic interventions to control small bowel bleeding, including electrocautery, argon plasma coagulation, clip application, and tattooing as a prelude to surgery. In this article, we clarify the recent approaches to the optimal diagnosis and management of patients with small bowel bleeding.
-
Citations
Citations to this article as recorded by- A practical approach for small bowel bleeding
Sung Eun Kim, Hyun Jin Kim, Myeongseok Koh, Min Cheol Kim, Joon Sung Kim, Ji Hyung Nam, Young Kwan Cho, A Reum Choe
Clinical Endoscopy.2023; 56(3): 283. CrossRef - Indication, Location of the Lesion, Diagnostic Yield, and Therapeutic Yield of Double-Balloon Enteroscopy: Seventeen Years of Experience
Sang Pyo Lee, Hyun Joo Jang, Sea Hyub Kae, Jae Gon Lee, Ji Hye Kwon
Diagnostics.2022; 12(9): 2224. CrossRef - Application of capsule endoscopy in patients with chronic and recurrent abdominal pain
Wei Yang, Zheng Li, Rui Liu, Xudong Tong, Wei Wang, Dongqiang Xu, Shan Gao
Medical Engineering & Physics.2022; 110: 103901. CrossRef
- A practical approach for small bowel bleeding
- 4,833 View
- 180 Download
- 3 Web of Science
- 3 Crossref

- Roles of Capsule Endoscopy and Device-Assisted Enteroscopy in the Diagnosis and Treatment of Small-Bowel Tumors
- Eun Ran Kim
- Clin Endosc 2020;53(4):410-416. Published online July 30, 2020
- DOI: https://doi.org/10.5946/ce.2020.161
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - With the development of capsule endoscopy (CE) and device-assisted enteroscopy (DAE), the incidence of small-bowel tumors has increased and the characteristics of these tumors have changed. In addition, the diagnostic and therapeutic approaches for small-bowel tumors have diversified. CE is a simple, noninvasive method that aid in the visualization the entire small bowel. CE is considered the initial approach for small-bowel tumors. DAE can be used to perform endoscopic procedures such as bleeding control, polypectomy, stent insertion, and tattooing, as well as for diagnosis through visualization or tissue sampling. Therapeutic intervention with DAE is particularly useful in polyposis syndromes such as familial adenomatous polyposis and Peutz-Jeghers syndrome. This review will discuss the roles of CE and DAE in the diagnosis and treatment of small-bowel tumors.
-
Citations
Citations to this article as recorded by- Cancer risk in patients with Peutz-Jeghers syndrome in Korea: a retrospective multi-center study
Su Hwan Kim, Eun Ran Kim, Jae Jun Park, Eun Sun Kim, Hyeon Jeong Goong, Kyeong Ok Kim, Ji Hyung Nam, Yehyun Park, Sang Pyo Lee, Hyun Joo Jang
The Korean Journal of Internal Medicine.2023; 38(2): 176. CrossRef - The first experience of small bowel adenoma removal by cold loop resection
A.A. Fedorenko, P.V. Pavlov, A.P. Kiryukhin, A.S. Tertychnyy
Endoskopicheskaya khirurgiya.2023; 29(2): 56. CrossRef - Intraoperative push enteroscopy for treatment of occult small bowel bleed due to hemorrhagic bleed and tumor: a report of two cases
Maribona A Sofia, Philip Dwight, Shatawi Zaineb, Seaver Christopher
Journal of Surgical Case Reports.2023;[Epub] CrossRef - Capsule Endoscopy for the Diagnosis of Suspected Small Bowel Bleeding
P. P. Polyakov, A. Ya. Alimetov, A. V. Onopriev, A. V. Avakimyan, A. Kh. Kade, S. A. Zanin, E. S. Zanina, Z. S. Popov, A. I. Trofimenko, Z. T. Jndoyan, A. A. Avagimyan
Innovative Medicine of Kuban.2023; (3): 121. CrossRef -
MUTYH-associated polyposis: Is it time to change upper gastrointestinal surveillance? A single-center case series and a literature overview
Lupe Sanchez-Mete, Lorenzo Mosciatti, Marco Casadio, Luigi Vittori, Aline Martayan, Vittoria Stigliano
World Journal of Gastrointestinal Oncology.2023; 15(11): 1891. CrossRef - Epidemiology, Risk Factors and Diagnosis of Small Bowel Adenocarcinoma
Thomas Aparicio, Atanas Pachev, Pierre Laurent-Puig, Magali Svrcek
Cancers.2022; 14(9): 2268. CrossRef - Small bowel lymphoma: clinical update and challenges for the gastroenterologist
Priya Oka, Reena Sidhu
Current Opinion in Gastroenterology.2022; 38(3): 270. CrossRef - Recent developments in small bowel endoscopy: the “black box” is now open!
Luigina Vanessa Alemanni, Stefano Fabbri, Emanuele Rondonotti, Alessandro Mussetto
Clinical Endoscopy.2022; 55(4): 473. CrossRef - Application of capsule endoscopy in patients with chronic and recurrent abdominal pain
Wei Yang, Zheng Li, Rui Liu, Xudong Tong, Wei Wang, Dongqiang Xu, Shan Gao
Medical Engineering & Physics.2022; 110: 103901. CrossRef - A Two Stream Fusion Assisted Deep Learning Framework for Stomach Diseases Classification
Muhammad Shahid Amin, Jamal Hussain Shah, Mussarat Yasmin, Ghulam Jillani Ansari, Muhamamd Attique Khan, Usman Tariq, Ye Jin Kim, Byoungchol Chang
Computers, Materials & Continua.2022; 73(2): 4423. CrossRef - Peutz-Jeghers syndrome
Ilja Tacheci, Marcela Kopacova, Jan Bures
Current Opinion in Gastroenterology.2021; 37(3): 245. CrossRef - Endoscopic Management of Hamartomatous Polyposis Syndromes
Elena G. Gibson, Judith Staub, Priyanka Kanth
Current Treatment Options in Gastroenterology.2021; 19(4): 543. CrossRef - Premalignant Lesions of the Small Intestine
Su Hwan Kim, Ji Won Kim
Journal of Digestive Cancer Reports.2021; 9(2): 60. CrossRef - Small Bowel Malignancies in Patients Undergoing Capsule Endoscopy for Iron Deficiency Anemia
Su Hwan Kim, Ji Won Kim
Diagnostics.2021; 12(1): 91. CrossRef
- Cancer risk in patients with Peutz-Jeghers syndrome in Korea: a retrospective multi-center study
- 4,494 View
- 116 Download
- 11 Web of Science
- 14 Crossref

Reviews
- Endoscopic Ultrasound-Guided Fine Needle Aspiration and Endoscopic Retrograde Cholangiopancreatography-Based Tissue Sampling in Suspected Malignant Biliary Strictures: A Meta-Analysis of Same-Session Procedures
- Diogo Turiani Hourneax de Moura, Marvin Ryou, Eduardo Guimarães Hourneaux de Moura, Igor Braga Ribeiro, Wanderlei Marques Bernardo, Christopher C. Thompson
- Clin Endosc 2020;53(4):417-428. Published online November 5, 2019
- DOI: https://doi.org/10.5946/ce.2019.053
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The diagnosis of biliary strictures can be challenging. There are no systematic reviews studying same-session endoscopic retrograde cholangiopancreatography (ERCP)-based tissue sampling and endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for the diagnosis of biliary strictures.
Methods
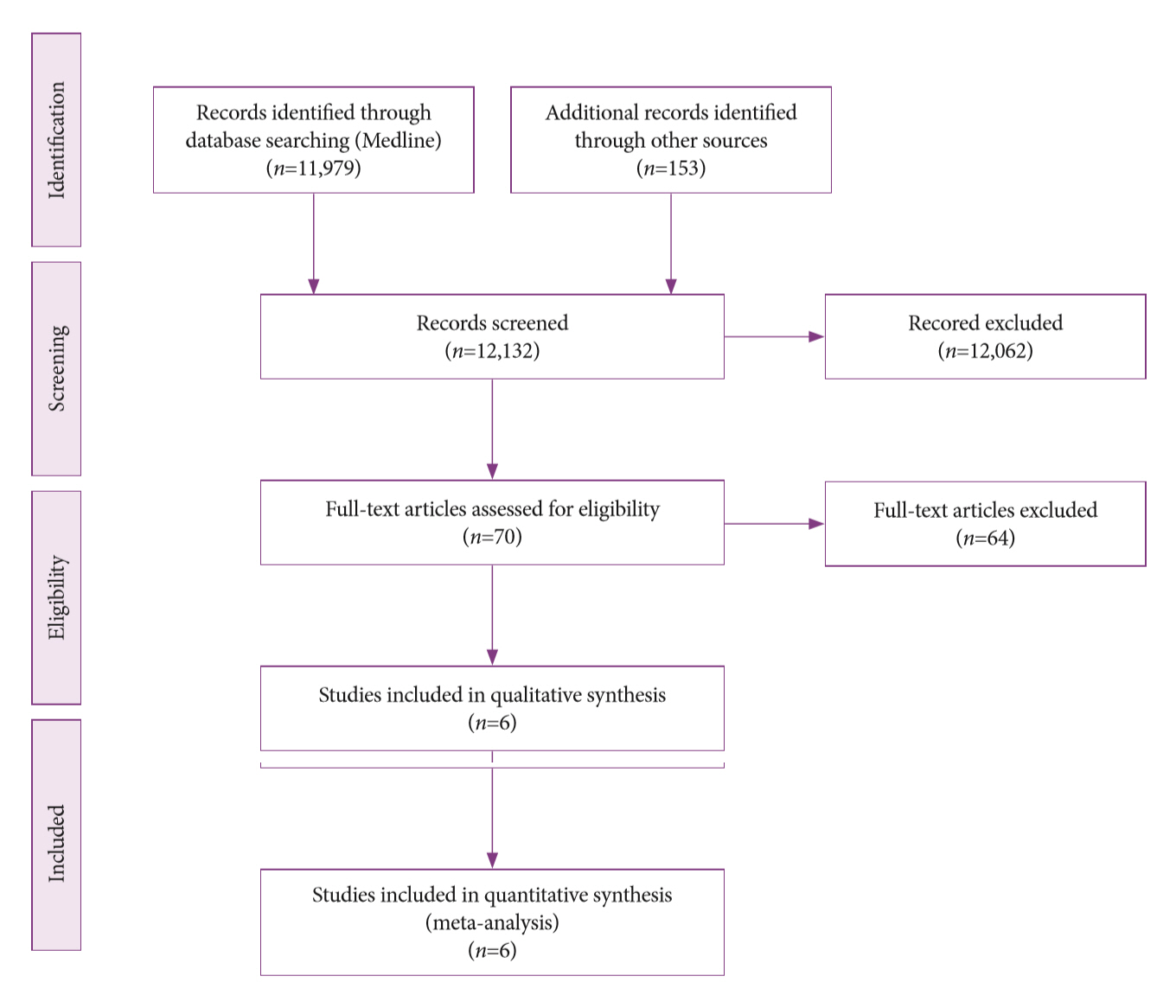
A systematic review was conducted on studies analyzing same-session EUS and ERCP for tissue diagnosis of suspected malignant biliary strictures. The primary outcome was the accuracy of each method individually compared to the two methods combined. The secondary outcome was the accuracy of each method in pancreatic and biliary etiologies. In the meta-analysis, we used Forest plots, summary receiver operating characteristic curves, and estimates of the area under the curve for intention-to-treat analysis.
Results
Of the 12,132 articles identified, six were included, resulting in a total of 497 patients analyzed. The sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and accuracy of the association between the two methods were: 86%, 98%, 12.50, 0.17, and 96.5%, respectively. For the individual analysis, the sensitivity, specificity and accuracy of EUS-FNA were 76%, 100%, and 94.5%, respectively; for ERCP-based tissue sampling, the sensitivity, specificity, and accuracy were 58%, 98%, and 78.1%, respectively. For pancreatic lesions, EUS-FNA was superior to ERCP-based tissue sampling. However, for biliary lesions, both methods had similar sensitivities.
Conclusions
Same-session EUS-FNA and ERCP-based tissue sampling is superior to either method alone in the diagnosis of suspected malignant biliary strictures. Considering these results, combination sampling should be performed when possible. -
Citations
Citations to this article as recorded by- British Society of Gastroenterology guidelines for the diagnosis and management of cholangiocarcinoma
Simon M Rushbrook, Timothy James Kendall, Yoh Zen, Raneem Albazaz, Prakash Manoharan, Stephen P Pereira, Richard Sturgess, Brian R Davidson, Hassan Z Malik, Derek Manas, Nigel Heaton, K Raj Prasad, John Bridgewater, Juan W Valle, Rebecca Goody, Maria Hawk
Gut.2024; 73(1): 16. CrossRef - Contrast-enhanced guided endoscopic ultrasound procedures
Marcel Ioan Gheorghiu, Andrada Seicean, Cristina Pojoga, Claudia Hagiu, Radu Seicean, Zeno Sparchez
World Journal of Gastroenterology.2024; 30(17): 2311. CrossRef - ACG Clinical Guideline: Diagnosis and Management of Biliary Strictures
B. Joseph Elmunzer, Jennifer L. Maranki, Victoria Gómez, Anna Tavakkoli, Bryan G. Sauer, Berkeley N. Limketkai, Emily A. Brennan, Elaine M. Attridge, Tara J. Brigham, Andrew Y. Wang
American Journal of Gastroenterology.2023; 118(3): 405. CrossRef - Endoscopic Ultrasound in the Diagnosis of Extrahepatic Cholangiocarcinoma: What Do We Know in 2023?
Rares Ilie Orzan, Cristina Pojoga, Renata Agoston, Radu Seicean, Andrada Seicean
Diagnostics.2023; 13(6): 1023. CrossRef - Endoscopic evaluation of indeterminate biliary strictures: Cholangioscopy, endoscopic ultrasound, or both?
Raymond S. Y. Tang
Digestive Endoscopy.2023;[Epub] CrossRef - Brush Cytology, Forceps Biopsy, or Endoscopic Ultrasound-Guided Sampling for Diagnosis of Bile Duct Cancer: A Meta-Analysis
Seung Bae Yoon, Sung-Hoon Moon, Sung Woo Ko, Hyun Lim, Ho Suk Kang, Jong Hyeok Kim
Digestive Diseases and Sciences.2022; 67(7): 3284. CrossRef - Managing adverse events after endoscopic ultrasound‐guided drainage of the biliary tract and pancreatic fluid collections: Narrative review (with video)
Mateus Pereira Funari, Igor Braga Ribeiro, Marcos Eduardo Lera dos Santos, Sergio Eiji Matuguma, Eduardo Guimarães Hourneaux de Moura
Digestive Endoscopy.2022; 34(2): 359. CrossRef - Endoscopic Ultrasound for the Diagnosis and Staging of Biliary Malignancy
Martin Coronel, Jeffrey H. Lee, Emmanuel Coronel
Clinics in Liver Disease.2022; 26(1): 115. CrossRef - Endoscopic Management of Pancreatobiliary Malignancies
Dong Wook Lee, Eun Young Kim
Digestive Diseases and Sciences.2022; 67(5): 1635. CrossRef - IgG4-related sclerosing cholangitis involving the gallbladder mimicking a hilar cholangiocarcinoma
Yun Chae Lee, Hyung Ku Chon, Keum Ha Choi
Endoscopy.2022; 54(12): E739. CrossRef - Promising Genomic Testing for Biliary Tract Cancer Using Endoscopic Ultrasound-Guided Fine-Needle Aspiration/Biopsy Specimens
Masaki Kuwatani, Kazumichi Kawakubo, Naoya Sakamoto
Diagnostics.2022; 12(4): 900. CrossRef - Endoscopic Ultrasound Plus Endoscopic Retrograde Cholangiopancreatography Based Tissue Sampling for Diagnosis of Proximal and Distal Biliary Stenosis Due to Cholangiocarcinoma: Results from a Retrospective Single-Center Study
Edoardo Troncone, Fabio Gadaleta, Omero Alessandro Paoluzi, Cristina Maria Gesuale, Vincenzo Formica, Cristina Morelli, Mario Roselli, Luca Savino, Giampiero Palmieri, Giovanni Monteleone, Giovanna Del Vecchio Blanco
Cancers.2022; 14(7): 1730. CrossRef - The Role of Cholangioscopy and EUS in the Evaluation of Indeterminate Biliary Strictures
Wilson Siu, Raymond S. Y. Tang
Gastroenterology Insights.2022; 13(2): 192. CrossRef - Current endoscopic approaches to biliary strictures
Tatsuya Sato, Yousuke Nakai, Mitsuhiro Fujishiro
Current Opinion in Gastroenterology.2022; 38(5): 450. CrossRef - Acute cholecystitis caused by gallbladder metastasis from non-small cell lung cancer: a case report
Kouki Imaoka, Daisuke Satoh, Ko Oshita, Takuya Yano, Tetsushi Kubota, Michihiro Ishida, Yasuhiro Choda, Masanori Yoshimitsu, Kanyu Nakano, Masao Harano, Hiroyoshi Matsukawa, Hitoshi Idani, Shigehiro Shiozaki, Masazumi Okajima
Clinical Journal of Gastroenterology.2021; 14(1): 351. CrossRef - Current Status and Research Progress of ERCP in the Diagnosis and Treatment of Biliary and Pancreatic System Diseases
跃华 李
Advances in Clinical Medicine.2021; 11(07): 3123. CrossRef - Same day endoscopic retrograde cholangio-pancreatography immediately after endoscopic ultrasound for choledocholithiasis is feasible, safe and cost-effective
Wisam Sbeit, Anas Kadah, Amir Shahin, Tawfik Khoury
Scandinavian Journal of Gastroenterology.2021; 56(10): 1243. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Tips and tricks for the diagnosis and management of biliary stenosis-state of the art review
Giovanna Del Vecchio Blanco, Michelangela Mossa, Edoardo Troncone, Renato Argirò, Andrea Anderloni, Alessandro Repici, Omero Alessandro Paoluzi, Giovanni Monteleone
World Journal of Gastrointestinal Endoscopy.2021; 13(10): 473. CrossRef - Stent versus Balloon Dilation for the Treatment of Dominant Strictures in Primary Sclerosing Cholangitis: A Systematic Review and Meta-Analysis
Marina Tucci Gammaro Baldavira Ferreira, Igor Braga Ribeiro, Diogo Turiani Hourneaux de Moura, Thomas R. McCarty, Alberto Machado da Ponte Neto, Galileu Ferreira Ayala Farias, Antônio Afonso de Miranda Neto, Pedro Victor Aniz Gomes de Oliveira, Wanderley
Clinical Endoscopy.2021; 54(6): 833. CrossRef - Endoscopic ultrasound fine needle aspiration vs fine needle biopsy in solid lesions: A multi-center analysis
Diogo Turiani Hourneaux Moura, Thomas R McCarty, Pichamol Jirapinyo, Igor Braga Ribeiro, Galileu Ferreira Ayala Farias, Antonio Coutinho Madruga-Neto, Marvin Ryou, Christopher C Thompson
World Journal of Clinical Cases.2021; 9(34): 10507. CrossRef - Efficacy of digital single-operator cholangioscopy in the visual interpretation of indeterminate biliary strictures: a systematic review and meta-analysis
Pedro Victor Aniz Gomes de Oliveira, Diogo Turiani Hourneaux de Moura, Igor Braga Ribeiro, Ahmad Najdat Bazarbashi, Tomazo Antonio Prince Franzini, Marcos Eduardo Lera dos Santos, Wanderley Marques Bernardo, Eduardo Guimarães Hourneaux de Moura
Surgical Endoscopy.2020; 34(8): 3321. CrossRef - Role of pancreatography in the endoscopic management of encapsulated pancreatic collections – review and new proposed classification
Igor Mendonça Proença, Marcos Eduardo Lera dos Santos, Diogo Turiani Hourneaux de Moura, Igor Braga Ribeiro, Sergio Eiji Matuguma, Spencer Cheng, Thomas R McCarty, Epifanio Silvino do Monte Junior, Paulo Sakai, Eduardo Guimarães Hourneaux de Moura
World Journal of Gastroenterology.2020; 26(45): 7104. CrossRef
- British Society of Gastroenterology guidelines for the diagnosis and management of cholangiocarcinoma
- 5,822 View
- 205 Download
- 21 Web of Science
- 23 Crossref

- Endoscopic Ultrasound-Guided Pancreatic Transmural Stenting and Transmural Intervention
- Takeshi Ogura, Hideko Ohama, Kazuhide Higuchi
- Clin Endosc 2020;53(4):429-435. Published online November 27, 2019
- DOI: https://doi.org/10.5946/ce.2019.130
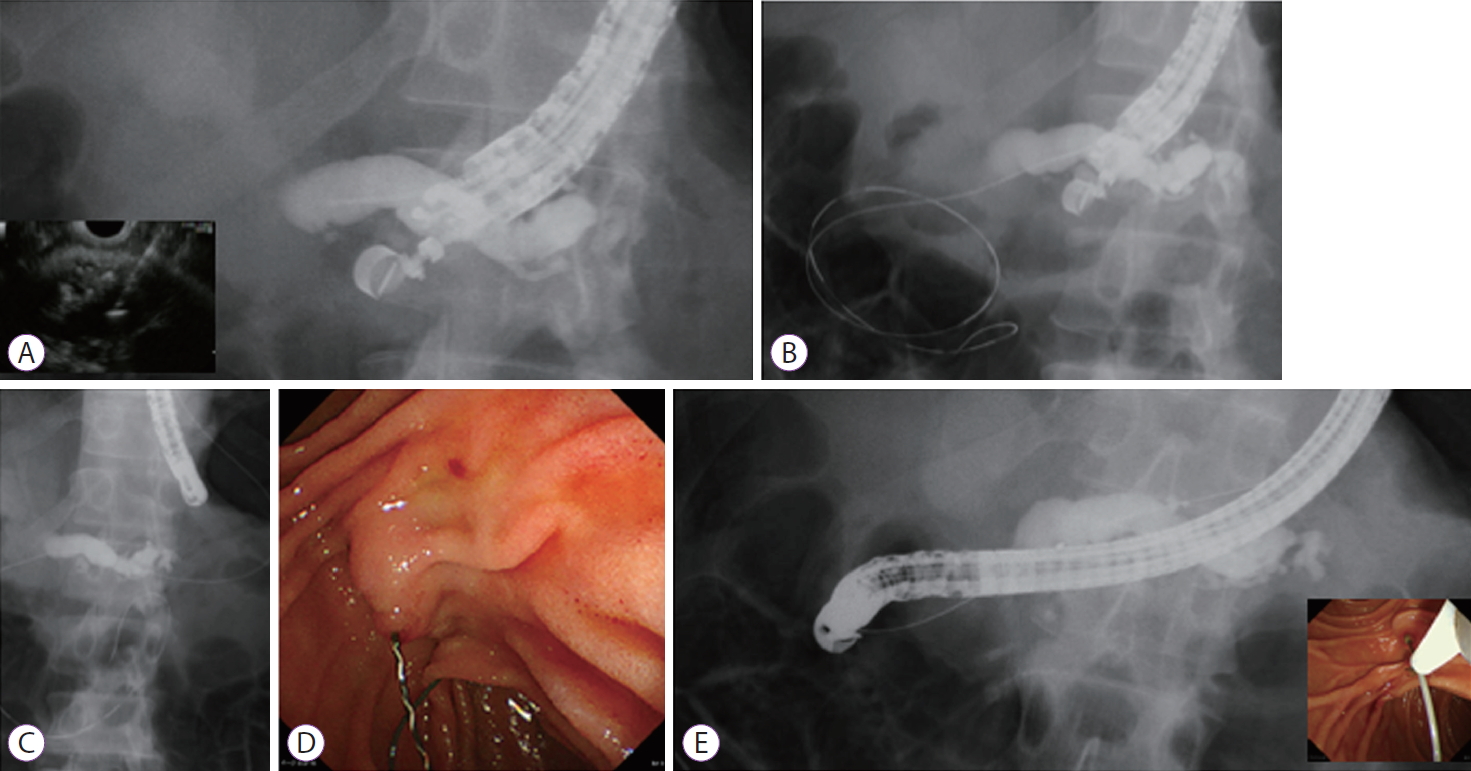
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic ultrasound (EUS)-guided pancreatic access is an emergent method that can be divided into the two main techniques of EUS-guided rendezvous and pancreatic transmural stenting (PTS). While many reports have described EUS-guided procedures, the indications, technical tips, clinical effects, and safety of EUS-guided pancreatic duct drainage (EUS-PD) remain controversial. This review describes the current status of and problems associated with EUS-PD, particularly PTS. We reviewed clinical data derived from a total of 334 patients. Rates of technical and clinical success ranged from 63% to 100% and 76% to 100%, respectively. In contrast, the rate of procedure-related adverse events was high at 26.7% (89/334). The most frequent adverse events comprised abdominal pain (n=38), acute pancreatitis (n=15), bleeding (n=9), and issues associated with pancreatic juice leakage such as perigastric fluid, pancreatic fluid collection, or pancreatic juice leaks (n=8). In conclusion, indications for EUS-PTS are limited, as is the evidence of its viability, due to the scarcity of expert operators. Despite improvements made to various devices, EUS-PTS remains technically challenging. Therefore, a long-term, large-scale, multicenter study is required to establish this technique as a viable alternative drainage method.
-
Citations
Citations to this article as recorded by- Needle-free technique for guidewire manipulation during endoscopic ultrasound-guided pancreatic duct drainage
Takeshi Ogura, Masahiro Yamamura, Mitsuki Tomita, Jun Sakamoto, Hiroki Nishikawa
Endoscopy.2024; 56(S 01): E184. CrossRef - Best Practices in Pancreatico-biliary Stenting and EUS-guided Drainage
Renato Medas, Joel Ferreira-Silva, Mohit Girotra, Monique Barakat, James H. Tabibian, Eduardo Rodrigues-Pinto
Journal of Clinical Gastroenterology.2023; 57(6): 553. CrossRef - Endoscopic treatment of pancreatholithiasis
Ichiro YASUDA, Toshiki ENTANI, Jun MATSUNO, Nobuhiko HAYASHI, Keisuke IWATA
Suizo.2023; 38(4): 201. CrossRef - Endoscopic pancreatic drainage
Toshifumi KIN, Kazuki HAMA, Kenta YOSHIDA, Risa NAKAMURA, Ryo ANDO, Kosuke IWANO, Haruka TOYONAGA, Tatsuya ISHII, Masayo MOTOYA, Tsuyoshi HAYASHI, Kuniyuki TAKAHASHI, Akio KATANUMA
Suizo.2023; 38(4): 192. CrossRef - Efficacy and Safety of Peroral Pancreatoscopy Through the Fistula Created by Endoscopic Ultrasound–Guided Pancreaticogastrostomy
Akinori Suzuki, Shigeto Ishii, Toshio Fujisawa, Hiroaki Saito, Yusuke Takasaki, Sho Takahashi, Wataru Yamagata, Kazushige Ochiai, Ko Tomishima, Hiroyuki Isayama
Pancreas.2022; 51(3): 228. CrossRef - Endoscopic ultrasound-guided pancreaticoduodenostomy with a forward-viewing echoendoscope
Shigenobu Yoshimura, So Nakaji, Toshiyasu Shiratori, Natsuki Kawamitsu, Shin Inoue
VideoGIE.2021; 6(12): 549. CrossRef - Endoscopic Ultrasound-Guided Pancreatic Duct Drainage: Techniques and Literature Review of Transmural Stenting
Akira Imoto, Takeshi Ogura, Kazuhide Higuchi
Clinical Endoscopy.2020; 53(5): 525. CrossRef - Technical tips for endoscopic ultrasound-guided pancreatic duct access and drainage
Yousuke Nakai
International Journal of Gastrointestinal Intervention.2020; 9(4): 154. CrossRef
- Needle-free technique for guidewire manipulation during endoscopic ultrasound-guided pancreatic duct drainage
- 5,603 View
- 273 Download
- 7 Web of Science
- 8 Crossref

Original Articles
- Endoscopic Yield, Appropriateness, and Complications of Pediatric Upper Gastrointestinal Endoscopy in an Adult Suite: A Retrospective Study of 822 Children
- Manzoor Ahmad Wani, Showkat Ali Zargar, Ghulam Nabi Yatoo, Inaamul Haq, Altaf Shah, Jaswinder Singh Sodhi, Ghulam Mohammad Gulzar, Mushtaq Khan
- Clin Endosc 2020;53(4):436-442. Published online April 7, 2020
- DOI: https://doi.org/10.5946/ce.2019.118

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: This study aimed to study the endoscopic yield, appropriateness, and complications of pediatric endoscopy performed by adult gastroenterologists in an adult endoscopic suite.
Methods
This a retrospective study in which records of all the patients less than 18 years of age who underwent endoscopy in the last 5 years were studied. The indications of endoscopy in children were categorized as appropriate or inappropriate per the latest guidelines by American Society for Gastrointestinal Endoscopy and North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Positive endoscopic yield was defined as the presence of any abnormality on endoscopy.
Results
Among the total of 822 children (age <18 years), the most common indications were variceal surveillance/eradication in 157 (19.1%), followed by dyspepsia in 143 (17.4%), upper gastrointestinal (UGI) bleeding in 136 (16.5%), recurrent abdominal pain in 94 (11.4%), unexplained anemia in 74 (9%), recurrent vomiting in 50 (6.08%), chronic refractory gastroesophageal reflux disease in 34 (4.1%) and others; 780 out of 822 endoscopic procedures (94.9%) done in children were appropriate as per the guidelines. The endoscopic yield was 45.8%, highest in patients with UGI bleeding (71.3%), followed by variceal surveillance (54.8%), recurrent vomiting (38%), dyspepsia (37.8%), and recurrent abdominal pain (36%). Minor adverse events occurred in 7.3% of children.
Conclusions
Pediatric endoscopy performed by an experienced adult gastroenterologist may be acceptable if done in cooperation with a pediatrician. -
Citations
Citations to this article as recorded by- Which Alarm Symptoms Are Associated With Abnormal Gastrointestinal Endoscopy Among Thai Children?
Anundorn Wongteerasut
Pediatric Gastroenterology, Hepatology & Nutrition.2024; 27(2): 113. CrossRef - Paediatric gastrointestinal endoscopy in the Asian-Pacific region: Recent advances in diagnostic and therapeutic techniques
James Guoxian Huang, Pornthep Tanpowpong
World Journal of Gastroenterology.2023; 29(18): 2717. CrossRef - Gastrointestinal Bleeding in Children: The Role of Endoscopy and the Sheffield Scoring System in a Resource-Limited Setting
Oluwafunmilayo Funke Adeniyi, Olufunmilayo Adenike Lesi, Emuobor Aghoghor Odeghe, Ganiyat Oyeleke, Nicholas Croft
JPGN Reports.2023; 4(4): e369. CrossRef - Pediatric esophagogastroduodenoscopy in china: indications, diagnostic yield, and factors associated with findings
Shengnan Wang, Xiaoxia Qiu, Jingfang Chen, Hong Mei, Haiyan Yan, Jieyu You, Ying Huang
BMC Pediatrics.2022;[Epub] CrossRef - Safety and Competency are the Main Priorities in Pediatric Endoscopy
Byung-Ho Choe
Clinical Endoscopy.2020; 53(4): 379. CrossRef
- Which Alarm Symptoms Are Associated With Abnormal Gastrointestinal Endoscopy Among Thai Children?
- 4,423 View
- 152 Download
- 6 Web of Science
- 5 Crossref

-
Determining the Safety and Effectiveness of Electrocautery Enhanced Scissors for Peroral Endoscopic Myotomy (with Video)

- Kelly E. Hathorn, Walter W. Chan, Hiroyuki Aihara, Christopher C. Thompson
- Clin Endosc 2020;53(4):443-451. Published online May 22, 2020
- DOI: https://doi.org/10.5946/ce.2019.214

-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Peroral endoscopic myotomy (POEM) has recently come to the forefront in the management of achalasia. We aimed to analyze the efficacy and safety of the use of electrocautery enhanced scissors (EES) for POEM.
Methods
This retrospective cohort study prospectively collected the data of all adult patients (aged ≥18 years) with normal foregut anatomy who underwent POEM using EES. The patients’ baseline characteristics and procedure details (time, tunnel length, myotomy length, depth, and location) were recorded. The primary outcome was clinical success (3-month post-procedure Eckardt score of ≤3). The secondary outcomes were technical success and adverse events. A paired Student’s t-test was performed.
Results
Fifteen patients were included in this study. The technical success rate of myotomy using EES was 100%. Fellows participated in the myotomy in all cases. The clinical success rate was 93.3% (14/15). The mean pre-Eckardt score was 5.4±2.5, while the mean post-Eckardt score was 1.3±1.3, which indicated a significant improvement (p≤0.0001). The most common treatment-related adverse events were post-procedure pain (4, 26.7%) and symptomatic reflux disease (4, 26.7%).
Conclusions
In the largest series to date on the use of EES in POEM, we demonstrated that this technique has both technical and clinical efficacy as well as an excellent safety profile. -
Citations
Citations to this article as recorded by- Peroral endoscopic myotomy using an endoscopic dissector: another novel device in our toolbox
Shruti Mony, Apurva Shrigiriwar, Andrew Canakis, Mouen A. Khashab
VideoGIE.2023; 8(1): 5. CrossRef - Peroral endoscopic myotomy (POEM) is more cost-effective than laparoscopic Heller myotomy in the short term for achalasia: economic evaluation from a randomized controlled trial
Tatiana Morgado Conte, Luciana Bertocco de Paiva Haddad, Igor Braga Ribeiro, Eduardo Turiani Hourneaux de Moura, Luiz Augusto Carneiro DʼAlbuquerque, Eduardo Guimarães Hourneaux de Moura
Endoscopy International Open.2020; 08(11): E1673. CrossRef
- Peroral endoscopic myotomy using an endoscopic dissector: another novel device in our toolbox
- 5,126 View
- 76 Download
- 2 Web of Science
- 2 Crossref

- Bleeding after Endoscopic Resection in Patients with End-Stage Renal Disease on Dialysis: A Multicenter Propensity Score-Matched Analysis
- In Kyung Yoo, Chan Gyoo Kim, Young Ju Suh, Younkyung Oh, Gwang Ho Baik, Sun Moon Kim, Young Dae Kim, Chul-Hyun Lim, Jung Won Jeon, Su Jin Hong, Byoung Wook Bang, Joon Sung Kim, Jun-Won Chung
- Clin Endosc 2020;53(4):452-457. Published online October 25, 2019
- DOI: https://doi.org/10.5946/ce.2019.107
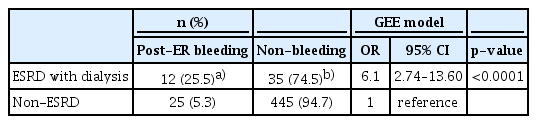
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Frequent bleeding after endoscopic resection (ER) has been reported in patients with end-stage renal disease (ESRD). We aimed to evaluate the association and clinical significance of bleeding with ER in ESRD patients on dialysis.
Methods
Between February 2008 and December 2018, 7,571 patients, including 47 ESRD patients on dialysis who underwent ER for gastric neoplasia, were enrolled. A total of 47 ESRDpatients on dialysis were propensity score-matched 1:10 to 470 non-ESRD patients, to adjust for between-group differences in variables such as age, sex, comorbidities, anticoagulation use, tumor characteristics, and ER method. Matching was performed using an optimal matching algorithm. For the matched data, clustered comparisons were performed using the generalized estimating equation method. Medical records were retrospectively reviewed. Frequency and outcomes of post-ER bleeding were evaluated.
Results
Bleeding was more frequent in the ESRD with dialysis group than in the non-ESRD group. ESRD with dialysis conferred a significant risk of post-ER bleeding (odds ratio, 6.1; 95% confidence interval, 2.7–13.6; p<0.0001). All post-ER bleeding events were controlled using endoscopic hemostasis except in 1 non-ESRD case that needed surgery.
Conclusions
ESRD with dialysis confers a bleeding risk after ER. However, all bleeding events could be managed endoscopically without sequelae. Concern about bleeding should not stop endoscopists from performing ER in ESRD patients on dialysis. -
Citations
Citations to this article as recorded by- Effect of renal insufficiency on the short‐ and long‐term outcomes of endoscopic submucosal dissection for early gastric cancer: Propensity score‐matched analysis
Tae‐Se Kim, Byung‐Hoon Min, Sun‐Young Baek, Kyunga Kim, Yang Won Min, Hyuk Lee, Poong‐Lyul Rhee, Jae J. Kim, Jun Haeng Lee
Digestive Endoscopy.2023; 35(7): 869. CrossRef - Safeness of Endoscopic Resection in Patients with End-Stage Renal Disease on Dialysis
Sun-Jin Boo
Clinical Endoscopy.2020; 53(4): 381. CrossRef
- Effect of renal insufficiency on the short‐ and long‐term outcomes of endoscopic submucosal dissection for early gastric cancer: Propensity score‐matched analysis
- 4,596 View
- 133 Download
- 2 Web of Science
- 2 Crossref

- Efficacy and Safety of Endoscopic Treatment for Gastrointestinal Stromal Tumors in the Upper Gastrointestinal Tract
- Cicilia Marcella, Shakeel Sarwar, Hui Ye, Rui Hua Shi
- Clin Endosc 2020;53(4):458-465. Published online March 17, 2020
- DOI: https://doi.org/10.5946/ce.2019.121
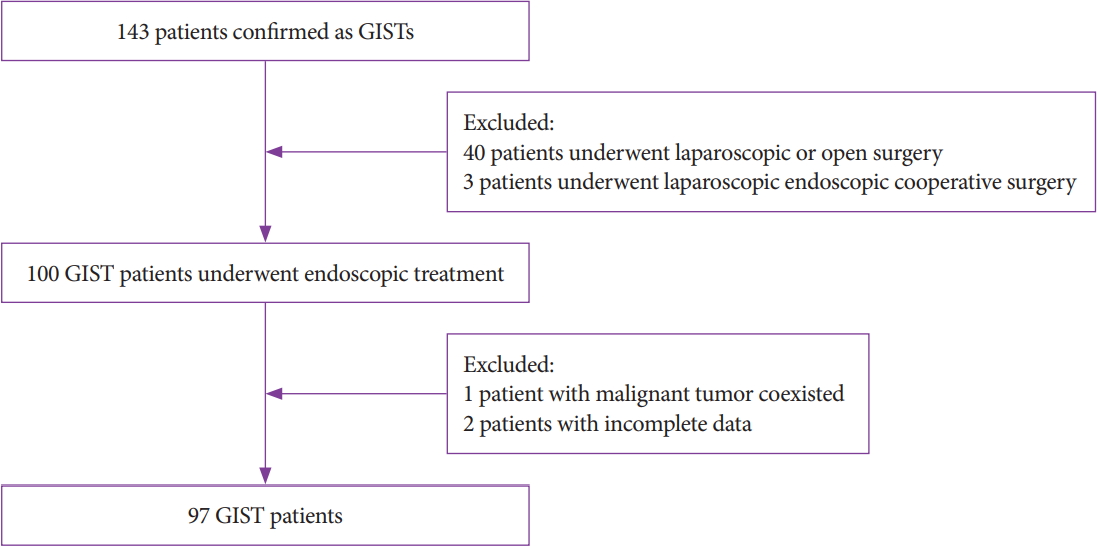
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic treatment (ET) has been applied for decades to treat subepithelial tumors, including gastrointestinal stromal tumors (GISTs). However, the efficacy of ET remains debatable. In this study, we evaluated the efficacy and safety of ET for GISTs in the upper gastrointestinal tract.
Methods
This retrospective single-center study included 97 patients who underwent ET. All patients were enrolled from July 2014 to July 2018. Parameters such as demographics, size, resection margin, complications, pathological features, procedure time, total cost, and follow-up were investigated and analyzed.
Results
Our study achieved 100% en bloc resection and 77.4% (72/93) R0 resection. The most common location was the fundus with a mean tumor size of 2.1±1.43 cm. The mean age, procedure time, hospital stay, and cost were 59.7±11.29 years, 64.7±35.23 minutes, 6.8 days, and 5,337 dollars, respectively. According to National Institutes of Health classification, 63 (64.9%), 26 (26.8%), 5 (5.2%), and 3 (3.1%) patients belonged to the very low, low, intermediate, and high risk classification, respectively. Immunohistochemistry results showed a 100% positive rate of CD34, DOG-1, CD117, and Ki67. A mean follow-up of 21.3±13.0 months showed no recurrence or metastasis.
Conclusions
ET is effective and safe for curative removal of GISTs in the upper gastrointestinal tract, and it can be a treatment of choice for patients with no metastasis. -
Citations
Citations to this article as recorded by- Comparison of endoscopic full-thickness resection and cap-assisted endoscopic full-thickness resection in the treatment of small (≤1.5 cm) gastric GI stromal tumors
Jinping Yang, Muhan Ni, Jingwei Jiang, Ximei Ren, Tingting Zhu, Shouli Cao, Shahzeb Hassan, Ying Lv, Xiaoqi Zhang, Yongyue Wei, Lei Wang, Guifang Xu
Gastrointestinal Endoscopy.2022; 95(4): 660. CrossRef - The necessarity of treatment for small gastric subepithelial tumors (1–2 cm) originating from muscularis propria: an analysis of 972 tumors
Jinlong Hu, Xinzhu Sun, Nan Ge, Sheng Wang, Jintao Guo, Xiang Liu, Guoxin Wang, Siyu Sun
BMC Gastroenterology.2022;[Epub] CrossRef - Natural History of Asymptomatic Esophageal Subepithelial Tumors of 30 mm or Less in Size
Seokin Kang, Do Hoon Kim, Yuri Kim, Dongsub Jeon, Hee Kyong Na, Jeong Hoon Lee, Ji Yong Ahn, Kee Wook Jung, Kee Don Choi, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung
Journal of Korean Medical Science.2022;[Epub] CrossRef - Massive Digestive Hemorrhagia Revealing a Gastro-Intestinal Stromal Tumor of the Jejunum
Yasmine Cherouaqi, Fatima zahra Belabbes, Hanane Delsa, Anass Nadi, Fedoua Rouibaa
Cureus.2021;[Epub] CrossRef - Endoscopic Treatment for Gastrointestinal Stromal Tumors in the Upper Gastrointestinal Tract
In Kyung Yoo, Joo Young Cho
Clinical Endoscopy.2020; 53(4): 383. CrossRef - Recent advances in the management of gastrointestinal stromal tumor
Monjur Ahmed
World Journal of Clinical Cases.2020; 8(15): 3142. CrossRef
- Comparison of endoscopic full-thickness resection and cap-assisted endoscopic full-thickness resection in the treatment of small (≤1.5 cm) gastric GI stromal tumors
- 4,658 View
- 150 Download
- 6 Web of Science
- 6 Crossref

- Endoscopic Findings in Patients Under the Age of 40 Years with Hematochezia in Singapore
- Man Hon Tang, Fung Joon Foo, Chee Yung Ng
- Clin Endosc 2020;53(4):466-470. Published online June 18, 2020
- DOI: https://doi.org/10.5946/ce.2019.029
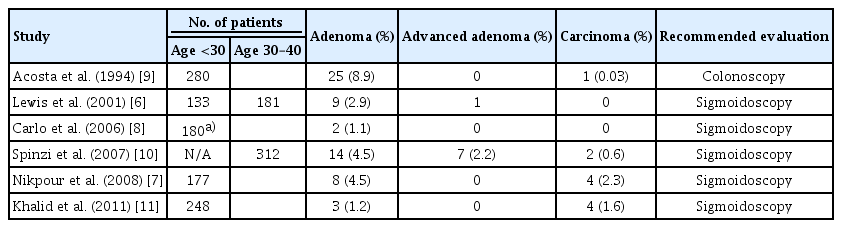
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Sigmoidoscopy is performed in most medical centers to evaluate the distal colons of young adults presenting with hematochezia who are at risk of developing proximal lesions. Colonoscopies offer more complete evaluations but are associated with a higher incidence of complications and possible low yield.
Methods
An analysis was conducted on colonoscopies performed in our center on patients 40 years of age or younger. The study population was sub-divided into 2 age groups for analysis: <30 years of age and 30–39 years of age.
Results
We recruited 453 patients for the study. Patients were 115 and 338 individuals that were <30 and 30–39 years of age, respectively. Hemorrhoids was identified as the cause of bleeding in the majority of cases. The overall incidence of polyps was 6.5%; this was significantly higher in the 30–39 age group (7.4% vs. 1.7%, p=0.026). There were two cases of advanced/malignant polyps. While the majority of the polyps were in the distal colon, 28% of the polyps in the older age group were found in the proximal colon. There was one case of colonic perforation.
Conclusions
Colonic polyps are more prevalent in patients aged 30–39. Colonoscopies should be considered for patients over the age of 30 with rectal bleeding. -
Citations
Citations to this article as recorded by- Comparing efficacy and factors of postoperative bleeding in endoscopic mucosal resection vs coagulation for intestinal polyps
Zhiang Li, Fei Yu, Chaoqian Wang, Zhang Du
Medicine.2023; 102(37): e34941. CrossRef - The role of colonoscopy in young patients with rectal bleeding: a systematic review and meta-analysis
Tuane Colles, Patrícia K. Ziegelmann, Daniel C. Damin
International Journal of Colorectal Disease.2023;[Epub] CrossRef - Usefulness of Colonoscopy in Patients with Hematochezia Aged under 40 Years
Hee Chan Yang, Sang Wook Kim
Clinical Endoscopy.2020; 53(4): 385. CrossRef
- Comparing efficacy and factors of postoperative bleeding in endoscopic mucosal resection vs coagulation for intestinal polyps
- 4,137 View
- 82 Download
- 2 Web of Science
- 3 Crossref

- Modified Endoscopic Ultrasound Needle to Obtain Histological Core Tissue Samples: A Retrospective Analysis
- Munish Ashat, Kaartik Soota, Jagpal S. Klair, Sarika Gupta, Chris Jensen, Arvind R. Murali, Randhir Jesudoss, Rami El-Abiad, Henning Gerke
- Clin Endosc 2020;53(4):471-479. Published online February 5, 2020
- DOI: https://doi.org/10.5946/ce.2019.108

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic ultrasound (EUS)-guided fine-needle aspiration is very effective for providing specimens for cytological evaluation. However, the ability to provide sufficient tissue for histological evaluation has been challenging due to the technical limitations of dedicated core biopsy needles. Recently, a modified EUS needle has been introduced to obtain tissue core samples for histological analysis. We aimed to determine (1) its ability to obtain specimens for histological assessment and (2) the diagnostic accuracy of EUS-guided fine-needle biopsy (EUS-FNB) using this needle.
Methods
We retrospectively analyzed consecutive cases of FNB using modified EUS needles for 342 lesions in 303 patients. The cytology and histological specimens were analyzed. Diagnostic accuracy was calculated.
Results
Adequate cytological and histological assessment was possible in 293/342 (86%) and 264/342 (77%) lesions, respectively. Diagnostic accuracy of the cytological specimen was 294/342 (86%) versus 254/342 (74%) for the histological specimen (p<0.01). Diagnostic accuracy of the combined cytological and histological assessment was 323/342 (94.4%), which was significantly higher than that of both histology alone (p<0.001) and cytology alone (p=0.001).
Conclusions
EUS-FNB with the modified EUS needle provided histologic tissue cores in the majority of cases and achieved excellent diagnostic accuracy with few needle passes. -
Citations
Citations to this article as recorded by- Effect of wet-heparinized suction on the quality of mediastinal solid tumor specimens obtained by endoscopic ultrasound-guided fine-needle aspiration: a retrospective study from a single center
Bo Xu, Qian Lu, Rong Fang, Xiaojuan Dai, Haiyan Xu, Xiangwu Ding, Huawei Gui
BMC Gastroenterology.2023;[Epub] CrossRef - Randomized controlled trial comparing the Franseen needle with the Fork-tip needle for EUS-guided fine-needle biopsy
Munish Ashat, Jagpal S. Klair, Sydney L. Rooney, Sagar J. Vishal, Chris Jensen, Nadav Sahar, Arvind R. Murali, Rami El-Abiad, Henning Gerke
Gastrointestinal Endoscopy.2021; 93(1): 140. CrossRef
- Effect of wet-heparinized suction on the quality of mediastinal solid tumor specimens obtained by endoscopic ultrasound-guided fine-needle aspiration: a retrospective study from a single center
- 4,587 View
- 112 Download
- 2 Web of Science
- 2 Crossref

- Efficacy and Safety of Lumen-Apposing Stents for Management of Pancreatic Fluid Collections in a Community Hospital Setting
- Rajat Garg, Abdelkader Chaar, Susan Szpunar, Babu P. Mohan, Mohammed Barawi
- Clin Endosc 2020;53(4):480-486. Published online October 16, 2019
- DOI: https://doi.org/10.5946/ce.2019.116
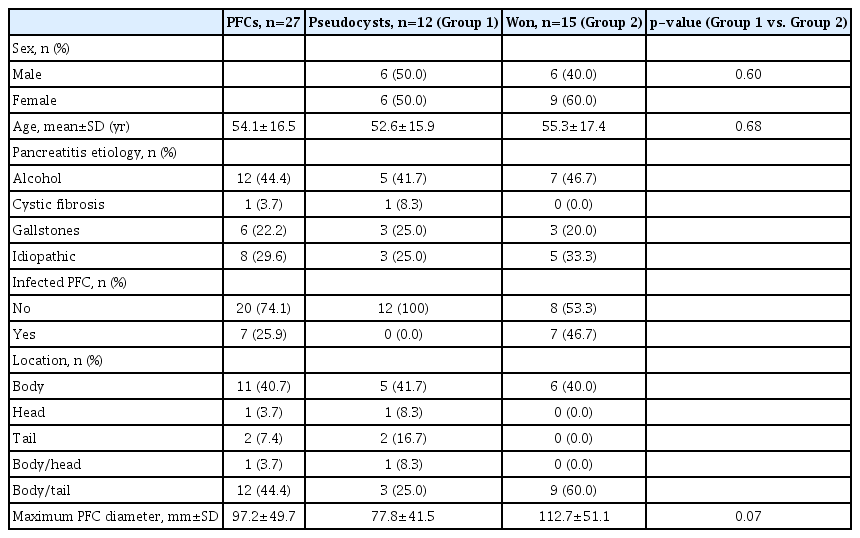
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic ultrasound-guided transmural drainage and necrosectomy employing lumen-apposing metal stent (LAMS) are used for treating pancreatic fluid collections (PFCs) with excellent results from academic centers. Herein, we report the efficacy and safety of LAMS in the treatment of PFCs at a community hospital.
Methods
We retrospectively reviewed the etiology of pancreatitis, type and size of PFCs, length of procedure, technical success, clinical success, adverse events, and stent removal. The primary outcome was the rate of clinical success, and secondary outcomes were technical success and adverse events.
Results
Twenty-seven patients with a mean age of 54.1±6.5 years were included, 44% of which were men. The mean size of the PFCs was 9.7±5.0 cm (range, 3–21). The most common etiology of pancreatitis was alcohol (44%) followed by idiopathic causes (30%) and presence of gallstones (22%). The diagnosis was pseudocyst in 44.4% (12/27) and walled off necrosis in 55.6% (15/27) of patients. There was 100% technical success without any complications. Clinical success was achieved in 22 of 27 patients (81.5%) who underwent stent removal.
Conclusions
Our study is the first to report that endoscopic therapy of PFCs using LAMS is safe and effective even in a community hospital setting with limited resources and support compared to large academic centers. -
Citations
Citations to this article as recorded by- EUS-guided interventional therapies for pancreatic diseases
Rongmin Xu, Kai Zhang, Nan Ge, Siyu Sun
Frontiers in Medicine.2024;[Epub] CrossRef - Trans-cavity lumen-apposing metal stent removal: an alternative safe modality
Giacomo Emanuele Maria Rizzo, Ilaria Tarantino
Clinical Endoscopy.2023; 56(1): 129. CrossRef - Lumen-apposing-metal stent misdeployment in endoscopic ultrasound-guided drainages: A systematic review focusing on issues and rescue management
Elia Armellini, Flavio Metelli, Andrea Anderloni, Anna Cominardi, Giovanni Aragona, Michele Marini, Fabio Pace
World Journal of Gastroenterology.2023; 29(21): 3341. CrossRef - Lumen-apposing metal stents: How far are we from standardization? An Italian survey
Carlo Fabbri, Chiara Coluccio, Cecilia Binda, Alessandro Fugazza, Andrea Anderloni, Ilaria Tarantino, NA i-EUS Group
Endoscopic Ultrasound.2022; 11(1): 59. CrossRef - Endoscopic Drainage of Giant Pancreatic Pseudocysts Using Both Lumen-Apposing Metal Stent and Plastic Stent: A Report of Two Cases and Review of the Current Literature
Hussam I. A. Alzeerelhouseini, Muawiyah Elqadi, Mohammad N. Elqadi, Sadi A. Abukhalaf, Hazem A. Ashhab, Yoshifumi Nakayama
Case Reports in Gastrointestinal Medicine.2021; 2021: 1. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Comparative outcomes of endoscopic ultrasound‐guided lumen‐apposing mental stents drainage for pancreatic pseudocysts and walled‐off necrosis: Case series and meta‐analysis
Jing Li, Qian Zhang, Anni Zhou, Guiping Zhao, Peng Li
Chronic Diseases and Translational Medicine.2021; 7(3): 157. CrossRef - Reply
Kazuki Takeishi, Toru Ikegami, Tomoharu Yoshizumi, Nao Fujimori, Masaki Mori
Liver Transplantation.2020; 26(5): 727. CrossRef - Safety and efficacy of lumen-apposing metal stents versus plastic stents to treat walled-off pancreatic necrosis: systematic review and meta-analysis
Vinay Chandrasekhara, Marc Barthet, Jacques Devière, Fateh Bazerbachi, Sundeep Lakhtakia, Jeffrey J. Easler, Joyce A. Peetermans, Edmund McMullen, Ornela Gjata, Margaret L. Gourlay, Barham K. Abu Dayyeh
Endoscopy International Open.2020; 08(11): E1639. CrossRef
- EUS-guided interventional therapies for pancreatic diseases
- 10,158 View
- 198 Download
- 8 Web of Science
- 9 Crossref

Case Reports
- Endoscopic Submucosal Dissection of a Colonic Calcifying Fibrous Tumor
- Jaeyoung Kim, Seongyul Ryu, Yeon-Ji Kim
- Clin Endosc 2020;53(4):487-490. Published online January 21, 2020
- DOI: https://doi.org/10.5946/ce.2019.138

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - A 49-year-old woman was referred to our hospital for further treatment due to the suspicion of a submucosal tumor in a routine screening colonoscopy. On colonoscopy, a 1-cm sized subepithelial mass with normal overlying mucosa in the hepatic flexure was found. Endoscopic ultrasonography (EUS) showed a homogenous hypoechoic lesion arising from the second and third layer. We were unable to make a final diagnosis because the lesion showed a small tumor with atypical macroscopic morphology including EUS findings. Therefore, endoscopic submucosal dissection was performed for the diagnostic treatment of the tumor. Submucosal dissection was performed just above the muscle layer, and the tumor was removed completely and reliably without any acute complications such as perforation. Based on histopathological findings, we diagnosed a benign, calcifying fibrous tumor (CFT). The present case is the first report of successful endoscopic diagnosis and treatment of colonic CFT mimicking a submucosal tumor.
-
Citations
Citations to this article as recorded by- Feasibility of endoscopic resection and impact of endoscopic ultrasound-based surveillance on colorectal subepithelial tumors
Eun Young Park, Dong Hoon Baek, Seung Min Hong, Bong Eun Lee, Moon Won Lee, Gwang Ha Kim, Geun Am Song
Surgical Endoscopy.2023; 37(9): 6867. CrossRef - Submucosal Necrotic Nodule of the Colon: An Enigmatic Entity Potentially Related to Anisakis Infection
Raul S. Gonzalez, Laura G. Pastrián, Sergey Pyatibrat, Hernan Dario Quiceno Arias, Yolanda Rodriguez Gil, Adam L. Booth, Itziar de la Peña Navarro, Maddi Garmendia-Irizar, Jennifer R. Lapointe, Mousa Mobarki, Luiz Miguel Nova-Camacho, Gina Parini, Estefan
Archives of Pathology & Laboratory Medicine.2023; 147(11): 1315. CrossRef
- Feasibility of endoscopic resection and impact of endoscopic ultrasound-based surveillance on colorectal subepithelial tumors
- 4,126 View
- 95 Download
- 2 Web of Science
- 2 Crossref

- Endoscopic Self-Expandable Metal Stent Placement for Malignant Afferent Loop Obstruction After Pancreaticoduodenectomy: A Case Series and Review
- Arata Sakai, Hideyuki Shiomi, Takao Iemoto, Ryota Nakano, Takuya Ikegawa, Takashi Kobayashi, Atsuhiro Masuda, Yuzo Kodama
- Clin Endosc 2020;53(4):491-496. Published online March 3, 2020
- DOI: https://doi.org/10.5946/ce.2019.145
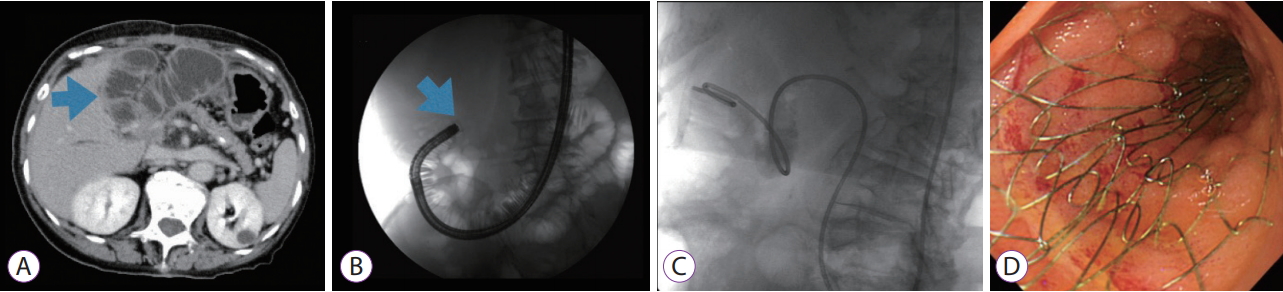
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - In this study, we assessed a series of our cases in which endoscopic self-expandable metal stents (SEMSs) were used to treat malignant afferent loop obstruction (ALO) that arose after pancreaticoduodenectomy (PD). We retrospectively examined the records of 7 patients who underwent endoscopic SEMS placement for malignant ALO following PD. Clinical success was achieved in all cases. The median procedure time was 30 min (range, 15–50 min). There were no cases of stent occlusion, and no procedure-related adverse events were encountered. All patients died of their primary disease, and the median overall survival period was 155 days (range, 96–374 days). A re-intervention involving endoscopic ultrasound-guided hepaticogastrostomy combined with antegrade stenting was performed for obstructive jaundice and acute cholangitis in 1 case. In conclusion, endoscopic SEMS placement may be an effective and safe treatment for malignant ALO that arises after PD.
-
Citations
Citations to this article as recorded by- Efficacy of endoscopic ultrasound‐guided gastroenterostomy using self‐expandable metallic stent for afferent loop syndrome: A single‐center retrospective study
Yuya Hagiwara, Susumu Hijioka, Yoshikuni Nagashio, Yuta Maruki, Akihiro Ohba, Yuki Kawasaki, Kotaro Takeshita, Tetsuro Takasaki, Daiki Agarie, Hidenobu Hara, Shin Yagi, Soma Fukuda, Masaru Kuwada, Daiki Yamashige, Kohei Okamoto, Mark Chatto, Shunsuke Kond
Journal of Gastroenterology and Hepatology.2024;[Epub] CrossRef - The Role of Endoscopic Management in Afferent Loop Syndrome
Clement Chun Ho Wu, Elizabeth Brindise, Rami El Abiad, Mouen A. Khashab
Gut and Liver.2023; 17(3): 351. CrossRef - Early and late effects of endoscopic interventions in patients with malignant afferent loop syndrome: A single‐center experience and literature review
Kenjiro Yamamoto, Takao Itoi, Yukitoshi Matsunami, Atsushi Sofuni, Takayoshi Tsuchiya, Shuntaro Mukai, Hiroyuki Kojima, Hirohito Minami, Ryosuke Nakatsubo, Ryosuke Tonozuka
Journal of Hepato-Biliary-Pancreatic Sciences.2023;[Epub] CrossRef - Simultaneous stent placement for biliary and afferent loop obstruction due to tumor recurrence after pancreatoduodenectomy
Tatsunori Satoh, Hirotoshi Ishiwatari, Kazuma Ishikawa, Hidenori Kimura, Hiroyuki Matsubayashi, Hiroyuki Ono
Endoscopy.2022; 54(09): E524. CrossRef - Extra-anatomic percutaneous stenting of a malignant afferent loop obstruction following pancreaticoduodenectomy
Stefan Lam, Sarah Khan, Robert Hutchins, Tim Fotheringham
International Journal of Gastrointestinal Intervention.2022; 11(2): 77. CrossRef - Endoscopic Transluminal Stent Placement for Malignant Afferent Loop Obstruction
Chinatsu Yonekura, Takashi Sasaki, Takafumi Mie, Takeshi Okamoto, Tsuyoshi Takeda, Takaaki Furukawa, Yuto Yamada, Akiyoshi Kasuga, Masato Matsuyama, Masato Ozaka, Naoki Sasahira
Journal of Clinical Medicine.2022; 11(21): 6357. CrossRef - Clinical management for malignant afferent loop obstruction
Arata Sakai, Hideyuki Shiomi, Atsuhiro Masuda, Takashi Kobayashi, Yasutaka Yamada, Yuzo Kodama
World Journal of Gastrointestinal Oncology.2021; 13(7): 684. CrossRef - Clinical management for malignant afferent loop obstruction
Arata Sakai, Hideyuki Shiomi, Atsuhiro Masuda, Takashi Kobayashi, Yasutaka Yamada, Yuzo Kodama
World Journal of Gastrointestinal Oncology.2021; 13(7): 509. CrossRef - Endoscopic Ultrasound-Guided Gastroenterostomy for Afferent Loop Syndrome
Hideyuki Shiomi, Arata Sakai, Ryota Nakano, Shogo Ota, Takashi Kobayashi, Atsuhiro Masuda, Hiroko Iijima
Clinical Endoscopy.2021; 54(6): 810. CrossRef - Percutaneous- and EUS-guided gastroenterostomy for malignant afferent limb syndrome
Dayyan Adoor, Zachary L. Smith
VideoGIE.2020; 5(11): 542. CrossRef
- Efficacy of endoscopic ultrasound‐guided gastroenterostomy using self‐expandable metallic stent for afferent loop syndrome: A single‐center retrospective study
- 4,513 View
- 99 Download
- 8 Web of Science
- 10 Crossref

Brief Report
- Effectiveness of Underwater Endoscopic Submucosal Dissection for a Superficial Cervical Esophageal Cancer
- Sho Sasaki, Jun Nishikawa, Kazuhiro Yamamoto, Isao Sakaida
- Clin Endosc 2020;53(4):497-498. Published online April 28, 2020
- DOI: https://doi.org/10.5946/ce.2020.031
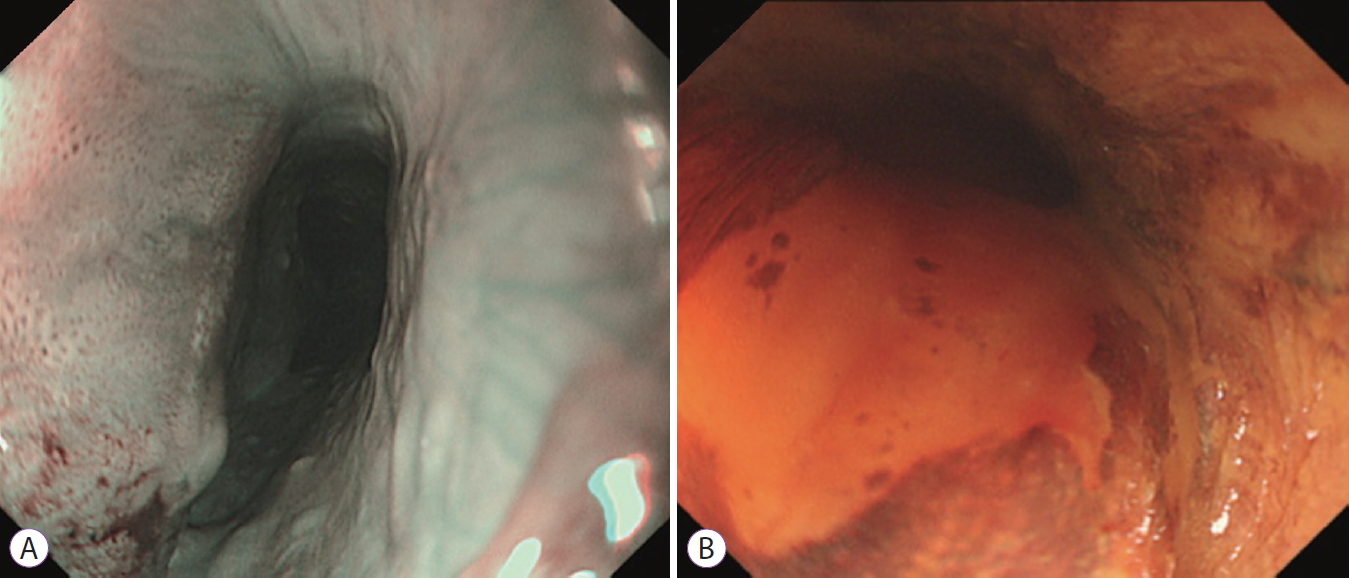
- 3,845 View
- 122 Download

Boost Your Learning with Quiz
- Rare Cause of a Colonic Laterally Spreading Tumor
- Sung Min Lee, Dong Hae Chung, Kwang An Kwon
- Clin Endosc 2020;53(4):499-501. Published online July 30, 2020
- DOI: https://doi.org/10.5946/ce.2020.197
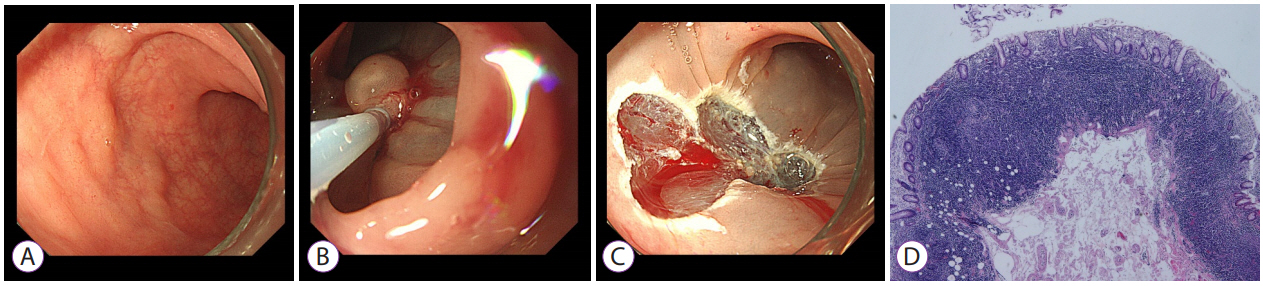
- 3,904 View
- 85 Download


 KSGE
KSGE




 First
First Prev
Prev



