Search
- Page Path
- HOME > Search
- Application of artificial intelligence for diagnosis of early gastric cancer based on magnifying endoscopy with narrow-band imaging
- Yusuke Horiuchi, Toshiaki Hirasawa, Junko Fujisaki
- Clin Endosc 2024;57(1):11-17. Published online January 5, 2024
- DOI: https://doi.org/10.5946/ce.2023.173

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Although magnifying endoscopy with narrow-band imaging is the standard diagnostic test for gastric cancer, diagnosing gastric cancer using this technology requires considerable skill. Artificial intelligence has superior image recognition, and its usefulness in endoscopic image diagnosis has been reported in many cases. The diagnostic performance (accuracy, sensitivity, and specificity) of artificial intelligence using magnifying endoscopy with narrow band still images and videos for gastric cancer was higher than that of expert endoscopists, suggesting the usefulness of artificial intelligence in diagnosing gastric cancer. Histological diagnosis of gastric cancer using artificial intelligence is also promising. However, previous studies on the use of artificial intelligence to diagnose gastric cancer were small-scale; thus, large-scale studies are necessary to examine whether a high diagnostic performance can be achieved. In addition, the diagnosis of gastric cancer using artificial intelligence has not yet become widespread in clinical practice, and further research is necessary. Therefore, in the future, artificial intelligence must be further developed as an instrument, and its diagnostic performance is expected to improve with the accumulation of numerous cases nationwide.
-
Citations
Citations to this article as recorded by- Pitfalls in Endoscopic Submucosal Dissection for Early Gastric Cancer with Papillary Adenocarcinoma
Gwang Ha Kim
Gut and Liver.2024; 18(3): 368. CrossRef
- Pitfalls in Endoscopic Submucosal Dissection for Early Gastric Cancer with Papillary Adenocarcinoma
- 2,469 View
- 184 Download
- 1 Web of Science
- 1 Crossref

- Computer-aided polyp characterization in colonoscopy: sufficient performance or not?
- Natalie Halvorsen, Yuichi Mori
- Clin Endosc 2024;57(1):18-23. Published online January 5, 2024
- DOI: https://doi.org/10.5946/ce.2023.092
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
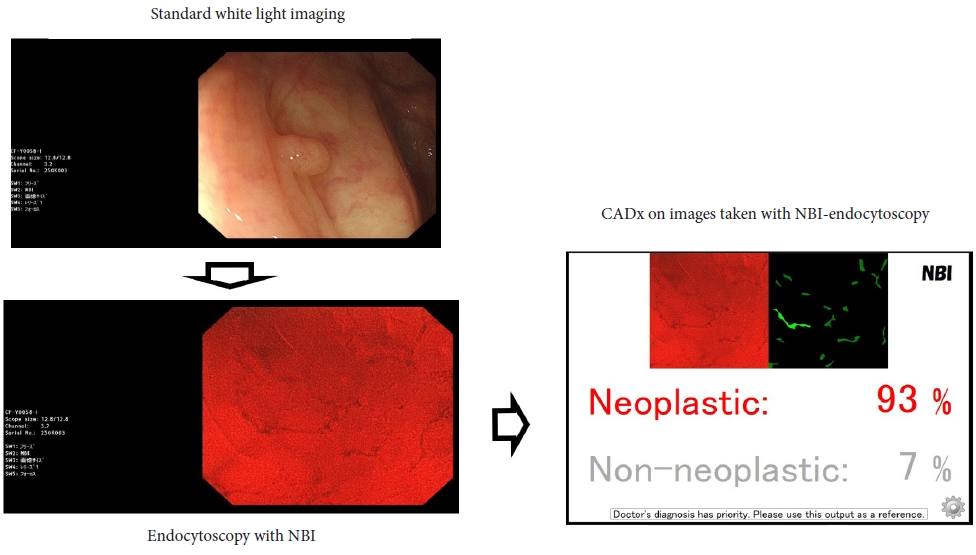
ePub - Computer-assisted polyp characterization (computer-aided diagnosis, CADx) facilitates optical diagnosis during colonoscopy. Several studies have demonstrated high sensitivity and specificity of CADx tools in identifying neoplastic changes in colorectal polyps. To implement CADx tools in colonoscopy, there is a need to confirm whether these tools satisfy the threshold levels that are required to introduce optical diagnosis strategies such as “diagnose-and-leave,” “resect-and-discard” or “DISCARD-lite.” In this article, we review the available data from prospective trials regarding the effect of multiple CADx tools and discuss whether they meet these thresholds.
- 2,113 View
- 159 Download

- Role of endoscopy in gastroesophageal reflux disease
- Daniel Martin Simadibrata, Elvira Lesmana, Ronnie Fass
- Clin Endosc 2023;56(6):681-692. Published online October 12, 2023
- DOI: https://doi.org/10.5946/ce.2023.182
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - In general, gastroesophageal reflux disease (GERD) is diagnosed clinically based on typical symptoms and/or response to proton pump inhibitor treatment. Upper gastrointestinal endoscopy is reserved for patients presenting with alarm symptoms, such as dysphagia, odynophagia, significant weight loss, gastrointestinal bleeding, or anorexia; those who meet the criteria for Barrett’s esophagus screening; those who report a lack or partial response to proton pump inhibitor treatment; and those with prior endoscopic or surgical anti-reflux interventions. Newer endoscopic techniques are primarily used to increase diagnostic yield and provide an alternative to medical or surgical treatment for GERD. The available endoscopic modalities for the diagnosis of GERD include conventional endoscopy with white-light imaging, high-resolution and high-magnification endoscopy, chromoendoscopy, image-enhanced endoscopy (narrow-band imaging, I- SCAN, flexible spectral imaging color enhancement, blue laser imaging, and linked color imaging), and confocal laser endomicroscopy. Endoscopic techniques for treating GERD include esophageal radiofrequency energy delivery/Stretta procedure, transoral incisionless fundoplication, and endoscopic full-thickness plication. Other novel techniques include anti-reflux mucosectomy, peroral endoscopic cardiac constriction, endoscopic submucosal dissection, and endoscopic band ligation. Currently, many of the new endoscopic techniques are not widely available, and their use is limited to centers of excellence.
-
Citations
Citations to this article as recorded by- Long-term efficacy of endoscopic radiofrequency Stretta therapy for patients with refractory gastroesophageal reflux disease
Sung Eun Kim
Clinical Endoscopy.2024; 57(1): 48. CrossRef - The role of ghrelin and leptin in the formation of morphological changes esophagus of patients with gastro-esophageal reflux disease against type 2 diabetes
Olha Bondar-Keleberda
EUREKA: Health Sciences.2023; (4): 24. CrossRef
- Long-term efficacy of endoscopic radiofrequency Stretta therapy for patients with refractory gastroesophageal reflux disease
- 3,414 View
- 331 Download
- 1 Web of Science
- 2 Crossref

- Preclinical study of a novel ingestible bleeding sensor for upper gastrointestinal bleeding
- Kimberly F. Schuster, Christopher C. Thompson, Marvin Ryou
- Clin Endosc 2024;57(1):73-81. Published online May 31, 2023
- DOI: https://doi.org/10.5946/ce.2022.293
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
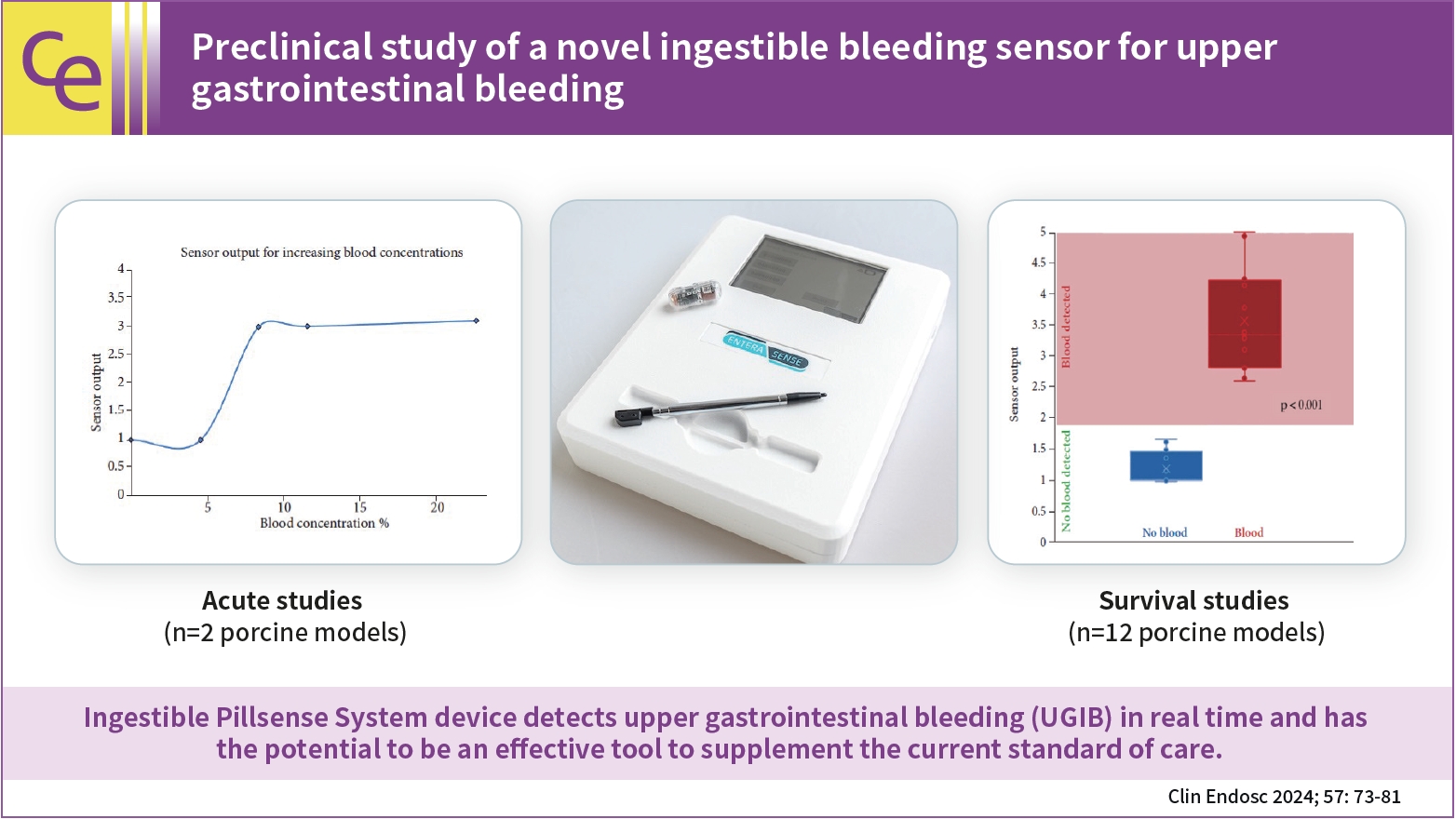
- Background
/Aims: Upper gastrointestinal bleeding (UGIB) is a life-threatening condition that necessitates early identification and intervention and is associated with substantial morbidity, mortality, and socioeconomic burden. However, several diagnostic challenges remain regarding risk stratification and the optimal timing of endoscopy. The PillSense System is a noninvasive device developed to detect blood in patients with UGIB in real time. This study aimed to assess the safety and performance characteristics of PillSense using a simulated bleeding model.
Methods
A preclinical study was performed using an in vivo porcine model (14 animals). Fourteen PillSense capsules were endoscopically placed in the stomach and blood was injected into the stomach to simulate bleeding. The safety and sensitivity of blood detection and pill excretion were also investigated.
Results
All the sensors successfully detected the presence or absence of blood. The minimum threshold was 9% blood concentration, with additional detection of increasing concentrations of up to 22.5% blood. All the sensors passed naturally through the gastrointestinal tract.
Conclusions
This study demonstrated the ability of the PillSense System sensor to detect UGIB across a wide range of blood concentrations. This ingestible device detects UGIB in real time and has the potential to be an effective tool to supplement the current standard of care. These favorable results will be further investigated in future clinical studies. -
Citations
Citations to this article as recorded by- Miniaturized Capsule System Toward Real‐Time Electrochemical Detection of H2S in the Gastrointestinal Tract
Justin M. Stine, Katie L. Ruland, Luke A. Beardslee, Joshua A. Levy, Hossein Abianeh, Santiago Botasini, Pankaj J. Pasricha, Reza Ghodssi
Advanced Healthcare Materials.2024;[Epub] CrossRef
- Miniaturized Capsule System Toward Real‐Time Electrochemical Detection of H2S in the Gastrointestinal Tract
- 2,187 View
- 141 Download
- 1 Web of Science
- 1 Crossref

-
Utility of narrow-band imaging with or without dual focus magnification in neoplastic prediction of small colorectal polyps: a Vietnamese experience

- Tien Manh Huynh, Quang Dinh Le, Nhan Quang Le, Huy Minh Le, Duc Trong Quach
- Clin Endosc 2023;56(4):479-489. Published online May 24, 2023
- DOI: https://doi.org/10.5946/ce.2022.212
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub 
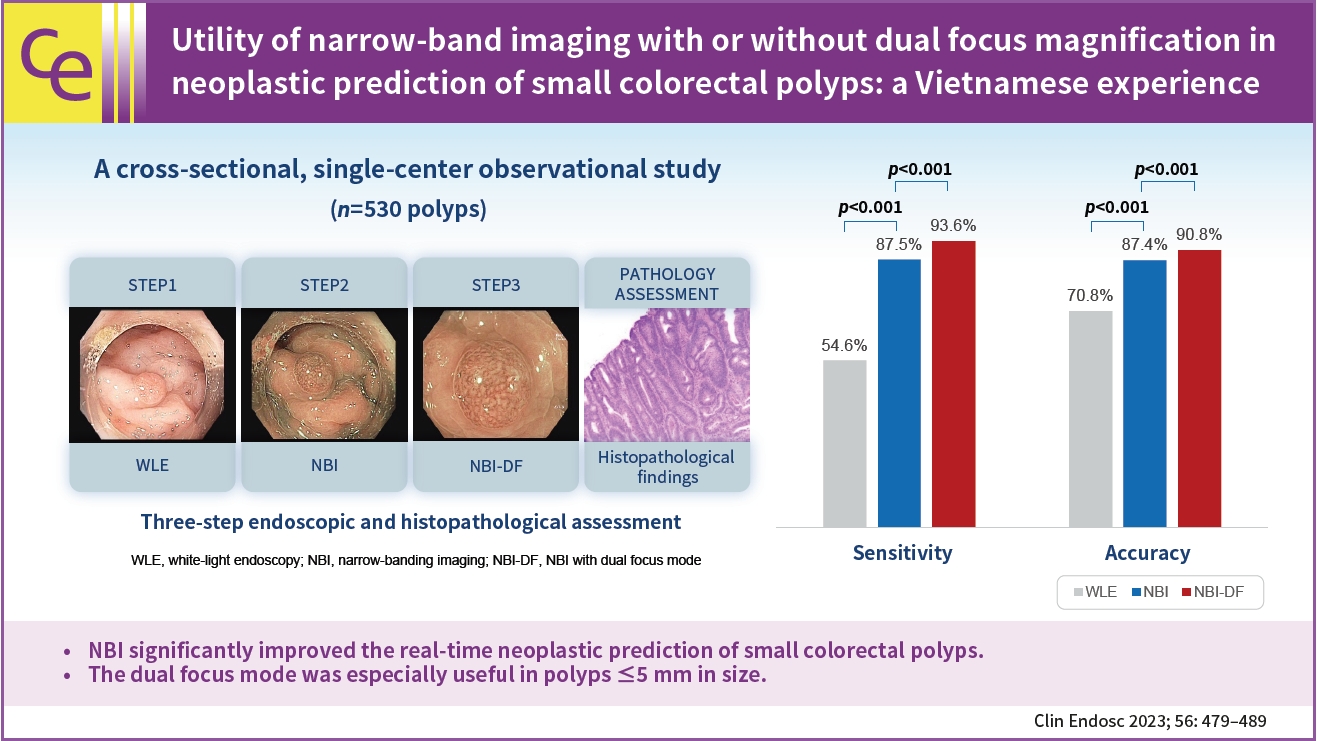
- Background
/Aims: Accurate neoplastic prediction can significantly decrease costs associated with pathology and unnecessary colorectal polypectomies. Narrow band imaging (NBI) and dual-focus (DF) mode are promising emerging optical technologies for recognizing neoplastic features of colorectal polyps digitally. This study aimed to clarify the clinical usefulness of NBI with and without DF assistance in the neoplastic prediction of small colorectal polyps (<10 mm).
Methods
This cross-sectional study included 530 small colorectal polyps from 343 consecutive patients who underwent colonoscopy at the University Medical Center from September 2020 to May 2021. Each polyp was endoscopically diagnosed in three successive steps using white-light endoscopy (WLE), NBI, and NBI-DF and retrieved for histopathological assessment. The diagnostic accuracy of each modality was evaluated with reference to histopathology.
Results
There were 295 neoplastic polyps and 235 non-neoplastic polyps. The overall accuracies of WLE, WLE+NBI, and WLE+NBI+NBI-DF in the neoplastic prediction of colorectal polyps were 70.8%, 87.4%, and 90.8%, respectively (p<0.001). The accuracy of WLE+NBI+NBI-DF was significantly higher than that of WLE+NBI in the polyp size ≤5 mm subgroup (87.3% vs. 90.1%, p<0.001).
Conclusions
NBI improved the real-time neoplastic prediction of small colorectal polyps. The DF mode was especially useful in polyps ≤5 mm in size. -
Citations
Citations to this article as recorded by- Effectiveness of Dual-Focus Magnification on Confidence Levels in Optical Diagnosis of Small Colorectal Polyps
Tien M Huynh, Quang D Le, Nhan Q Le , Huy M Le , Duc T Quach
Cureus.2024;[Epub] CrossRef - Implementing narrow banding imaging with dual focus magnification for histological prediction of small rectosigmoid polyps in Vietnamese setting
Tien Manh Huynh, Quang Dinh Le, Nhan Quang Le, Huy Minh Le, Duc Trong Quach
JGH Open.2024;[Epub] CrossRef - The role of narrow-band imaging with or without dual focus in the detection of polyps smaller than 10 mm, especially diminutive polyps
Jin Hwa Park
Clinical Endoscopy.2023; 56(4): 455. CrossRef - Strategy for post-polypectomy colonoscopy surveillance: focus on the revised Korean guidelines
Yong Soo Kwon, Su Young Kim
Journal of the Korean Medical Association.2023; 66(11): 652. CrossRef
- Effectiveness of Dual-Focus Magnification on Confidence Levels in Optical Diagnosis of Small Colorectal Polyps
- 2,505 View
- 83 Download
- 4 Web of Science
- 4 Crossref

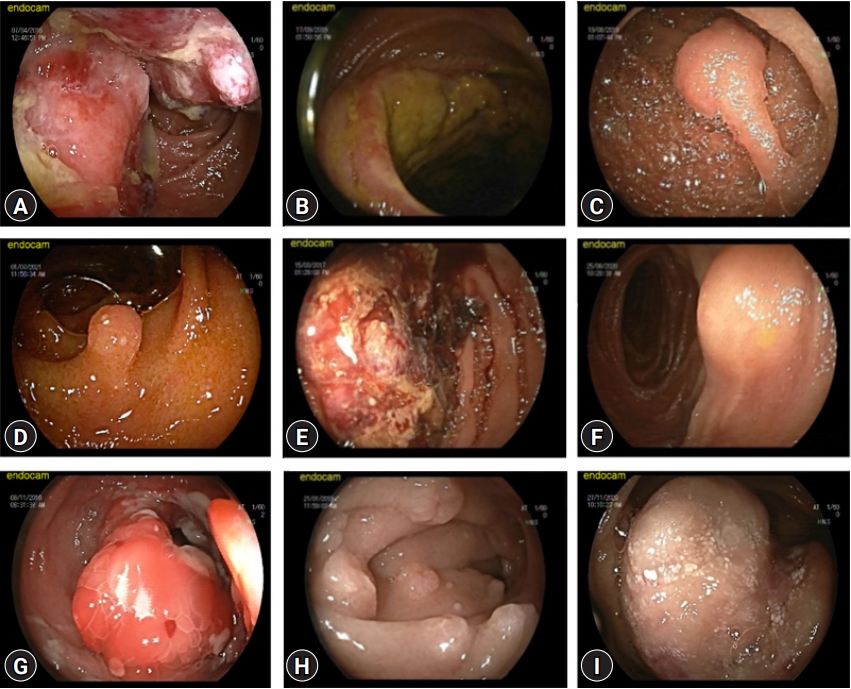
- Endoscopic and histological characteristics of small bowel tumors diagnosed by double-balloon enteroscopy
- Suleyman Dolu, Soner Onem, Zarni Htway, Farid Hajıyev, Ali Bilgen, Hatice Cilem Binicier, Ecem Kalemoglu, Ozgul Sagol, Mesut Akarsu
- Clin Endosc 2023;56(1):83-91. Published online October 27, 2022
- DOI: https://doi.org/10.5946/ce.2022.131
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Double-balloon enteroscopy (DBE) allows for the diagnoses and treatment of small bowel tumors (SBTs). This study aimed to evaluate the utility of DBE for the diagnosis and treatment of SBTs.
Methods
Patients diagnosed with SBTs who underwent DBE were included in this study. According to their endoscopic appearances, they were categorized as polyps or masses, and according to their histological characteristics, they were categorized as benign or malignant SBTs.
Results
A total of 704 patients were retrospectively analyzed, and 90 (12.8%) were diagnosed with SBTs. According to their endoscopic appearance, 48 (53.3%) had polyps and 42 (46.7%) had masses. Additionally, 53 (58.9%) and 37 (41.1%) patients had malignant and benign SBTs, respectively, depending on their histological characteristics. Patients diagnosed with polyps were younger than those diagnosed with masses (p<0.001). Patients diagnosed with benign SBTs were younger than those diagnosed with malignant SBT (p<0.001). Overall, histological diagnosis was determined using DBE in 73 (81.1%) patients.
Conclusions
DBE is a useful method for diagnosing SBTs. Additionally, the histological type of the lesion can be determined using DBE.
- 1,823 View
- 118 Download
- 1 Web of Science

- Optical diagnosis by near-focus versus normal-focus narrow band imaging colonoscopy in colorectal polyps based on combined NICE and WASP classification: a randomized controlled trial
- Nisa Netinatsunton, Natcha Cheewasereechon, Tanawat Pattarapuntakul, Jaksin Sottisuporn, Kanet Kanjanapradit, Bancha Ovartlarnporn
- Clin Endosc 2022;55(5):645-654. Published online September 8, 2022
- DOI: https://doi.org/10.5946/ce.2022.048

-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Narrow Band Imaging (NBI) International Colorectal Endoscopic (NICE) and Workgroup Serrated Polyps and Polyposis (WASP) classifications were developed for optical diagnosis of neoplastic and sessile serrated polyps, respectively. Near-focus NBI with NICE combined with WASP criteria for optical diagnosis of colonic polyps has not yet been evaluated. We aimed to compare the accuracy of near-focus NBI (group A) with normal-focus NBI (group B) in real-time optical diagnosis of colorectal polyps using combined NICE and WASP criteria.
Methods
Among 362 patients, 118 with 227 polyps were recruited. Groups A and B included 62 patients with 130 polyps (three lost polyps) and 56 patients with 106 polyps (six lost polyps), respectively. Optical diagnoses were compared with pathological reports.
Results
The accuracy of optical diagnosis of neoplastic polyps in groups A and B was not significantly different (76% vs. 71%, p=0.52). WASP criteria provided all false positive diagnoses of sessile polyps as serrated polyps in 31 (16.2%) patients.
Conclusions
Near-focus NBI was not superior to normal-focus NBI in optical diagnostics of neoplastic polyps using NICE criteria. In our study, WASP classification yielded all false positives in the diagnosis of sessile serrated adenomas/polyps. Routine real-life optical diagnosis of polyps is still unadvisable. -
Citations
Citations to this article as recorded by- Colonoscopy Quality, Innovation, and the Assessment of New Technology
Sanjay R.V. Gadi, Sriya S. Muralidharan, Jeremy R. Glissen Brown
Techniques and Innovations in Gastrointestinal Endoscopy.2024; 26(2): 177. CrossRef - Classification and endoscopic diagnosis of colorectal polyps
Ji Hyun Kim, Sung Chul Park
Journal of the Korean Medical Association.2023; 66(11): 633. CrossRef - Understanding colorectal polyps to prevent colorectal cancer
Dong-Hoon Yang
Journal of the Korean Medical Association.2023; 66(11): 626. CrossRef - AI-powered medical devices for practical clinicians including the diagnosis of colorectal polyps
Donghwan Kim, Eunsun Kim
Journal of the Korean Medical Association.2023; 66(11): 658. CrossRef - Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials
Mizuki Nagai, Sho Suzuki, Yohei Minato, Fumiaki Ishibashi, Kentaro Mochida, Ken Ohata, Tetsuo Morishita
Clinical Endoscopy.2023; 56(5): 553. CrossRef
- Colonoscopy Quality, Innovation, and the Assessment of New Technology
- 2,343 View
- 144 Download
- 5 Web of Science
- 5 Crossref

- Preparation of image databases for artificial intelligence algorithm development in gastrointestinal endoscopy
- Chang Bong Yang, Sang Hoon Kim, Yun Jeong Lim
- Clin Endosc 2022;55(5):594-604. Published online May 31, 2022
- DOI: https://doi.org/10.5946/ce.2021.229

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Over the past decade, technological advances in deep learning have led to the introduction of artificial intelligence (AI) in medical imaging. The most commonly used structure in image recognition is the convolutional neural network, which mimics the action of the human visual cortex. The applications of AI in gastrointestinal endoscopy are diverse. Computer-aided diagnosis has achieved remarkable outcomes with recent improvements in machine-learning techniques and advances in computer performance. Despite some hurdles, the implementation of AI-assisted clinical practice is expected to aid endoscopists in real-time decision-making. In this summary, we reviewed state-of-the-art AI in the field of gastrointestinal endoscopy and offered a practical guide for building a learning image dataset for algorithm development.
-
Citations
Citations to this article as recorded by- Use of artificial intelligence in the management of T1 colorectal cancer: a new tool in the arsenal or is deep learning out of its depth?
James Weiquan Li, Lai Mun Wang, Katsuro Ichimasa, Kenneth Weicong Lin, James Chi-Yong Ngu, Tiing Leong Ang
Clinical Endoscopy.2024; 57(1): 24. CrossRef - Computer‐aided diagnosis in real‐time endoscopy for all stages of gastric carcinogenesis: Development and validation study
Eun Jeong Gong, Chang Seok Bang, Jae Jun Lee
United European Gastroenterology Journal.2024; 12(4): 487. CrossRef - Assessing Endoscopic Response in Locally Advanced Rectal Cancer Treated with Total Neoadjuvant Therapy: Development and Validation of a Highly Accurate Convolutional Neural Network
Hannah Williams, Hannah M. Thompson, Christina Lee, Aneesh Rangnekar, Jorge T. Gomez, Maria Widmar, Iris H. Wei, Emmanouil P. Pappou, Garrett M. Nash, Martin R. Weiser, Philip B. Paty, J. Joshua Smith, Harini Veeraraghavan, Julio Garcia-Aguilar
Annals of Surgical Oncology.2024;[Epub] CrossRef - As how artificial intelligence is revolutionizing endoscopy
Jean-Francois Rey
Clinical Endoscopy.2024; 57(3): 302. CrossRef - Next-Generation Endoscopy in Inflammatory Bowel Disease
Irene Zammarchi, Giovanni Santacroce, Marietta Iacucci
Diagnostics.2023; 13(15): 2547. CrossRef - Public Imaging Datasets of Gastrointestinal Endoscopy for Artificial Intelligence: a Review
Shiqi Zhu, Jingwen Gao, Lu Liu, Minyue Yin, Jiaxi Lin, Chang Xu, Chunfang Xu, Jinzhou Zhu
Journal of Digital Imaging.2023; 36(6): 2578. CrossRef - AI-powered medical devices for practical clinicians including the diagnosis of colorectal polyps
Donghwan Kim, Eunsun Kim
Journal of the Korean Medical Association.2023; 66(11): 658. CrossRef - Impact of the Volume and Distribution of Training Datasets in the Development of Deep-Learning Models for the Diagnosis of Colorectal Polyps in Endoscopy Images
Eun Jeong Gong, Chang Seok Bang, Jae Jun Lee, Young Joo Yang, Gwang Ho Baik
Journal of Personalized Medicine.2022; 12(9): 1361. CrossRef
- Use of artificial intelligence in the management of T1 colorectal cancer: a new tool in the arsenal or is deep learning out of its depth?
- 3,395 View
- 249 Download
- 8 Web of Science
- 8 Crossref

- Peroral Pancreatoscopy with Videoscopy and Narrow-Band Imaging in Intraductal Papillary Mucinous Neoplasms with Dilatation of the Main Pancreatic Duct
- Yui Kishimoto, Naoki Okano, Ken Ito, Kensuke Takuma, Seiichi Hara, Susumu Iwasaki, Kensuke Yoshimoto, Yuto Yamada, Koji Watanabe, Yusuke Kimura, Hiroki Nakagawa, Yoshinori Igarashi
- Clin Endosc 2022;55(2):270-278. Published online December 6, 2021
- DOI: https://doi.org/10.5946/ce.2021.083
- Correction in: Clin Endosc 2023;56(2):261

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic evaluation of intraductal papillary mucinous neoplasms (IPMNs) is useful in determining whether the lesions are benign or malignant. This study aimed to examine the usefulness of peroral pancreatoscopy (POPS) in determining the prognosis of IPMNs.
Methods
POPS with videoscopy was performed using the mother–baby scope technique. After surgery, computed tomography/magnetic resonance cholangiopancreatography or ultrasonography and blood tests were performed every 6 months during the follow-up.
Results
A total of 39 patients with main pancreatic duct (MPD)–type IPMNs underwent POPS using a videoscope, and the protrusions in the MPD were observed in 36 patients. The sensitivity and specificity of cytology/biopsy performed at the time of POPS were 85% and 87.5%, respectively. Of 19 patients who underwent surgery, 18 (95%) patients had negative surgical margins and 1 (5%) patient had a positive margin.
Conclusions
In IPMNs with dilatation of the MPD, POPS is considered effective if the lesions can be directly observed. The diagnosis of benign and malignant lesions is possible depending on the degree of lesion elevation. However, in some cases, slightly elevated lesions may increase in size during the follow-up or multiple lesions may be simultaneously present; therefore, careful follow-up is necessary. -
Citations
Citations to this article as recorded by- Pancreatoscopy-Guided Endotherapies for Pancreatic Diseases
Yuri Hanada, Raj J. Shah
Gastrointestinal Endoscopy Clinics of North America.2024;[Epub] CrossRef - Peroral pancreatoscopy with videoscopy and narrow-band imaging in intraductal papillary mucinous neoplasms with dilatation of the main pancreatic duct
Yui Kishimoto, Naoki Okano, Ken Ito, Kensuke Takuma, Seiichi Hara, Susumu Iwasaki, Kensuke Yoshimoto, Yuto Yamada, Koji Watanabe, Yusuke Kimura, Hiroki Nakagawa, Yoshinori Igarashi
Clinical Endoscopy.2023; 56(2): 261. CrossRef - Predicting Malignancy by Peroral Pancreatoscopy of an Intraductal Papillary Mucinous Neoplasm with a Dilated Main Pancreatic Duct: Is Seeing Enough?
Yun Nah Lee, Jong Ho Moon
Clinical Endoscopy.2022; 55(2): 213. CrossRef
- Pancreatoscopy-Guided Endotherapies for Pancreatic Diseases
- 3,637 View
- 202 Download
- 2 Web of Science
- 3 Crossref

- Value of Fecal Calprotectin Measurement During the Initial Period of Therapeutic Anti-Tubercular Trial
- Hyeong Ho Jo, Eun Young Kim, Jin Tae Jung, Joong Goo Kwon, Eun Soo Kim, Hyun Seok Lee, Yoo Jin Lee, Kyeong Ok Kim, Byung Ik Jang, the Crohn’s and Colitis Association in Daegu-Gyeongbuk
- Clin Endosc 2022;55(2):256-262. Published online November 5, 2021
- DOI: https://doi.org/10.5946/ce.2021.061

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The diagnosis of intestinal tuberculosis (ITB) is often challenging. Therapeutic anti-tubercular trial (TATT) is sometimes used for the diagnosis of ITB. We aimed to evaluate the changing pattern of fecal calprotectin (FC) levels during TATT in patients with ITB.
Methods
A retrospective review was performed on the data of 39 patients who underwent TATT between September 2015 and November 2018 in five university hospitals in Daegu, South Korea. The analysis was performed for 33 patients with serial FC measurement reports.
Results
The mean age of the participants was 48.8 years. The final diagnosis of ITB was confirmed in 30 patients based on complete mucosal healing on follow-up colonoscopy performed after 2 months of TATT. Before starting TATT, the mean FC level of the ITB patients was 170.2 μg/g (range, 11.5-646.5). It dropped to 25.4 μg/g (range, 11.5-75.3) and then 23.3 μg/g (range, 11.5-172.2) after one and two months of TATT, respectively. The difference in mean FC before and one month after TATT was statistically significant (p<0.001), and FC levels decreased to below 100 μg/g in all patients after one month of TATT.
Conclusions
All ITB patients showed FC decline after only 1 month of TATT, and this finding correlated with complete mucosal healing in the follow-up colonoscopy after 2 months of TATT. -
Citations
Citations to this article as recorded by- Primary Gastric Tuberculosis in an Immunocompetent Patient: The Truth Lying beneath the Surface
Fábio Pereira Correia, Luísa Martins Figueiredo, Luís Carvalho Lourenço, Sofia Santos, Rita Theias Manso, David Horta
GE - Portuguese Journal of Gastroenterology.2024; 31(3): 191. CrossRef - Evidence-based approach to diagnosis and management of abdominal tuberculosis
Daya Krishna Jha, Mythili Menon Pathiyil, Vishal Sharma
Indian Journal of Gastroenterology.2023; 42(1): 17. CrossRef - Fecal Calprotectin as a Surrogate Marker for Mucosal Healing After Initiating the Therapeutic Anti-Tubercular Trial
Satimai Aniwan
Clinical Endoscopy.2022; 55(2): 210. CrossRef
- Primary Gastric Tuberculosis in an Immunocompetent Patient: The Truth Lying beneath the Surface
- 3,056 View
- 281 Download
- 3 Web of Science
- 3 Crossref

- Application of Current Image-Enhanced Endoscopy in Gastric Diseases
- Wansik Lee
- Clin Endosc 2021;54(4):477-487. Published online July 28, 2021
- DOI: https://doi.org/10.5946/ce.2021.160

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Image-enhanced endoscopy (IEE) plays an integral role in endoscopic diagnosis and treatment. IEE enables an early and accurate detection of cancer and characterization of lesions prior to therapeutic decisions. Ideal IEE can serve as an optical or digital chromoscopic endoscopy, as well as an optical biopsy that predicts exact histopathology. Several IEE modalities have recently been developed and are used in the clinical field. The stomach is a challenging organ for imaging because of its complex secretion function and status of Helicobacter pylori infection. Therefore, understanding the current IEE modalities for their clinical applicability in an evidence-based approach is warranted. Along with technology refinements, the new paradigm will be available for the diagnosis of gastric cancer or other conditions in the stomach in the near future.
-
Citations
Citations to this article as recorded by- The Diagnostic Performance of Linked Color Imaging Compared to White Light Imaging in Endoscopic Diagnosis of Helicobacter pylori Infection: A Systematic Review and Meta-Analysis
Jae Gon Lee, In Kyung Yoo, Abdullah Ozgur Yeniova, Sang Pyo Lee
Gut and Liver.2024; 18(3): 444. CrossRef - Magnifying Endoscopy with Narrow-Band Imaging for Duodenal Neuroendocrine Tumors
Gwang Ha Kim, Kiyoun Yi, Dong Chan Joo, Moon Won Lee, Hye Kyung Jeon, Bong Eun Lee
Journal of Clinical Medicine.2023; 12(9): 3106. CrossRef - Endoscopic Resection for Gastric Adenocarcinoma of the Fundic Gland Type: A Case Series
Hwa Jin Lee, Gwang Ha Kim, Dong Chan Joo, Moon Won Lee, Bong Eun Lee, Kyungbin Kim
The Korean Journal of Gastroenterology.2023; 81(6): 259. CrossRef - Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials
Mizuki Nagai, Sho Suzuki, Yohei Minato, Fumiaki Ishibashi, Kentaro Mochida, Ken Ohata, Tetsuo Morishita
Clinical Endoscopy.2023; 56(5): 553. CrossRef - Role of linked color imaging for upper gastrointestinal disease: present and future
Sang Pyo Lee
Clinical Endoscopy.2023; 56(5): 546. CrossRef - Endoscopic submucosal dissection for early gastric cancer: It is time to consider the quality of its outcomes
Gwang Ha Kim
World Journal of Gastroenterology.2023; 29(43): 5800. CrossRef - Quality indicators in esophagogastroduodenoscopy
Sang Yoon Kim, Jae Myung Park
Clinical Endoscopy.2022; 55(3): 319. CrossRef - Endoscopic treatment for early gastric cancer
Ji Yong Ahn
Journal of the Korean Medical Association.2022; 65(5): 276. CrossRef - Endoscopic diagnosis of early gastric cancer
Dong Chan Joo, Gwang Ha Kim
Journal of the Korean Medical Association.2022; 65(5): 267. CrossRef - Risk factors for early gastric cancer: focus on Helicobacter pylori gastritis
Hee Seok Moon
Journal of the Korean Medical Association.2022; 65(5): 259. CrossRef - Current status of the gastric cancer screening program in Korea
Young-Il Kim, Il Ju Choi
Journal of the Korean Medical Association.2022; 65(5): 250. CrossRef - Paneth Cell Carcinoma of the Stomach
Jun Wan Kim, Gwang Ha Kim, Kyung Bin Kim
The Korean Journal of Gastroenterology.2022; 80(1): 34. CrossRef - Current Evidence for a Paradigm Shift in Gastric Cancer Prevention From Endoscopic Screening toHelicobacter pyloriEradication in Korea
Young-Il Kim, Il Ju Choi
Journal of Gastric Cancer.2022; 22(3): 169. CrossRef
- The Diagnostic Performance of Linked Color Imaging Compared to White Light Imaging in Endoscopic Diagnosis of Helicobacter pylori Infection: A Systematic Review and Meta-Analysis
- 4,416 View
- 194 Download
- 14 Web of Science
- 13 Crossref

- Artificial Intelligence in Gastrointestinal Endoscopy
- Alexander P. Abadir, Mohammed Fahad Ali, William Karnes, Jason B. Samarasena
- Clin Endosc 2020;53(2):132-141. Published online March 30, 2020
- DOI: https://doi.org/10.5946/ce.2020.038
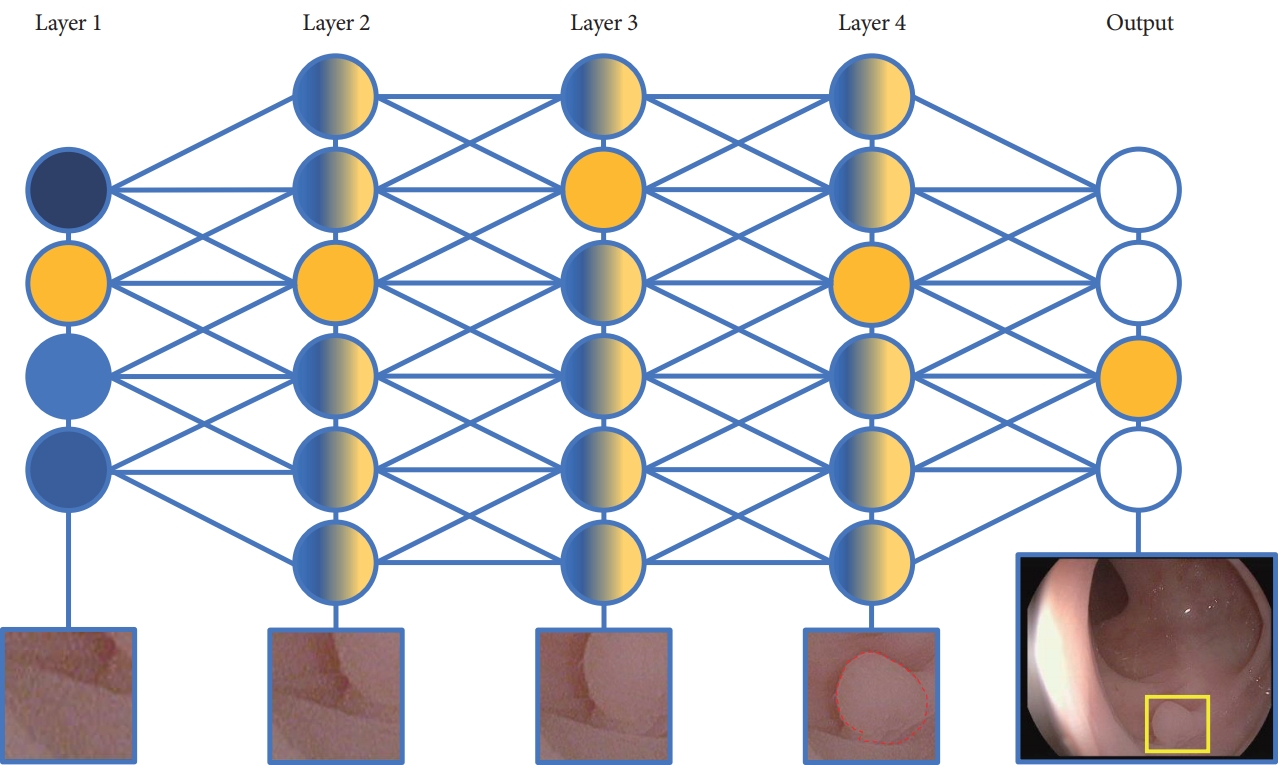
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Artificial intelligence (AI) is rapidly integrating into modern technology and clinical practice. Although in its nascency, AI has become a hot topic of investigation for applications in clinical practice. Multiple fields of medicine have embraced the possibility of a future with AI assisting in diagnosis and pathology applications.
In the field of gastroenterology, AI has been studied as a tool to assist in risk stratification, diagnosis, and pathologic identification. Specifically, AI has become of great interest in endoscopy as a technology with substantial potential to revolutionize the practice of a modern gastroenterologist. From cancer screening to automated report generation, AI has touched upon all aspects of modern endoscopy.
Here, we review landmark AI developments in endoscopy. Starting with broad definitions to develop understanding, we will summarize the current state of AI research and its potential applications. With innovation developing rapidly, this article touches upon the remarkable advances in AI-assisted endoscopy since its initial evaluation at the turn of the millennium, and the potential impact these AI models may have on the modern clinical practice. As with any discussion of new technology, its limitations must also be understood to apply clinical AI tools successfully. -
Citations
Citations to this article as recorded by- Artificial intelligence‐aided diagnostic imaging: A state‐of‐the‐art technique in precancerous screening
Yang‐Bor Lu, Si‐Cun Lu, Fu‐Dong Li, Puo‐Hsien Le, Kai‐Hua Zhang, Zi‐Zheng Sun, Yung‐Ning Huang, Yu‐Chieh Weng, Wei‐Ting Chen, Yi‐Wei Fu, Jun‐Bo Qian, Bin Hu, Hong Xu, Cheng‐Tang Chiu, Qin‐Wei Xu, Wei Gong
Journal of Gastroenterology and Hepatology.2024; 39(3): 544. CrossRef - Development of convolutional neural network models that recognize normal anatomic structures during real-time radial-array and linear-array EUS (with videos)
Carlos Robles-Medranda, Jorge Baquerizo-Burgos, Miguel Puga-Tejada, Raquel Del Valle, Juan C. Mendez, Maria Egas-Izquierdo, Martha Arevalo-Mora, Domenica Cunto, Juan Alcívar-Vasquez, Hannah Pitanga-Lukashok, Daniela Tabacelia
Gastrointestinal Endoscopy.2024; 99(2): 271. CrossRef - Repurposing of Drug Aspirin in Colon Cancer: Therapeutic Approach
Vrushali Neve, Abhijeet Kamble, Pawan Karwa
Clinical Cancer Investigation Journal.2024; 13(1): 23. CrossRef - Consensus statements on the current landscape of artificial intelligence applications in endoscopy, addressing roadblocks, and advancing artificial intelligence in gastroenterology
Sravanthi Parasa, Tyler Berzin, Cadman Leggett, Seth Gross, Alessandro Repici, Omer F. Ahmad, Austin Chiang, Nayantara Coelho-Prabhu, Jonathan Cohen, Evelien Dekker, Rajesh N. Keswani, Charles E. Kahn, Cesare Hassan, Nicholas Petrick, Peter Mountney, Jona
Gastrointestinal Endoscopy.2024;[Epub] CrossRef - Colorectal Polyp Detection Model by Using Super-Resolution Reconstruction and YOLO
Shaofang Wang, Jun Xie, Yanrong Cui, Zhongju Chen
Electronics.2024; 13(12): 2298. CrossRef - Artificial intelligence in pancreatic cancer: diagnosis, limitations, and the future prospects—a narrative review
Maanya Rajasree Katta, Pavan Kumar Reddy Kalluru, Divyaraj Amber Bavishi, Maha Hameed, Sai Sudha Valisekka
Journal of Cancer Research and Clinical Oncology.2023; 149(9): 6743. CrossRef - Deep learning-based clinical decision support system for gastric neoplasms in real-time endoscopy: development and validation study
Eun Jeong Gong, Chang Seok Bang, Jae Jun Lee, Gwang Ho Baik, Hyun Lim, Jae Hoon Jeong, Sung Won Choi, Joonhee Cho, Deok Yeol Kim, Kang Bin Lee, Seung-Il Shin, Dick Sigmund, Byeong In Moon, Sung Chul Park, Sang Hoon Lee, Ki Bae Bang, Dae-Soon Son
Endoscopy.2023; 55(08): 701. CrossRef - Update zur Navigation im OP-Saal
Philipp Anthony Wise, Alexander Studier-Fischer, Thilo Hackert, Felix Nickel
Zentralblatt für Chirurgie - Zeitschrift für Allgemeine, Viszeral-, Thorax- und Gefäßchirurgie.2023;[Epub] CrossRef - ДІАГНОСТИКА ДОБРОЯКІСНИХ ПУХЛИН ТОНКОГО КИШЕЧНИКА: СУЧАСНИЙ СТАН ПРОБЛЕМИ
В. Ю. ІЛЬЇНА-СТОГНІЄНКО, О. М. ЧАЙКА
Шпитальна хірургія. Журнал імені Л. Я. Ковальчука.2023; (1): 96. CrossRef - Future Directions in EndoHepatology
Ahmad Najdat Bazarbashi, Lolwa Al-Obaid, Marvin Ryou
Techniques and Innovations in Gastrointestinal Endoscopy.2022; 24(1): 98. CrossRef - El papel emergente de la inteligencia artificial en la endoscopia gastrointestinal: una revisión de la literatura
María José Aguilera-Chuchuca, Sergio A. Sánchez-Luna, Begoña González Suárez, Kenneth Ernest-Suárez, Andres Gelrud, Tyler M. Berzin
Gastroenterología y Hepatología.2022; 45(6): 492. CrossRef - Artificial Intelligence-Based Colorectal Polyp Histology Prediction: High Accuracy in Larger Polyps
Naoki Muguruma, Tetsuji Takayama
Clinical Endoscopy.2022; 55(1): 45. CrossRef - Assessment of the Risk of Severe Dengue Using Intrahost Viral Population in Dengue Virus Serotype 2 Patients via Machine Learning
Su-Jhen Hung, Huey-Pin Tsai, Ya-Fang Wang, Wen-Chien Ko, Jen-Ren Wang, Sheng-Wen Huang
Frontiers in Cellular and Infection Microbiology.2022;[Epub] CrossRef - Artificial intelligence in colorectal cancer screening in patients with inflammatory bowel disease
Kêmily Fuentes Marques, Alana Fuentes Marques, Marina Amorim Lopes, Rodrigo Fedatto Beraldo, Talles Bazeia Lima, Ligia Yukie Sassaki
Artificial Intelligence in Gastrointestinal Endoscopy.2022; 3(1): 1. CrossRef - Weakly supervised end-to-end artificial intelligence in gastrointestinal endoscopy
Lukas Buendgens, Didem Cifci, Narmin Ghaffari Laleh, Marko van Treeck, Maria T. Koenen, Henning W. Zimmermann, Till Herbold, Thomas Joachim Lux, Alexander Hann, Christian Trautwein, Jakob Nikolas Kather
Scientific Reports.2022;[Epub] CrossRef - Automated Detection of Bowel Preparation Scoring and Adequacy With Deep Convolutional Neural Networks
Daniel J Low, Zhuoqiao Hong, Sechiv Jugnundan, Anjishnu Mukherjee, Samir C Grover
Journal of the Canadian Association of Gastroenterology.2022; 5(6): 256. CrossRef - The emerging role of artificial intelligence in gastrointestinal endoscopy: a review
María José Aguilera-Chuchuca, Sergio A. Sánchez-Luna, Begoña González Suárez, Kenneth Ernest-Suárez, Andres Gelrud, Tyler M. Berzin
Gastroenterología y Hepatología (English Edition).2022; 45(6): 492. CrossRef - Artificial Intelligence for Colonoscopy: Past, Present, and Future
Wallapak Tavanapong, JungHwan Oh, Michael A. Riegler, Mohammed Khaleel, Bhuvan Mittal, Piet C. de Groen
IEEE Journal of Biomedical and Health Informatics.2022; 26(8): 3950. CrossRef - Künstliche Intelligenz in der Viszeralmedizin – „brave new world“ oder digitaler Horror?
R. Jakobs, M. Fried, J. Hampe
Der Gastroenterologe.2021; 16(1): 1. CrossRef - Challenges in Crohn’s Disease Management after Gastrointestinal Cancer Diagnosis
Claudio Fiorillo, Carlo Alberto Schena, Giuseppe Quero, Vito Laterza, Daniela Pugliese, Giuseppe Privitera, Fausto Rosa, Tommaso Schepis, Lisa Salvatore, Brunella Di Stefano, Luigi Larosa, Laura Maria Minordi, Luigi Natale, Giampaolo Tortora, Alessandro A
Cancers.2021; 13(3): 574. CrossRef - Robotics and Artificial Intelligence in Gastrointestinal Endoscopy: Updated Review of the Literature and State of the Art
Ivo Boškoski, Beatrice Orlandini, Luigi Giovanni Papparella, Maria Valeria Matteo, Martina De Siena, Valerio Pontecorvi, Guido Costamagna
Current Robotics Reports.2021; 2(1): 43. CrossRef - Use of Endoscopic Images in the Prediction of Submucosal Invasion of Gastric Neoplasms: Automated Deep Learning Model Development and Usability Study
Chang Seok Bang, Hyun Lim, Hae Min Jeong, Sung Hyeon Hwang
Journal of Medical Internet Research.2021; 23(4): e25167. CrossRef - Artificial intelligence in brachytherapy: a summary of recent developments
Susovan Banerjee, Shikha Goyal, Saumyaranjan Mishra, Deepak Gupta, Shyam Singh Bisht, Venketesan K, Kushal Narang, Tejinder Kataria
The British Journal of Radiology.2021;[Epub] CrossRef - Artificial Intelligence in Colorectal Cancer Screening, Diagnosis and Treatment. A New Era
Athanasia Mitsala, Christos Tsalikidis, Michail Pitiakoudis, Constantinos Simopoulos, Alexandra K. Tsaroucha
Current Oncology.2021; 28(3): 1581. CrossRef - Predicting Colorectal Cancer Occurrence in IBD
Mehmet Yalchin, Ann-Marie Baker, Trevor A. Graham, Ailsa Hart
Cancers.2021; 13(12): 2908. CrossRef - Usefulness of artificial intelligence in gastric neoplasms
Ji Hyun Kim, Seung-Joo Nam, Sung Chul Park
World Journal of Gastroenterology.2021; 27(24): 3543. CrossRef - Deep neural network approaches for detecting gastric polyps in endoscopic images
Serdar Durak, Bülent Bayram, Tolga Bakırman, Murat Erkut, Metehan Doğan, Mert Gürtürk, Burak Akpınar
Medical & Biological Engineering & Computing.2021; 59(7-8): 1563. CrossRef - Use of artificial intelligence in endoscopic ultrasound evaluation of pancreatic pathologies
Ravinder Mankoo, Ahmad H Ali, Ghassan M Hammoud
Artificial Intelligence in Gastrointestinal Endoscopy.2021; 2(3): 89. CrossRef - Role of Artificial Intelligence in Video Capsule Endoscopy
Ioannis Tziortziotis, Faidon-Marios Laskaratos, Sergio Coda
Diagnostics.2021; 11(7): 1192. CrossRef - Use of Artificial Intelligence to Improve the Quality Control of Gastrointestinal Endoscopy
Ya-qi Song, Xin-li Mao, Xian-bin Zhou, Sai-qin He, Ya-hong Chen, Li-hui Zhang, Shi-wen Xu, Ling-ling Yan, Shen-ping Tang, Li-ping Ye, Shao-wei Li
Frontiers in Medicine.2021;[Epub] CrossRef - Use of artificial intelligence in endoscopic ultrasound evaluation of pancreatic pathologies
Ravinder Mankoo, Ahmad H Ali, Ghassan M Hammoud
Artificial Intelligence in Gastrointestinal Endoscopy.2021; 2(3): 88. CrossRef - Endoscopic diagnosis and treatment of gastric dysplasia and early cancer: Current evidence and what the future may hold
Edward Young, Hamish Philpott, Rajvinder Singh
World Journal of Gastroenterology.2021; 27(31): 5126. CrossRef - Computer-Aided Diagnosis of Diminutive Colorectal Polyps in Endoscopic Images: Systematic Review and Meta-analysis of Diagnostic Test Accuracy
Chang Seok Bang, Jae Jun Lee, Gwang Ho Baik
Journal of Medical Internet Research.2021; 23(8): e29682. CrossRef - Applications of Artificial Intelligence for the Diagnosis of Gastrointestinal Diseases
Silvia Pecere, Sebastian Manuel Milluzzo, Gianluca Esposito, Emanuele Dilaghi, Andrea Telese, Leonardo Henry Eusebi
Diagnostics.2021; 11(9): 1575. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Artificial Intelligence in Capsule Endoscopy: A Practical Guide to Its Past and Future Challenges
Sang Hoon Kim, Yun Jeong Lim
Diagnostics.2021; 11(9): 1722. CrossRef - Digital surgery for gastroenterological diseases
Niall Philip Hardy, Ronan Ambrose Cahill
World Journal of Gastroenterology.2021; 27(42): 7240. CrossRef - Choledochoscopy: An update
Tsinrong Lee, Thomas Zheng Jie Teng, Vishal G Shelat
World Journal of Gastrointestinal Endoscopy.2021; 13(12): 571. CrossRef - Prediction of Submucosal Invasion for Gastric Neoplasms in Endoscopic Images Using Deep-Learning
Bum-Joo Cho, Chang Seok Bang, Jae Jun Lee, Chang Won Seo, Ju Han Kim
Journal of Clinical Medicine.2020; 9(6): 1858. CrossRef - The Impact of Artificial Intelligence in the Endoscopic Assessment of Premalignant and Malignant Esophageal Lesions: Present and Future
Daniela Cornelia Lazăr, Mihaela Flavia Avram, Alexandra Corina Faur, Adrian Goldiş, Ioan Romoşan, Sorina Tăban, Mărioara Cornianu
Medicina.2020; 56(7): 364. CrossRef - Techniques to integrate artificial intelligence systems with medical information in gastroenterology
Hong-Yu Jin, Man Zhang, Bing Hu
Artificial Intelligence in Gastrointestinal Endoscopy.2020; 1(1): 19. CrossRef - Assessing the risk of dengue severity using demographic information and laboratory test results with machine learning
Sheng-Wen Huang, Huey-Pin Tsai, Su-Jhen Hung, Wen-Chien Ko, Jen-Ren Wang, Michael R. Holbrook
PLOS Neglected Tropical Diseases.2020; 14(12): e0008960. CrossRef - Emerging use of artificial intelligence in inflammatory bowel disease
Arushi Kohli, Erik A Holzwanger, Alexander N Levy
World Journal of Gastroenterology.2020; 26(44): 6923. CrossRef
- Artificial intelligence‐aided diagnostic imaging: A state‐of‐the‐art technique in precancerous screening
- 8,286 View
- 284 Download
- 32 Web of Science
- 43 Crossref

- Endoscopic Ultrasound-Guided Fine Needle Aspiration and Endoscopic Retrograde Cholangiopancreatography-Based Tissue Sampling in Suspected Malignant Biliary Strictures: A Meta-Analysis of Same-Session Procedures
- Diogo Turiani Hourneax de Moura, Marvin Ryou, Eduardo Guimarães Hourneaux de Moura, Igor Braga Ribeiro, Wanderlei Marques Bernardo, Christopher C. Thompson
- Clin Endosc 2020;53(4):417-428. Published online November 5, 2019
- DOI: https://doi.org/10.5946/ce.2019.053
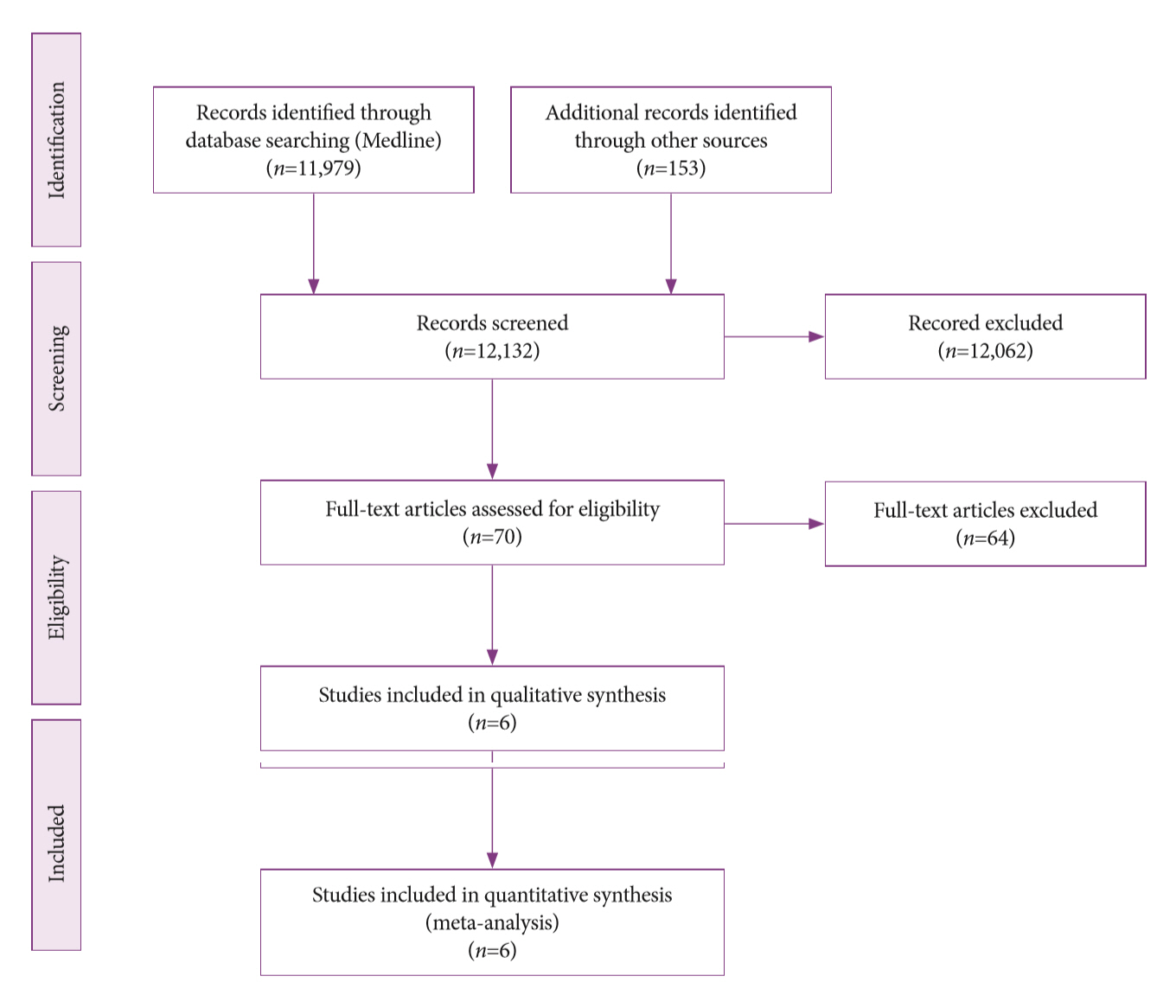
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The diagnosis of biliary strictures can be challenging. There are no systematic reviews studying same-session endoscopic retrograde cholangiopancreatography (ERCP)-based tissue sampling and endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for the diagnosis of biliary strictures.
Methods
A systematic review was conducted on studies analyzing same-session EUS and ERCP for tissue diagnosis of suspected malignant biliary strictures. The primary outcome was the accuracy of each method individually compared to the two methods combined. The secondary outcome was the accuracy of each method in pancreatic and biliary etiologies. In the meta-analysis, we used Forest plots, summary receiver operating characteristic curves, and estimates of the area under the curve for intention-to-treat analysis.
Results
Of the 12,132 articles identified, six were included, resulting in a total of 497 patients analyzed. The sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and accuracy of the association between the two methods were: 86%, 98%, 12.50, 0.17, and 96.5%, respectively. For the individual analysis, the sensitivity, specificity and accuracy of EUS-FNA were 76%, 100%, and 94.5%, respectively; for ERCP-based tissue sampling, the sensitivity, specificity, and accuracy were 58%, 98%, and 78.1%, respectively. For pancreatic lesions, EUS-FNA was superior to ERCP-based tissue sampling. However, for biliary lesions, both methods had similar sensitivities.
Conclusions
Same-session EUS-FNA and ERCP-based tissue sampling is superior to either method alone in the diagnosis of suspected malignant biliary strictures. Considering these results, combination sampling should be performed when possible. -
Citations
Citations to this article as recorded by- British Society of Gastroenterology guidelines for the diagnosis and management of cholangiocarcinoma
Simon M Rushbrook, Timothy James Kendall, Yoh Zen, Raneem Albazaz, Prakash Manoharan, Stephen P Pereira, Richard Sturgess, Brian R Davidson, Hassan Z Malik, Derek Manas, Nigel Heaton, K Raj Prasad, John Bridgewater, Juan W Valle, Rebecca Goody, Maria Hawk
Gut.2024; 73(1): 16. CrossRef - Contrast-enhanced guided endoscopic ultrasound procedures
Marcel Ioan Gheorghiu, Andrada Seicean, Cristina Pojoga, Claudia Hagiu, Radu Seicean, Zeno Sparchez
World Journal of Gastroenterology.2024; 30(17): 2311. CrossRef - ACG Clinical Guideline: Diagnosis and Management of Biliary Strictures
B. Joseph Elmunzer, Jennifer L. Maranki, Victoria Gómez, Anna Tavakkoli, Bryan G. Sauer, Berkeley N. Limketkai, Emily A. Brennan, Elaine M. Attridge, Tara J. Brigham, Andrew Y. Wang
American Journal of Gastroenterology.2023; 118(3): 405. CrossRef - Endoscopic Ultrasound in the Diagnosis of Extrahepatic Cholangiocarcinoma: What Do We Know in 2023?
Rares Ilie Orzan, Cristina Pojoga, Renata Agoston, Radu Seicean, Andrada Seicean
Diagnostics.2023; 13(6): 1023. CrossRef - Endoscopic evaluation of indeterminate biliary strictures: Cholangioscopy, endoscopic ultrasound, or both?
Raymond S. Y. Tang
Digestive Endoscopy.2023;[Epub] CrossRef - Brush Cytology, Forceps Biopsy, or Endoscopic Ultrasound-Guided Sampling for Diagnosis of Bile Duct Cancer: A Meta-Analysis
Seung Bae Yoon, Sung-Hoon Moon, Sung Woo Ko, Hyun Lim, Ho Suk Kang, Jong Hyeok Kim
Digestive Diseases and Sciences.2022; 67(7): 3284. CrossRef - Managing adverse events after endoscopic ultrasound‐guided drainage of the biliary tract and pancreatic fluid collections: Narrative review (with video)
Mateus Pereira Funari, Igor Braga Ribeiro, Marcos Eduardo Lera dos Santos, Sergio Eiji Matuguma, Eduardo Guimarães Hourneaux de Moura
Digestive Endoscopy.2022; 34(2): 359. CrossRef - Endoscopic Ultrasound for the Diagnosis and Staging of Biliary Malignancy
Martin Coronel, Jeffrey H. Lee, Emmanuel Coronel
Clinics in Liver Disease.2022; 26(1): 115. CrossRef - Endoscopic Management of Pancreatobiliary Malignancies
Dong Wook Lee, Eun Young Kim
Digestive Diseases and Sciences.2022; 67(5): 1635. CrossRef - IgG4-related sclerosing cholangitis involving the gallbladder mimicking a hilar cholangiocarcinoma
Yun Chae Lee, Hyung Ku Chon, Keum Ha Choi
Endoscopy.2022; 54(12): E739. CrossRef - Promising Genomic Testing for Biliary Tract Cancer Using Endoscopic Ultrasound-Guided Fine-Needle Aspiration/Biopsy Specimens
Masaki Kuwatani, Kazumichi Kawakubo, Naoya Sakamoto
Diagnostics.2022; 12(4): 900. CrossRef - Endoscopic Ultrasound Plus Endoscopic Retrograde Cholangiopancreatography Based Tissue Sampling for Diagnosis of Proximal and Distal Biliary Stenosis Due to Cholangiocarcinoma: Results from a Retrospective Single-Center Study
Edoardo Troncone, Fabio Gadaleta, Omero Alessandro Paoluzi, Cristina Maria Gesuale, Vincenzo Formica, Cristina Morelli, Mario Roselli, Luca Savino, Giampiero Palmieri, Giovanni Monteleone, Giovanna Del Vecchio Blanco
Cancers.2022; 14(7): 1730. CrossRef - The Role of Cholangioscopy and EUS in the Evaluation of Indeterminate Biliary Strictures
Wilson Siu, Raymond S. Y. Tang
Gastroenterology Insights.2022; 13(2): 192. CrossRef - Current endoscopic approaches to biliary strictures
Tatsuya Sato, Yousuke Nakai, Mitsuhiro Fujishiro
Current Opinion in Gastroenterology.2022; 38(5): 450. CrossRef - Acute cholecystitis caused by gallbladder metastasis from non-small cell lung cancer: a case report
Kouki Imaoka, Daisuke Satoh, Ko Oshita, Takuya Yano, Tetsushi Kubota, Michihiro Ishida, Yasuhiro Choda, Masanori Yoshimitsu, Kanyu Nakano, Masao Harano, Hiroyoshi Matsukawa, Hitoshi Idani, Shigehiro Shiozaki, Masazumi Okajima
Clinical Journal of Gastroenterology.2021; 14(1): 351. CrossRef - Current Status and Research Progress of ERCP in the Diagnosis and Treatment of Biliary and Pancreatic System Diseases
跃华 李
Advances in Clinical Medicine.2021; 11(07): 3123. CrossRef - Same day endoscopic retrograde cholangio-pancreatography immediately after endoscopic ultrasound for choledocholithiasis is feasible, safe and cost-effective
Wisam Sbeit, Anas Kadah, Amir Shahin, Tawfik Khoury
Scandinavian Journal of Gastroenterology.2021; 56(10): 1243. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Tips and tricks for the diagnosis and management of biliary stenosis-state of the art review
Giovanna Del Vecchio Blanco, Michelangela Mossa, Edoardo Troncone, Renato Argirò, Andrea Anderloni, Alessandro Repici, Omero Alessandro Paoluzi, Giovanni Monteleone
World Journal of Gastrointestinal Endoscopy.2021; 13(10): 473. CrossRef - Stent versus Balloon Dilation for the Treatment of Dominant Strictures in Primary Sclerosing Cholangitis: A Systematic Review and Meta-Analysis
Marina Tucci Gammaro Baldavira Ferreira, Igor Braga Ribeiro, Diogo Turiani Hourneaux de Moura, Thomas R. McCarty, Alberto Machado da Ponte Neto, Galileu Ferreira Ayala Farias, Antônio Afonso de Miranda Neto, Pedro Victor Aniz Gomes de Oliveira, Wanderley
Clinical Endoscopy.2021; 54(6): 833. CrossRef - Endoscopic ultrasound fine needle aspiration vs fine needle biopsy in solid lesions: A multi-center analysis
Diogo Turiani Hourneaux Moura, Thomas R McCarty, Pichamol Jirapinyo, Igor Braga Ribeiro, Galileu Ferreira Ayala Farias, Antonio Coutinho Madruga-Neto, Marvin Ryou, Christopher C Thompson
World Journal of Clinical Cases.2021; 9(34): 10507. CrossRef - Efficacy of digital single-operator cholangioscopy in the visual interpretation of indeterminate biliary strictures: a systematic review and meta-analysis
Pedro Victor Aniz Gomes de Oliveira, Diogo Turiani Hourneaux de Moura, Igor Braga Ribeiro, Ahmad Najdat Bazarbashi, Tomazo Antonio Prince Franzini, Marcos Eduardo Lera dos Santos, Wanderley Marques Bernardo, Eduardo Guimarães Hourneaux de Moura
Surgical Endoscopy.2020; 34(8): 3321. CrossRef - Role of pancreatography in the endoscopic management of encapsulated pancreatic collections – review and new proposed classification
Igor Mendonça Proença, Marcos Eduardo Lera dos Santos, Diogo Turiani Hourneaux de Moura, Igor Braga Ribeiro, Sergio Eiji Matuguma, Spencer Cheng, Thomas R McCarty, Epifanio Silvino do Monte Junior, Paulo Sakai, Eduardo Guimarães Hourneaux de Moura
World Journal of Gastroenterology.2020; 26(45): 7104. CrossRef
- British Society of Gastroenterology guidelines for the diagnosis and management of cholangiocarcinoma
- 5,824 View
- 205 Download
- 21 Web of Science
- 23 Crossref

- Comparison of the Diagnostic Ability of Endoscopic Ultrasonography and Abdominopelvic Computed Tomography in the Diagnosis of Gastric Subepithelial Tumors
- Sang Yoon Kim, Ki-Nam Shim, Joo-Ho Lee, Ji Young Lim, Tae Oh Kim, A. Reum Choe, Chung Hyun Tae, Hye-Kyung Jung, Chang Mo Moon, Seong-Eun Kim, Sung-Ae Jung
- Clin Endosc 2019;52(6):565-573. Published online July 17, 2019
- DOI: https://doi.org/10.5946/ce.2019.019

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic ultrasonography (EUS) is the most efficient imaging modality for gastric subepithelial tumors (SETs). However, abdominopelvic computed tomography (APCT) has other advantages in evaluating the characteristics, local extension, or invasion of SETs to adjacent organs. This study aimed to compare the diagnostic ability of EUS and APCT based on surgical histopathology results.
Methods
We retrospectively reviewed data from 53 patients who underwent both EUS and APCT before laparoscopic wedge resection for gastric SETs from January 2010 to December 2017 at a single institution. On the basis of histopathology results, we assessed the diagnostic ability of the 2 tests.
Results
The overall accuracy of EUS and APCT was 64.2% and 50.9%, respectively. In particular, the accuracy of EUS vs. APCT for the diagnosis of gastrointestinal stromal tumors (GISTs), leiomyomas, and ectopic pancreas was 83.9% vs. 74.2%, 37.5% vs. 0.0%, and 57.1% vs. 14.3%, respectively. Most of the incorrect diagnoses with EUS involved hypoechoic lesions originating in the fourth echolayer, with the most common misdiagnosed lesions being GISTs mistaken for leiomyomas and vice versa.
Conclusions
APCT showed a lower overall accuracy than EUS; however, APCT remains a useful modality for malignant/potentially malignant gastric SETs. -
Citations
Citations to this article as recorded by- Guidelines in Practice: The Diagnosis and Management of Gastrointestinal Subepithelial Lesions
Brian C. Jacobson, Vanessa M. Shami
American Journal of Gastroenterology.2024; 119(3): 397. CrossRef - Advances in Endoscopic Diagnosis and Treatment of Gastric Neuroendocrine Neoplasms
Xinrui Guo, Xiaohan Zhao, Gang Huang, Yanbo Yu
Digestive Diseases and Sciences.2024; 69(1): 27. CrossRef - Diagnostic Endoscopic Ultrasound (EUS) of the Luminal Gastrointestinal Tract
Giovanna Impellizzeri, Giulio Donato, Claudio De Angelis, Nico Pagano
Diagnostics.2024; 14(10): 996. CrossRef - The value of contrast-enhanced harmonic endoscopic ultrasound in differential diagnosis and evaluation of malignant risk of gastrointestinal stromal tumors (<50mm)
Jiali Wu, Mengqi Zhuang, Yubao Zhou, Xiang Zhan, Weiwei Xie
Scandinavian Journal of Gastroenterology.2023; 58(5): 542. CrossRef - ACG Clinical Guideline: Diagnosis and Management of Gastrointestinal Subepithelial Lesions
Brian C. Jacobson, Amit Bhatt, Katarina B. Greer, Linda S. Lee, Walter G. Park, Bryan G. Sauer, Vanessa M. Shami
American Journal of Gastroenterology.2023; 118(1): 46. CrossRef - Approach to Small Gastric Subepithelial Lesions
Moon Won Lee, Bong Eun Lee
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2023; 23(1): 28. CrossRef - Computed tomography features of gastric leiomyoma versus gastric stromal tumor: a case–control study with propensity score matching
Qi Wang, Lijia Wang, Xiaohui Qi, Xiang Liu, Qiao Xie, Yifeng Wang, Gaofeng Shi
Journal of International Medical Research.2023; 51(5): 030006052311710. CrossRef - EUS-Guided Diagnosis of Gastric Subepithelial Lesions, What Is New?
Thomas Vasilakis, Dimitrios Ziogas, Georgios Tziatzios, Paraskevas Gkolfakis, Eleni Koukoulioti, Christina Kapizioni, Konstantinos Triantafyllou, Antonio Facciorusso, Ioannis S. Papanikolaou
Diagnostics.2023; 13(13): 2176. CrossRef - The effect of endoscopic ultrasound on the precise selection of endoscopic treatment for submucosal tumors in the upper gastrointestinal tract
Jian-Hua Li, Shu-Min Qin, Tian-Wen Liu, Jun-Qian Chen, Ying-Ting Li
BMC Surgery.2023;[Epub] CrossRef - Systematic Endoscopic Approach for Diagnosing Gastric Subepithelial Tumors
Gwang Ha Kim
Gut and Liver.2022; 16(1): 19. CrossRef - Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline
Pierre H. Deprez, Leon M.G. Moons, Dermot OʼToole, Rodica Gincul, Andrada Seicean, Pedro Pimentel-Nunes, Gloria Fernández-Esparrach, Marcin Polkowski, Michael Vieth, Ivan Borbath, Tom G. Moreels, Els Nieveen van Dijkum, Jean-Yves Blay, Jeanin E. van Hooft
Endoscopy.2022; 54(04): 412. CrossRef - Prediction of Gastric Gastrointestinal Stromal Tumors before Operation: A Retrospective Analysis of Gastric Subepithelial Tumors
Yu-Ning Lin, Ming-Yan Chen, Chun-Yi Tsai, Wen-Chi Chou, Jun-Te Hsu, Chun-Nan Yeh, Ta-Sen Yeh, Keng-Hao Liu
Journal of Personalized Medicine.2022; 12(2): 297. CrossRef - Advancements in the Diagnosis of Gastric Subepithelial Tumors
Osamu Goto, Mitsuru Kaise, Katsuhiko Iwakiri
Gut and Liver.2022; 16(3): 321. CrossRef - DIAGNOSTIC AND THERAPEUTIC MANAGEMENT FOR LEIOMYOMA OF THE UPPER GASTROINTESTINAL TRACT
V. O. Shaprynskyi, Yu. V. Babii
Kharkiv Surgical School.2022; (4-5): 46. CrossRef - A scoring model for radiologic diagnosis of gastric leiomyomas (GLMs) with contrast-enhanced computed tomography (CE-CT): Differential diagnosis from gastrointestinal stromal tumors (GISTs)
Jian-Xia Xu, Qiao-Ling Ding, Yuan-Fei Lu, Shu-Feng Fan, Qin-Pan Rao, Ri-Sheng Yu
European Journal of Radiology.2021; 134: 109395. CrossRef - A Nomogram for Predicting Laparoscopic and Endoscopic Cooperative Surgery during the Endoscopic Resection of Subepithelial Tumors of the Upper Gastrointestinal Tract
Shun-Wen Hsiao, Mei-Wen Chen, Chia-Wei Yang, Kuo-Hua Lin, Yang-Yuan Chen, Chew-Teng Kor, Siou-Ping Huang, Hsu-Heng Yen
Diagnostics.2021; 11(11): 2160. CrossRef - Ultrasonido endoscópico, aplicaciones actuales en tumores sólidos gastrointestinales
Gabriel Alonso Mosquera-Klinger, Jhon Jaime Carvajal Gutiérrez, Alavaro Andrés Gómez Venegas, Sebastián Niño Ramírez, Raúl Cañadas Garrido
Revista Colombiana de Gastroenterología.2020; 35(4): 506. CrossRef - Diagnosis of Gastric Subepithelial Tumors Using Endoscopic Ultrasonography or Abdominopelvic Computed Tomography: Which is Better?
Eun Young Park, Gwang Ha Kim
Clinical Endoscopy.2019; 52(6): 519. CrossRef
- Guidelines in Practice: The Diagnosis and Management of Gastrointestinal Subepithelial Lesions
- 6,588 View
- 186 Download
- 15 Web of Science
- 18 Crossref

- Accuracy of Endoscopic Diagnosis for Mild Atrophic Gastritis Infected with Helicobacter pylori
- Takuma Okamura, Yugo Iwaya, Kei Kitahara, Tomoaki Suga, Eiji Tanaka
- Clin Endosc 2018;51(4):362-367. Published online April 26, 2018
- DOI: https://doi.org/10.5946/ce.2017.177
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: This study examined the accuracy of endoscopic evaluation for determining the Helicobacter pylori infection status in patients with mild atrophy who might not exhibit characteristic endoscopic findings.
Methods
Forty endoscopists determined the H. pylori infection status of 50 randomly presented H. pylori-positive and H. pylorinegative cases on the basis of a list of established findings.
Results
The median clinical endoscopy experience was 7 years (range, 1–35 years), including 22 board-certified endoscopists (55%) of the Japan Gastroenterological Endoscopy Society. The mean accuracy rate of endoscopic diagnosis was 67% and was unrelated to experience status (experienced vs. trainee: 69% vs. 65%, p=0.089) and total years of experience (R2 =0.022). The most frequently selected endoscopic findings were regular arrangement of collecting venules (59%), atrophy (45%), and red streak (22%), which had fair accuracy rates of 67%, 65%, and 73%, respectively. By contrast, the accuracy rates of nodularity (89%) and mucosal swelling (77%) were highest. The 20 endoscopists who more frequently identified these findings diagnosed H. pylori infection significantly more accurately than did the other endoscopists (71% vs. 64%, p=0.008).
Conclusions
Careful attention to nodularity and mucosal swelling in patients with mild atrophy may enhance diagnosis, enable prompt treatment, and avoid possible long-term carcinogenesis. -
Citations
Citations to this article as recorded by- The Diagnostic Performance of Linked Color Imaging Compared to White Light Imaging in Endoscopic Diagnosis of Helicobacter pylori Infection: A Systematic Review and Meta-Analysis
Jae Gon Lee, In Kyung Yoo, Abdullah Ozgur Yeniova, Sang Pyo Lee
Gut and Liver.2024; 18(3): 444. CrossRef - Endoscopic Features According to Helicobacter pylori Infection Status
Jun-young Seo, Ji Yong Ahn
The Korean Journal of Medicine.2023; 98(3): 117. CrossRef - Role of linked color imaging for upper gastrointestinal disease: present and future
Sang Pyo Lee
Clinical Endoscopy.2023; 56(5): 546. CrossRef - Clinical usefulness of linked color imaging in identifying Helicobacter pylori infection: A systematic review and meta-analysis
Yu Zhang, Jing-Zhai Wang, Xuan Bai, Peng-Li Zhang, Qiang Guo
World Journal of Gastrointestinal Endoscopy.2023; 15(12): 735. CrossRef - Characterization of the cagA-gene in Helicobacter pylori in Mongolia and detection of two EPIYA-A enriched CagA types
Oyunbaatar Altanbayar, Avarzed Amgalanbaatar, Chimeddorj Battogtokh, Narmandakh Bayarjargal, Dana Belick, Malte Kohns Vasconcelos, Colin R. Mackenzie, Klaus Pfeffer, Birgit Henrich
International Journal of Medical Microbiology.2022; 312(3): 151552. CrossRef - Risk factors for early gastric cancer: focus on Helicobacter pylori gastritis
Hee Seok Moon
Journal of the Korean Medical Association.2022; 65(5): 259. CrossRef - Usefulness of the Kyoto Classification Score for Prediction of Current Helicobacter pylori Infection
Heejun Kang, Chul-Hyun Lim, Sukil Kim, Arum Choi, Jung-Hwan Oh
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2022; 22(4): 281. CrossRef - Clinical Features of False-Negative Early Gastric Cancers: A Retrospective Study of Endoscopic Submucosal Dissection Cases
Kohei Oka, Naoto Iwai, Takashi Okuda, Tasuku Hara, Yutaka Inada, Toshifumi Tsuji, Toshiyuki Komaki, Junichi Sakagami, Yuji Naito, Keizo Kagawa, Yoshito Itoh, Fabiana Zingone
Gastroenterology Research and Practice.2021; 2021: 1. CrossRef - Gastritis: The clinico-pathological spectrum
Massimo Rugge, Edoardo Savarino, Marta Sbaraglia, Ludovica Bricca, Peter Malfertheiner
Digestive and Liver Disease.2021; 53(10): 1237. CrossRef - What Is New in Helicobacter pylori Diagnosis. An Overview
Maria Pina Dore, Giovanni Mario Pes
Journal of Clinical Medicine.2021; 10(10): 2091. CrossRef - Deep learning for diagnosis of precancerous lesions in upper gastrointestinal endoscopy: A review
Tao Yan, Pak Kin Wong, Ye-Ying Qin
World Journal of Gastroenterology.2021; 27(20): 2531. CrossRef - In situ Diagnosis of Helicobacter pylori Infection Using the Endoscopic Kyoto Scoring System
Eunsun Lim, Ik Hyun Jo, Yeon-Ji Kim, Woo Chul Chung
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2021; 21(4): 322. CrossRef - Helicobacter pylori Gastritis in Children—The Link between Endoscopy and Histology
Ana-Maria Teodora Domșa, Raluca Lupușoru, Dan Gheban, Radu Șerban, Cristina Maria Borzan
Journal of Clinical Medicine.2020; 9(3): 784. CrossRef - The role of linked color imaging in endoscopic diagnosis of Helicobacter pylori associated gastritis
Sang Pyo Lee, Jin Lee, Sea Hyub Kae, Hyun Joo Jang, Dong Hee Koh, Jang Han Jung, Sun-Ju Byeon
Scandinavian Journal of Gastroenterology.2020; 55(9): 1114. CrossRef - Gastritis: An Update in 2020
Massimo Rugge, Kentaro Sugano, Diana Sacchi, Marta Sbaraglia, Peter Malfertheiner
Current Treatment Options in Gastroenterology.2020; 18(3): 488. CrossRef - The endoscopic predictors of Helicobacter pylori status: a meta-analysis of diagnostic performance
Ben Glover, Julian Teare, Hutan Ashrafian, Nisha Patel
Therapeutic Advances in Gastrointestinal Endoscopy.2020; 13: 263177452095084. CrossRef - Helicobacter pylori Infection and the Kyoto Classification of Gastritis
Sun-Young Lee
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2019; 19(2): 81. CrossRef - Review: Diagnosis of Helicobacter pylori infection
Athanasios Makristathis, Alexander M. Hirschl, Francis Mégraud, Emilie Bessède
Helicobacter.2019;[Epub] CrossRef - Accuracy of Endoscopic Diagnosis of Mild Atrophic Gastritis with Helicobacter pylori Infection
Dae Bum Kim, Woo Chul Chung
Clinical Endoscopy.2018; 51(4): 310. CrossRef
- The Diagnostic Performance of Linked Color Imaging Compared to White Light Imaging in Endoscopic Diagnosis of Helicobacter pylori Infection: A Systematic Review and Meta-Analysis
- 10,966 View
- 268 Download
- 16 Web of Science
- 19 Crossref

- Raman Spectroscopy for the Endoscopic Diagnosis of Esophageal, Gastric, and Colonic Diseases
- Neel Sharma, Nobuyoshi Takeshita, Khek Yu Ho
- Clin Endosc 2016;49(5):404-407. Published online September 22, 2016
- DOI: https://doi.org/10.5946/ce.2016.100
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Globally white-light endoscopy with biopsy sampling is the gold standard diagnostic modality for esophageal, gastric, and colonic pathologies. However, there is overwhelming evidence to highlight the deficiencies of an approach based predominantly on eyeball visualization. Biopsy sampling is also problematic due in part to excessive sampling and hence attendant cost. Various innovations are currently taking place in the endoscopic domain to aid operators in diagnosis forming. These include narrow band imaging which aims to enhance the surface anatomy and vasculature, and confocal laser endomicroscopy which provides real time histological information. However, both of these tools are limited by the skill of the operator and the extensive learning curve associated with their use. There is a gap therefore for a new form of technology that relies solely on an objective measure of disease and reduces the need for biopsy sampling. Raman spectroscopy (RS) is a potential platform that aims to satisfy these criteria. It enables a fingerprint capture of tissue in relation to the protein, DNA, and lipid content. This focused review highlights the strong potential for the use of RS during endoscopic gastroenterological examination.
-
Citations
Citations to this article as recorded by- Biomedical applications, perspectives and tag design concepts in the cell – silent Raman window
Martha Z. Vardaki, Vasilis G. Gregoriou, Christos L. Chochos
RSC Chemical Biology.2024; 5(4): 273. CrossRef - Toward a New Era of SERS and TERS at the Nanometer Scale: From Fundamentals to Innovative Applications
Tamitake Itoh, Marek Procházka, Zhen-Chao Dong, Wei Ji, Yuko S. Yamamoto, Yao Zhang, Yukihiro Ozaki
Chemical Reviews.2023; 123(4): 1552. CrossRef - Performance assessment of probe-based Raman spectroscopy systems for biomedical analysis
Sean Fitzgerald, Eric Marple, Anita Mahadevan-Jansen
Biomedical Optics Express.2023; 14(7): 3597. CrossRef - Raman opportunities in the field of pathological calcifications
Ivan T. Lucas, Dominique Bazin, Michel Daudon
Comptes Rendus. Chimie.2022; 25(S1): 83. CrossRef - Mevastatin in colon cancer by spectroscopic and microscopic methods – Raman imaging and AFM studies
K. Beton, P. Wysocki, B. Brozek-Pluska
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy.2022; 270: 120726. CrossRef - In Vitro Detection of Biochemical Effect in Human CaCo-2 Cell Line after Exposure to a Low Concentration of a Deltamethrin-Based Pesticide
Giuseppe Perna, Vito Capozzi, Maria Lasalvia
Chemosensors.2022; 10(11): 438. CrossRef - Advances in optical gastrointestinal endoscopy: a technical review
Yubo Tang, Sharmila Anandasabapathy, Rebecca Richards‐Kortum
Molecular Oncology.2021; 15(10): 2580. CrossRef - Label-free detection of human enteric nerve system using Raman spectroscopy: A pilot study for diagnosis of Hirschsprung disease
Katsuhiro Ogawa, Yusuke Oshima, Tsuyoshi Etoh, Yushi Kaisyakuji, Manabu Tojigamori, Yasuharu Ohno, Norio Shiraishi, Masafumi Inomata
Journal of Pediatric Surgery.2021; 56(7): 1150. CrossRef - Non-invasive diagnosis of colorectal cancer by Raman spectroscopy: Recent developments in liquid biopsy and endoscopy approaches
Hemanth Noothalapati, Keita Iwasaki, Tatsuyuki Yamamoto
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy.2021; 258: 119818. CrossRef - From Raman to SESORRS: moving deeper into cancer detection and treatment monitoring
Sian Sloan-Dennison, Stacey Laing, Duncan Graham, Karen Faulds
Chemical Communications.2021; 57(93): 12436. CrossRef - Present and Future of Surface-Enhanced Raman Scattering
Judith Langer, Dorleta Jimenez de Aberasturi, Javier Aizpurua, Ramon A. Alvarez-Puebla, Baptiste Auguié, Jeremy J. Baumberg, Guillermo C. Bazan, Steven E. J. Bell, Anja Boisen, Alexandre G. Brolo, Jaebum Choo, Dana Cialla-May, Volker Deckert, Laura Fabris
ACS Nano.2020; 14(1): 28. CrossRef - Implementation of a classification strategy of Raman data collected in different clinical conditions: application to the diagnosis of chronic lymphocytic leukemia
M. Féré, C. Gobinet, L. H. Liu, A. Beljebbar, V. Untereiner, D. Gheldof, M. Chollat, J. Klossa, B. Chatelain, O. Piot
Analytical and Bioanalytical Chemistry.2020; 412(4): 949. CrossRef - Virtual spectral histopathology of colon cancer - biomedical applications of Raman spectroscopy and imaging
Beata Brozek-Pluska, Adam Dziki, Halina Abramczyk
Journal of Molecular Liquids.2020; 303: 112676. CrossRef - BPC 157 Rescued NSAID-cytotoxicity Via Stabilizing Intestinal Permeability and Enhancing Cytoprotection
Jong M. Park, Ho J. Lee, Predrag Sikiric, Ki B. Hahm
Current Pharmaceutical Design.2020; 26(25): 2971. CrossRef - Challenges to diagnostic standardization of Barrett's esophagus in Asia
Yu Sen Alex Soh, Yeong Yeh Lee, Takuji Gotoda, Prateek Sharma, Khek‐Yu Ho
Digestive Endoscopy.2019; 31(6): 609. CrossRef - Biochemical Changes in Human Cells Exposed to Low Concentrations of Gold Nanoparticles Detected by Raman Microspectroscopy
Maria Lasalvia, Giuseppe Perna, Vito Capozzi
Sensors.2019; 19(10): 2418. CrossRef - Intelligent magnetic manipulation for gastrointestinal ultrasound
Joseph C. Norton, Piotr R. Slawinski, Holly S. Lay, James W. Martin, Benjamin F. Cox, Gerard Cummins, Marc P.Y. Desmulliez, Richard E. Clutton, Keith L. Obstein, Sandy Cochran, Pietro Valdastri
Science Robotics.2019;[Epub] CrossRef - Raman spectroscopic analysis of cataract lens: A compendious review
Chia-Chi Huang, Wenlung Chen
Applied Spectroscopy Reviews.2018; 53(9): 689. CrossRef - Histochemical analysis of human breast tissue samples by IR and Raman spectroscopies. Protocols discussion
Beata Brozek-Pluska, Monika Kopec, Jakub Surmacki, Halina Abramczyk
Infrared Physics & Technology.2018; 93: 247. CrossRef - Potential Application of Raman Spectroscopy for Real-time Diagnosis and Classification of Colorectal Cancer
Ryuichi SEKINE, Sumito SATO, Jun-ichi TANAKA, Hirotada KAGOSHIMA, Takeshi AOKI, Masahiko MURAKAMI
The Showa University Journal of Medical Sciences.2018; 30(3): 381. CrossRef - Linked colour imaging benefits the endoscopic diagnosis of distal gastric diseases
Xiaotian Sun, Yiliang Bi, Tenghui Dong, Min Min, Wei Shen, Yang Xu, Yan Liu
Scientific Reports.2017;[Epub] CrossRef - Endoscopic imaging using surface-enhanced Raman scattering
Yong-il Kim, Sinyoung Jeong, Bong-Hyun Jun, Yun-Sang Lee, Yoon-Sik Lee, Dae Hong Jeong, Dong Soo Lee
European Journal of Nanomedicine.2017;[Epub] CrossRef
- Biomedical applications, perspectives and tag design concepts in the cell – silent Raman window
- 8,338 View
- 215 Download
- 27 Web of Science
- 22 Crossref

- Endoscopic Ultrasonography in the Diagnosis of Gastric Subepithelial Lesions
- Eun Jeong Gong, Do Hoon Kim
- Clin Endosc 2016;49(5):425-433. Published online September 5, 2016
- DOI: https://doi.org/10.5946/ce.2016.065
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Subepithelial lesions occasionally found in the stomach of patients undergoing endoscopy may be either benign lesions or tumors with malignant potential. They may also appear due to extrinsic compression. Discrimination of gastric subepithelial lesions begins with meticulous endoscopic examination for size, shape, color, mobility, consistency, and appearance of the overlying mucosa. Accurate diagnosis can be achieved with endoscopic ultrasonography, which provides useful information on the exact size, layer-of-origin, and characteristic morphologic features to support a definitive diagnosis. Endoscopic ultrasonography also aids in the prediction of malignant potential, especially in gastrointestinal stromal tumors. Features of subepithelial lesions identified on endoscopic ultrasonography can be used to determine whether further diagnostic procedures such as endoscopic resection, fine needle aspiration, or core biopsy are required. Endoscopic ultrasonography is a valuable tool for diagnosis and clinical decision making during follow-up of gastric subepithelial lesions.
-
Citations
Citations to this article as recorded by- Clinical features can distinguish gastrointestinal stromal tumor from other subepithelial gastric tumors
Richard J. Straker, Amr K. El Jack, Giorgos C. Karakousis, Cimarron E. Sharon, Nuzhat A. Ahmad, Ronald P. DeMatteo, Robert E. Roses
Journal of Gastrointestinal Surgery.2024; 28(3): 276. CrossRef - Endoscopically resected duodenal lipoma as an uncommon cause of upper gastrointestinal bleeding: a case report
Dong Chan Joo, Gwang Ha Kim, Bong Eun Lee, Moon Won Lee, Cheolung Kim
The Ewha Medical Journal.2024;[Epub] CrossRef - Compression from a retroperitoneal schwannoma presenting as a subepithelial lesion of the gastric fundus
Xue-Mei Lin, Juan Liu, Chun-Hui Xi, Jun Wang, Guo-Dong Yang, Xian-Fei Wang, Cong Yuan
Endoscopy.2024; 56(S 01): E236. CrossRef - Gastrointestinal Glomus Tumors: A Single Institution, 20-Year Retrospective Study
Andrea Zironda, Travis E. Grotz, Andrew L. Folpe, Cornelius A. Thiels
Journal of Surgical Research.2023; 283: 982. CrossRef - Diagnostic Obscurity of Gastrointestinal Subepithelial Tumors: An Organizing Gastric Hematoma Requiring En Bloc Resection
Clive Jude Miranda, William Schertzing, Anushi Shah, Paul Anthony Reyes Del Prado
ACG Case Reports Journal.2023; 10(4): e01024. CrossRef - Coexistence of early gastric cancer and benign submucosal lesions mimic invasive cancer: a retrospective multicenter experience
Huawei Yang, Zhen Li, Zhi Wei, Guodong Li, Yi Li, Shanbin Wu, Rui Ji
BMC Gastroenterology.2023;[Epub] CrossRef - Spontaneous hemoperitoneum as a rare presentation of gastric lesions: Two case reports
Joana Isabel Almeida, Catarina Lima, Paula Pinto, Isabel Armas, Tatiana Santos, Carla Freitas
International Journal of Surgery Case Reports.2022; 91: 106769. CrossRef - Usefulness of endoscopic ultrasound in children with pancreatobiliary and gastrointestinal symptoms
Ankit Dalal, Nagesh Kamat, Gaurav Patil, Rajen Daftary, Amit Maydeo
Endoscopy International Open.2022; 10(02): E192. CrossRef - Efficiency of an endoscopic resection strategy for management of submucosal tumors < 20 mm in the upper gastrointestinal tract
Fabrice Caillol, Elise Meunier, Christophe Zemmour, Jean-Philippe Ratone, Jerome Guiramand, Solene Hoibian, Yanis Dahel, Flora Poizat, Marc Giovannini
Endoscopy International Open.2022; 10(04): E347. CrossRef - Scoring systems for differentiating gastrointestinal stromal tumors and schwannomas from leiomyomas in the stomach
Shotaro Okanoue, Masaya Iwamuro, Takehiro Tanaka, Takuya Satomi, Kenta Hamada, Hiroyuki Sakae, Makoto Abe, Yoshiyasu Kono, Hiromitsu Kanzaki, Seiji Kawano, Yoshiro Kawahara, Hiroyuki Okada
Medicine.2021; 100(40): e27520. CrossRef - Role of endoscopic ultrasonography for differential diagnosis of upper gastrointestinal submucosal lesions
Qian Su, Jin Peng, Xiong Chen, Zhiming Xiao, Rui Liu, Fen Wang
BMC Gastroenterology.2021;[Epub] CrossRef - More than Just a Hole in the Wall: Evolving Management and Treatment Paradigms of Suppurative Gastritis
Michael Coles, Victoria Madray, Kayla Cox, Pearl Uy, Amol Sharma
Digestive Diseases and Sciences.2020; 65(8): 2203. CrossRef - Accessory spleen originating from the intrinsic muscularis of the stomach misdiagnosed as gastrointestinal stromal tumor: a case report
Jing Zhang, Jin-Wei Zhong, Guang-Rong Lu, Yu-Hui Zhou, Zhan-Xiong Xue, Meng-Si Ye
Journal of International Medical Research.2020; 48(8): 030006052093530. CrossRef - Immunoglobulin G4-related gastric pseudotumor – An impostor: Case report
Manuel Santiago Mosquera, Andrea Suarez Gómez, Hugo Herrera, Karen Moreno-Medina, Alejandro González-Orozco, Carlos J-Perez Rivera
International Journal of Surgery Case Reports.2020; 75: 333. CrossRef - Prepyloric gastric inflammatory fibroid polyp presenting as chronic epigastric discomfort in a 5th decade aged female: A case report
Jad J. Terro, Etienne El-Helou, Alaa Kansoun, Alaa Taha, Jocelyne Karaki, Jessica Naccour, Nahed Damaj, Houssam Khodor Abtar
International Journal of Surgery Case Reports.2020; 76: 49. CrossRef - Endoscopic diagnosis and management of gastric subepithelial lesions
Thomas R. McCarty, Marvin Ryou
Current Opinion in Gastroenterology.2020; 36(6): 530. CrossRef - Current Status of Endoscopic Ultrasonography in Gastrointestinal Subepithelial Tumors
Sang Gyun Kim, Ji Hyun Song, Joo Ha Hwang
Clinical Endoscopy.2019; 52(4): 301. CrossRef - An Esophageal Squamous Cell Carcinoma with Lymph Node Metastasis Presenting as a Small Subepithelial Tumor
Jang Won Park, Eun Jeong Gong, Myeongsook Seo, Baek Gyu Jun, Hyun Il Seo, Jong Kyu Park, Koon Hee Han, Sang Jin Lee, Young Don Kim, Woo Jin Jeong, Gab Jin Cheon
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2019; 19(4): 272. CrossRef - Gastric subepitelyal lezyonlar: Endoskopik Ultrasonografi’nin yeri ve etkinliği
Nurettin TUNÇ, Mehmet YALNIZ, İbrahim BAHÇECİOĞLU, Ulvi DEMİREL
Endoskopi Gastrointestinal.2019; 27(3): 69. CrossRef - Diagnostic Endoscopic Ultrasound: Technique, Current Status and Future Directions
Tiing Leong Ang, Andrew Boon Eu Kwek, Lai Mun Wang
Gut and Liver.2018; 12(5): 483. CrossRef - Accessory Spleen Presenting as a Submucosal Lesion on Stomach Wall after Splenectomy
Ya-Ting Shen, Chun-Hua Zhou, Wen Tang, Wei Wu, Guang-Qiang Chen, Duan-Min Hu
Chinese Medical Journal.2018; 131(7): 869. CrossRef - Identification of significant biomarkers and pathways associated with gastric carcinogenesis by whole genome-wide expression profiling analysis
Hong-Jun Fei, Song-Chang Chen, Jun-Yu Zhang, Shu-Yuan Li, Lan-Lan Zhang, Yi-Yao Chen, Chun-Xin Chang, Chen-Ming Xu
International Journal of Oncology.2018;[Epub] CrossRef - Spontaneous peeled ileal giant lipoma caused by lower gastrointestinal bleeding
Jung Ho Kim, Hyun Hwa Yoon, Seok Hoo Jeong, Hyun Sun Woo, Won-Suk Lee, Seung Joon Choi, Seog Gyun Kim, Seung Yeon Ha, Kwang An Kwon
Medicine.2017; 96(51): e9253. CrossRef
- Clinical features can distinguish gastrointestinal stromal tumor from other subepithelial gastric tumors
- 16,486 View
- 527 Download
- 20 Web of Science
- 23 Crossref

- Endoscopic Diagnosis and Differentiation of Inflammatory Bowel Disease
- Ji Min Lee, Kang-Moon Lee
- Clin Endosc 2016;49(4):370-375. Published online July 29, 2016
- DOI: https://doi.org/10.5946/ce.2016.090
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Patients with inflammatory bowel disease have significantly increased in recent decades in Korea. Intestinal tuberculosis (ITB) and intestinal Behcet’s disease (BD), which should be differentiated from Crohn’s disease (CD), are more frequent in Korea than in the West. Thus, the accurate diagnosis of these inflammatory diseases is problematic in Korea and clinicians should fully understand their clinical and endoscopic characteristics. Ulcerative colitis mostly presents with rectal inflammation and continuous lesions, while CD presents with discontinuous inflammatory lesions and frequently involves the ileocecal area. Involvement of fewer than four segments, a patulous ileocecal valve, transverse ulcers, and scars or pseudopolyps are more frequently seen in ITB than in CD. A few ulcers with discrete margins are a typical endoscopic finding of intestinal BD. However, the differential diagnosis is difficult in many clinical situations because typical endoscopic findings are not always observed. Therefore, clinicians should also consider symptoms and laboratory, pathological, and radiological findings, in addition to endoscopic findings.
-
Citations
Citations to this article as recorded by- Recent Trends in Non-Invasive Methods of Diagnosis and Evaluation of Inflammatory Bowel Disease: A Short Review
Dan Vălean, Roxana Zaharie, Roman Țaulean, Lia Usatiuc, Florin Zaharie
International Journal of Molecular Sciences.2024; 25(4): 2077. CrossRef - Differential diagnosis of Crohn’s disease and intestinal tuberculosis based on ATR-FTIR spectroscopy combined with machine learning
Yuan-Peng Li, Tian-Yu Lu, Fu-Rong Huang, Wei-Min Zhang, Zhen-Qiang Chen, Pei-Wen Guang, Liang-Yu Deng, Xin-Hao Yang
World Journal of Gastroenterology.2024; 30(10): 1377. CrossRef - The quality of life of patients with inflammatory bowel diseases and the multidisciplinary team
Roxana-Ioana Ghenade, Ilie-Marius Ciorbă
Medic.ro.2024; 3(159): 24. CrossRef - A Case Report and Literature Review of Rectosigmoid Crohn’s Disease: A Diagnostic Pitfall Ultimately Leading to Spontaneous Colonic Perforation
Muhammad Z Ali, Muhammad Usman Tariq, Muhammad Hasan Abid, Hamma Abdulaziz, Mohmmad AlAdwani, Arif Khurshid, Muhammad Rashid, Fawaz Al Thobaiti , Amjad Althagafi
Cureus.2023;[Epub] CrossRef - Identification of cuproptosis-associated subtypes and signature genes for diagnosis and risk prediction of Ulcerative colitis based on machine learning
Dadong Tang, Baoping Pu, Shiru Liu, Hongyan Li
Frontiers in Immunology.2023;[Epub] CrossRef - Endoscopic features of the intestinal mucosa in patients with ulcerative colitis depending on the level of IgG4
Yu.M. Stepanov, M.V. Stoykevich, Yu.A. Gaidar, T.S. Tarasova, O.V. Simonova, O.M. Tatarchuk, O.P. Petishko
GASTROENTEROLOGY.2023; 57(1): 30. CrossRef - Standardizing Scoring Conventions for Crohn’s Disease Endoscopy: An International RAND/UCLA Appropriateness Study
Reena Khanna, Christopher Ma, Malcolm Hogan, Guangyong Zou, Talat Bessissow, Brian Bressler, Jean-Frédéric Colombel, Silvio Danese, Marco Daperno, James E. East, Lawrence Hookey, Edward V. Loftus, John W.D. McDonald, Remo Panaccione, Laurent Peyrin-Biroul
Clinical Gastroenterology and Hepatology.2023; 21(11): 2938. CrossRef - Creeping fat exhibits distinct Inflammation-specific adipogenic preadipocytes in Crohn’s disease
Nahee Hwang, Dongwoo Kang, Su-Jin Shin, Bo Kyung Yoon, Jaeyoung Chun, Jae-woo Kim, Sungsoon Fang
Frontiers in Immunology.2023;[Epub] CrossRef - PD-1-positive cells contribute to the diagnosis of inflammatory bowel disease and can aid in predicting response to vedolizumab
Min Kyu Kim, Su In Jo, Sang-Yeob Kim, Hyun Lim, Ho Suk Kang, Sung‑Hoon Moon, Byong Duk Ye, Jae Seung Soh, Sung Wook Hwang
Scientific Reports.2023;[Epub] CrossRef - Inflammatory bowel disease in adults
Umang Qazi
InnovAiT: Education and inspiration for general practice.2022; 15(2): 97. CrossRef - Integrative computational approach identifies immune‐relevant biomarkers in ulcerative colitis
Tianzhen He, Kai Wang, Peng Zhao, Guanqun Zhu, Xinbao Yin, Yulian Zhang, Zongliang Zhang, Kai Zhao, Zhenlin Wang, Ke Wang
FEBS Open Bio.2022; 12(2): 500. CrossRef - Absent X‐linked inhibitor of apoptosis protein expression in T cell blasts and causal mutations including non‐coding deletion
Shimaa Said Mohamed Ali Abdrabou, Nariaki Toita, Shin Ichihara, Yusuke Tozawa, Michiko Takahashi, Shin‐ichi Fujiwara, Toshifumi Ashida, Osamu Ohara, Tadashi Ariga, Atsushi Manabe, Mutsuko Konno, Masafumi Yamada
Pediatrics International.2022;[Epub] CrossRef - Mechanisms of mucosal healing: treating inflammatory bowel disease without immunosuppression?
Eduardo J. Villablanca, Katja Selin, Charlotte R. H. Hedin
Nature Reviews Gastroenterology & Hepatology.2022; 19(8): 493. CrossRef - Role of multidetector computed tomography in patients with acute infectious colitis
Seung Jung Yu, Jae Hyuk Heo, Eun Jeong Choi, Jong Hyuk Kim, Hong Sub Lee, Sun Young Kim, Jae Hoon Lim
World Journal of Clinical Cases.2022; 10(12): 3686. CrossRef - Differential Diagnosis of Crohn’s Disease and Ulcerative Primary Intestinal Lymphoma: A Scoring Model Based on a Multicenter Study
Hong Yang, Huimin Zhang, Wei Liu, Bei Tan, Tao Guo, Xiang Gao, Rui Feng, Kaichun Wu, Qian Cao, Zhihua Ran, Zhanju Liu, Naizhong Hu, Liangru Zhu, Yamin Lai, Congling Wang, Wei Han, Jiaming Qian
Frontiers in Oncology.2022;[Epub] CrossRef - Gut inflammation induced by drugs: Can pathology help to differentiate from inflammatory bowel disease?
Naoimh Herlihy, Roger Feakins
United European Gastroenterology Journal.2022; 10(5): 451. CrossRef - A Rare Case of New-Onset Ulcerative Colitis in a Nonagenarian
Emmanuel U Emeasoba, Cece E Ibeson, Sanchit Kundal, Stefanie Biondi, Ifeanyi Nwosu, Shmuel Golfeyz, Michael Kantrowitz, Dimitry Khodorskiy
Cureus.2022;[Epub] CrossRef - Application value of tissue tuberculosis antigen combined with Xpert MTB/RIF detection in differential diagnoses of intestinal tuberculosis and Crohn’s disease
Baoying Fei, Lin Zhou, Yu Zhang, Linhe Luo, Yuanyuan Chen
BMC Infectious Diseases.2021;[Epub] CrossRef - De Novo Inflammatory Bowel Disease Rarely Occurs During Posttransplant Immunosuppression
Jiayun M Fang, Laura Lamps, Amoah Yeboah-Korang, Jerome Cheng, Maria Westerhoff
American Journal of Clinical Pathology.2021; 156(6): 1113. CrossRef - Diterpenoids Isolated from Podocarpus macrophyllus Inhibited the Inflammatory Mediators in LPS-Induced HT-29 and RAW 264.7 Cells
ChoEen Kim, DucDat Le, Mina Lee
Molecules.2021; 26(14): 4326. CrossRef - Retrospective study of the differential diagnosis between cryptogenic multifocal ulcerous stenosing enteritis and small bowel Crohn’s disease
Dan Chen, Wei Liu, Weixun Zhou, Weiyang Zheng, Dong Wu, Jiaming Qian
BMC Gastroenterology.2020;[Epub] CrossRef - PREVALENCE AND FACTORS ASSOCIATED WITH SMALL INTESTINAL BACTERIAL OVERGROWTH IN PATIENTS WITH CROHN’S DISEASE: A RETROSPECTIVE STUDY AT A REFERRAL CENTER
Erika Ruback BERTGES, Júlio Maria Fonseca CHEBLI
Arquivos de Gastroenterologia.2020; 57(3): 283. CrossRef - Can natural language processing help differentiate inflammatory intestinal diseases in China? Models applying random forest and convolutional neural network approaches
Yuanren Tong, Keming Lu, Yingyun Yang, Ji Li, Yucong Lin, Dong Wu, Aiming Yang, Yue Li, Sheng Yu, Jiaming Qian
BMC Medical Informatics and Decision Making.2020;[Epub] CrossRef - Chronological Review of Endoscopic Indices in Inflammatory Bowel Disease
Joon Seop Lee, Eun Soo Kim, Won Moon
Clinical Endoscopy.2019; 52(2): 129. CrossRef - Illuminating an Invisible Epidemic: A Systemic Review of the Clinical and Economic Benefits of Early Diagnosis and Treatment in Inflammatory Disease and Related Syndromes
Wylezinski, Gray, Polk, Harmata, Spurlock
Journal of Clinical Medicine.2019; 8(4): 493. CrossRef - Anti-IL-13Rα2 therapy promotes recovery in a murine model of inflammatory bowel disease
Erik P. Karmele, Trisha S. Pasricha, Thirumalai R. Ramalingam, Robert W. Thompson, Richard L. Gieseck, Kayla J. Knilans, Martin Hegen, Mark Farmer, Fang Jin, Aaron Kleinman, David A. Hinds, Thiago Almeida Pereira, Rafael de Queiroz Prado, Nan Bing, Lioudm
Mucosal Immunology.2019; 12(5): 1174. CrossRef - A challenge in diagnosis and management of ulcerative colitis in elderly patient with atypical presentation: A reported case
Panutchaya Kongon, Vorapatu Tangsirapat, Vittawat Ohmpornuwat, Kannakrit Sumtong, Vichack Chakrapan Na Ayudhya, Kobkool Chakrapan Na Ayudhya, Paiboon Sookpotarom, Paisarn Vejchapipat
International Journal of Surgery Case Reports.2019; 61: 234. CrossRef - Primary hypertrophic osteoarthropathy related gastrointestinal complication has distinctive clinical and pathological characteristics: two cases report and review of the literature
Qiang Wang, Ying-he Li, Guo-le Lin, Yue Li, Wei-xun Zhou, Jia-ming Qian, Wei-bo Xia, Dong Wu
Orphanet Journal of Rare Diseases.2019;[Epub] CrossRef - Intestinal granulomatous disease: what is the first call
Alex Guri, Michal Kori, Pearl Herskovitz, Oren Zimhony
BMJ Case Reports.2018; : bcr-2017-223094. CrossRef - Aspecific ileitis: Crohn’s disease or not Crohn’s disease? A prospective study
Cristina Bezzio, Ilaria Arena, Massimo Devani, Barbara Omazzi, Gianpiero Manes, Simone Saibeni
International Journal of Colorectal Disease.2017; 32(7): 1025. CrossRef - Endoscopic Bamboo Joint-like Appearance of the Stomach in Crohn's Disease
Kwang Il Seo, Won Moon
The Korean Journal of Gastroenterology.2017; 69(2): 151. CrossRef - Management of Crohn's disease in Taiwan: consensus guideline of the Taiwan Society of Inflammatory Bowel Disease
Shu-Chen Wei, Ting-An Chang, Te-Hsin Chao, Jinn-Shiun Chen, Jen-Wei Chou, Yenn-Hwei Chou, Chiao-Hsiung Chuang, Wen-Hung Hsu, Tien-Yu Huang, Tzu-Chi Hsu, Chun-Chi Lin, Hung-Hsin Lin, Jen-Kou Lin, Wei-Chen Lin, Yen-Hsuan Ni, Ming-Jium Shieh, I-Lun Shih, Chi
Intestinal Research.2017; 15(3): 285. CrossRef
- Recent Trends in Non-Invasive Methods of Diagnosis and Evaluation of Inflammatory Bowel Disease: A Short Review
- 26,414 View
- 774 Download
- 30 Web of Science
- 32 Crossref

- Advances in the Management of Upper Gastrointestinal Subepithelial Tumor: Pathologic Diagnosis Using Endoscopy without Endoscopic Ultrasound-Guided Biopsy
- Hang Lak Lee
- Clin Endosc 2016;49(3):216-219. Published online May 30, 2016
- DOI: https://doi.org/10.5946/ce.2016.064
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Until now, biopsy methods for subepithelial tumors (SETs) have focused on endoscopic ultrasound (EUS)-guided biopsy; however, these methods have several limitations. We devised a simple method for pathologic diagnosis of SETs. SETs are occasionally diagnosed during endoscopy, and lesions are generally small and asymptomatic. It can be challenging to decide on a management plan for large asymptomatic SETs. EUS imaging provides information regarding the size, layer, and echo pattern of the lesions. Patient management plans have traditionally been determined based on EUS images, whereby the endoscopist chooses to either monitor or remove the tumor. However, EUS alone cannot diagnose and evaluate upper gastrointestinal SETs with high accuracy. As sufficient tissue samples are required for the accurate diagnosis of SETs, EUS-guided biopsy techniques such as EUS fine-needle aspiration and trucut biopsy are currently used. However, these methods have a relatively low diagnostic accuracy and do not always provide information upon immunohistochemical staining. Endoscopists can easily detect a submucosal mass after creating an iatrogenic mucosal ulcer, after which tissue sampling is performed by using endoscopic biopsy. Furthermore, pathologic results can differentiate between benign and premalignant lesions. Here, we introduce a simple method for the pathologic diagnosis of SETs.
-
Citations
Citations to this article as recorded by- Risk factors for the failure of endoscopic resection of gastric submucosal tumors: a long-term retrospective case–control study
Yuzhu Yuan, Lixin Sun, Xiaoying Zhou, Han Chen, Xinmin Si, Weifeng Zhang, Yun Wang, Bixing Ye, Nana Tang, Guoxin Zhang, Xueliang Li, Hongjie Zhang, Chunhua Jiao
Gastric Cancer.2022; 25(5): 929. CrossRef - Controversies in EUS: Do we need miniprobes?
Hans Seifert, Pietro Fusaroli, PaoloGiorgio Arcidiacono, Barbara Braden, Felix Herth, Michael Hocke, Alberto Larghi, Bertrand Napoleon, Mihai Rimbas, BogdanSilvio Ungureanu, Adrian Sãftoiu, AnandV Sahai, ChristophF Dietrich
Endoscopic Ultrasound.2021; 10(4): 246. CrossRef - Overcoming the Challenge of Full-Thickness Resection of Gastric Lesions Using a Colonic Full-Thickness Resection Device
Yazan Fahmawi, Patel Krutika, Manoj Kumar, Lindsey Merritt, Meir Mizrahi
ACG Case Reports Journal.2020; 7(3): e00329. CrossRef - Digital image analysis-based scoring system for endoscopic ultrasonography is useful in predicting gastrointestinal stromal tumors
Moon Won Lee, Gwang Ha Kim, Kwang Baek Kim, Yoon Ho Kim, Do Youn Park, Chang In Choi, Dae Hwan Kim, Tae Yong Jeon
Gastric Cancer.2019; 22(5): 980. CrossRef - Endoscopic full‐thickness resection for gastrointestinal submucosal tumors
Ming‐Yan Cai, Francisco Martin Carreras‐Presas, Ping‐Hong Zhou
Digestive Endoscopy.2018; 30(S1): 17. CrossRef
- Risk factors for the failure of endoscopic resection of gastric submucosal tumors: a long-term retrospective case–control study
- 7,250 View
- 180 Download
- 6 Web of Science
- 5 Crossref

- Mucosal Incision and Forceps Biopsy for Reliable Tissue Sampling of Gastric Subepithelial Tumors
- Sa Young Shin, Sang Jin Lee, Jae Hyuck Jun, Jong Kyu Park, Hyun Il Seo, Koon Hee Han, Young Don Kim, Woo Jin Jeong, Gab Jin Cheon
- Clin Endosc 2017;50(1):64-68. Published online March 4, 2016
- DOI: https://doi.org/10.5946/ce.2015.094
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The diagnostic efficacy of current tissue sampling techniques for gastric subepithelial tumors (SETs) is limited. Better tissue sampling techniques are needed to improve pathological diagnosis. The aim of this study was to evaluate the safety and efficacy of a new technique, mucosal incision and forceps biopsy, for reliable tissue sampling of gastric SETs.
Methods
This study enrolled 12 consecutive patients who underwent mucosal incision and forceps biopsy of gastric SETs between November 2011 and September 2014 at Gangneung Asan Hospital. The medical records of patients were reviewed retrospectively. The safety and diagnostic yield of this method were evaluated.
Results
By performing mucosal incision and forceps biopsy, we were able to provide a definitive histological diagnosis for 11 out of 12 cases. The pathological diagnoses were leiomyoma (3/11), gastrointestinal stromal tumor (GIST; 2/11), lipoma (2/11), schwannoma (1/11), and ectopic pancreas (3/11). In cases of leiomyoma (n=3) and GIST (n=2), tissue samples were of sufficient size to allow immunohistochemical staining. In addition, the mitotic index was evaluated in two cases of GIST. There were no procedure-related complications.
Conclusions
Mucosal incision and forceps biopsy can be used as one of several methods to obtain adequate tissue samples from gastric SETs. -
Citations
Citations to this article as recorded by- Natural history of gastric leiomyoma
Kwangbeom Park, Ji Yong Ahn, Hee Kyong Na, Kee Wook Jung, Jeong Hoon Lee, Do Hoon Kim, Kee Don Choi, Ho June Song, Gin Hyug Lee, Hwwon-Yong Jung
Surgical Endoscopy.2024; 38(5): 2726. CrossRef - Diagnostic yield of endoscopic and EUS-guided biopsy techniques in subepithelial lesions of the upper GI tract: a systematic review
Cynthia A. Verloop, Jacqueline A.C. Goos, Marco J. Bruno, Rutger Quispel, Lydi M.J.W. van Driel, Lieke Hol
Gastrointestinal Endoscopy.2024; 99(6): 895. CrossRef - Advancements in the Diagnosis of Gastric Subepithelial Tumors
Osamu Goto, Mitsuru Kaise, Katsuhiko Iwakiri
Gut and Liver.2022; 16(3): 321. CrossRef - Endoscopic full-thickness resection of gastric subepithelial tumors with the gFTRD-system: a prospective pilot study (RESET trial)
Benjamin Meier, Arthur Schmidt, Nicolas Glaser, Alexander Meining, Benjamin Walter, Andreas Wannhoff, Bettina Riecken, Karel Caca
Surgical Endoscopy.2020; 34(2): 853. CrossRef - Comparison of the Diagnostic Ability of Endoscopic Ultrasonography and Abdominopelvic Computed Tomography in the Diagnosis of Gastric Subepithelial Tumors
Sang Yoon Kim, Ki-Nam Shim, Joo-Ho Lee, Ji Young Lim, Tae Oh Kim, A. Reum Choe, Chung Hyun Tae, Hye-Kyung Jung, Chang Mo Moon, Seong-Eun Kim, Sung-Ae Jung
Clinical Endoscopy.2019; 52(6): 565. CrossRef - Diagnosis of Gastric Subepithelial Tumors Using Endoscopic Ultrasonography or Abdominopelvic Computed Tomography: Which is Better?
Eun Young Park, Gwang Ha Kim
Clinical Endoscopy.2019; 52(6): 519. CrossRef - How Can We Obtain Tissue from a Subepithelial Lesion for Pathologic Diagnosis?
Eun Young Kim
Clinical Endoscopy.2017; 50(1): 6. CrossRef
- Natural history of gastric leiomyoma
- 8,044 View
- 160 Download
- 8 Web of Science
- 7 Crossref

- The Role of Capsule Endoscopy in Patients with Obscure Gastrointestinal Bleeding
- Yang Won Min, Dong Kyung Chang
- Clin Endosc 2016;49(1):16-20. Published online January 28, 2016
- DOI: https://doi.org/10.5946/ce.2016.49.1.16
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Obscure gastrointestinal bleeding (OGIB) accounts for 5% of all gastrointestinal (GI) bleeding cases and is often caused by small bowel lesions. Capsule endoscopy (CE), which allows non-invasive visualization of the small bowel mucosa, has revolutionized the evaluation of OGIB. CE is preferred by both patients and physicians mainly because of its non-invasiveness, and is widely used as the first-line diagnostic modality for OGIB. The diagnostic yield of CE in OGIB has been reported to be in the range of 32% to 83%. Although no direct comparison has been made, a meta-analysis showed similar diagnostic yields between CE and double-balloon enteroscopy (DBE) for OGIB. However, CE could enhance the yield of subsequent DBE and serve as a guide for optimizing the insertion route for DBE. Even after negative CE, selected patients could benefit from second-look CE for OGIB. In terms of outcomes, a favorable clinical impact after CE has been reported in several studies. However, observations indicate that CE might not influence clinical outcomes directly, but rather play a role in selecting patients with OGIB who are likely to benefit from subsequent evaluation and intervention.
-
Citations
Citations to this article as recorded by- Experiencia con el uso de videocápsula endoscópica en pacientes con anemia ferropénica inexplicada
Rosangela Ramirez Barranco, Valeria Atenea Costa Barney, Reinaldo Andres Rincón
Revista colombiana de Gastroenterología.2022; 37(1): 33. CrossRef - Concordancia diagnóstica entre la videocápsula endoscópica y enteroscopia mono y de doble balón en la hemorragia de intestino delgado en un hospital de alta complejidad en Bogotá, Colombia
R. Cañadas Garrido, R.A. Rincón Sánchez, V.A. Costa Barney, P.A. Roa Ballestas, C.A. Espinosa Martínez, D.F. Pinzón Arenas, R. Ramirez Barranco
Revista de Gastroenterología de México.2021; 86(1): 51. CrossRef - Effect of Nonsteroidal Anti-inflammatory Agents on Small Intestinal Injuries as Evaluated by Capsule Endoscopy
Sang Pyo Lee, Jin Lee, Sea Hyub Kae, Hyun Joo Jang, Eun Suk Jung
Digestive Diseases and Sciences.2021; 66(8): 2724. CrossRef - Diagnostic agreement between video capsule endoscopy and single and double balloon enteroscopy for small bowel bleeding at a tertiary care hospital in Bogota, Colombia
R. Cañadas Garrido, R.A. Rincón Sánchez, V.A. Costa Barney, P.A. Roa Ballestas, C.A. Espinosa Martínez, D.F. Pinzón Arenas, R. Ramírez Barranco
Revista de Gastroenterología de México (English Edition).2021; 86(1): 51. CrossRef - CapsoCam SV-1 Versus PillCam SB 3 in the Detection of Obscure Gastrointestinal Bleeding
Lilli L. Zwinger, Britta Siegmund, Andrea Stroux, Andreas Adler, Winfried Veltzke-Schlieker, Robert Wentrup, Christian Jürgensen, Bertram Wiedenmann, Felix Wiedbrauck, Stephan Hollerbach, Thomas Liceni, Christian Bojarski
Journal of Clinical Gastroenterology.2019; 53(3): e101. CrossRef - Clinical Utility of Emergency Capsule Endoscopy for Diagnosing the Source and Nature of Ongoing Overt Obscure Gastrointestinal Bleeding
Sumio Iio, Shiro Oka, Shinji Tanaka, Akiyoshi Tsuboi, Ichiro Otani, Sayoko Kunihara, Kazuaki Chayama
Gastroenterology Research and Practice.2019; 2019: 1. CrossRef - Diagnostic Benefit of Simultaneous Capsule Endoscopy Using Two Different Systems
Seung Han Kim, Hyuk Soon Choi, Hoon Jai Chun, Eun Sun Kim, Bora Keum, Yeon Seok Seo, Yoon Tae Jeen, Hong Sik Lee, Soon Ho Um, Chang Duck Kim
Gastroenterology Research and Practice.2018; 2018: 1. CrossRef - Management of occult obscure gastrointestinal bleeding patients based on long-term outcomes
Sayoko Kunihara, Shiro Oka, Shinji Tanaka, Akiyoshi Tsuboi, Ichiro Otani, Kazuaki Chayama
Therapeutic Advances in Gastroenterology.2018; 11: 175628481878740. CrossRef - Magnetic-Guided Capsule Endoscopy in the Diagnosis of Gastrointestinal Diseases in Minors
Yuting Qian, Tingting Bai, Juanjuan Li, Yi Zang, Tong Li, Mingping Xie, Qi Wang, Lifu Wang, Ruizhe Shen
Gastroenterology Research and Practice.2018; 2018: 1. CrossRef - What is the Role of Double-Balloon Endoscopy in Patients Presenting with Obscure Gastrointestinal Bleeding?
Jung Ho Kim, Kwang An Kwon
Clinical Endoscopy.2017; 50(1): 8. CrossRef - Intérêt de l’exploration de l’intestin grêle dans les hémorragies digestives
Chloé Leandri, Benoit Bordacahar, Sophie Ribiere, Ammar Oudjit, Marie-Anne Guillaumot, Bertrand Brieau, Frédéric Prat, Vered Abitbol, Stanislas Chaussade, Romain Coriat
La Presse Médicale.2017; 46(10): 903. CrossRef - Development and validation of a new scoring system to determine the necessity of small-bowel endoscopy in obscure gastrointestinal bleeding
Genta Uchida, Masanao Nakamura, Osamu Watanabe, Takeshi Yamamura, Takuya Ishikawa, Kazuhiro Furukawa, Kohei Funasaka, Eizaburo Ohno, Hiroki Kawashima, Ryoji Miyahara, Hidemi Goto, Yoshiki Hirooka
Digestive and Liver Disease.2017; 49(11): 1218. CrossRef
- Experiencia con el uso de videocápsula endoscópica en pacientes con anemia ferropénica inexplicada
- 9,229 View
- 192 Download
- 13 Web of Science
- 12 Crossref

- An Ultrathin Endoscope with a 2.4-mm Working Channel Shortens the Esophagogastroduodenoscopy Time by Shortening the Suction Time
- Satoshi Shinozaki, Yoshimasa Miura, Yuji Ino, Kenjiro Shinozaki, Alan Kawarai Lefor, Hironori Yamamoto
- Clin Endosc 2015;48(6):516-521. Published online November 30, 2015
- DOI: https://doi.org/10.5946/ce.2015.48.6.516
- Correction in: Clin Endosc 2016;49(1):100
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Poor suction ability through a narrow working channel prolongs esophagogastroduodenoscopy (EGD). The aim of this study was to evaluate suction with a new ultrathin endoscope (EG-580NW2; Fujifilm Corp.) having a 2.4-mm working channel in clinical practice.
Methods
To evaluate in vitro suction, 200 mL water was suctioned and the suction time was measured. The clinical data of 117 patients who underwent EGD were retrospectively reviewed on the basis of recorded video, and the suction time was measured by using a stopwatch.
Results
In vitro, the suction time with the EG-580NW2 endoscope was significantly shorter than that with the use of an ultrathin endoscope with a 2.0-mm working channel (EG-580NW; mean ± standard deviation, 22.7±1.1 seconds vs. 34.7±2.2 seconds; p<0.001). We analyzed the total time and the suction time for routine EGD in 117 patients (50 in the EG-580NW2 group and 67 in the EG-580NW group). In the EG-580NW2 group, the total time for EGD was significantly shorter than that in the EG-580NW group (275.3±42.0 seconds vs. 300.6±46.5 seconds, p=0.003). In the EG-580NW2 group, the suction time was significantly shorter than that in the EG-580NW group (19.2±7.6 seconds vs. 38.0±15.9 seconds, p<0.001).
Conclusions
An ultrathin endoscope with a 2.4-mm working channel considerably shortens the routine EGD time by shortening the suction time, in comparison with an endoscope with a 2.0-mm working channel. -
Citations
Citations to this article as recorded by- Optical spectroscopy for in vivo medical diagnosis—a review of the state of the art and future perspectives
Jang Ah Kim, Dominic J Wales, Guang-Zhong Yang
Progress in Biomedical Engineering.2020; 2(4): 042001. CrossRef - Necessity of transnasal gastroscopy in routine diagnostics: a patient-centred requirement analysis
Anna-Livia Schuldt, Holger Kirsten, Jan Tuennemann, Mario Heindl, Florian van Bommel, Juergen Feisthammel, Marcus Hollenbach, Albrecht Hoffmeister
BMJ Open Gastroenterology.2019; 6(1): e000264. CrossRef
- Optical spectroscopy for in vivo medical diagnosis—a review of the state of the art and future perspectives
- 7,538 View
- 50 Download
- 3 Web of Science
- 2 Crossref

- Polyp Detection, Characterization, and Management Using Narrow-Band Imaging with/without Magnification
- Takahiro Utsumi, Mineo Iwatate, Wataru Sano, Hironori Sunakawa, Santa Hattori, Noriaki Hasuike, Yasushi Sano
- Clin Endosc 2015;48(6):491-497. Published online November 30, 2015
- DOI: https://doi.org/10.5946/ce.2015.48.6.491
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Narrow-band imaging (NBI) is a new imaging technology that was developed in 2006 and has since spread worldwide. Because of its convenience, NBI has been replacing the role of chromoendoscopy. Here we review the efficacy of NBI with/without magnification for detection, characterization, and management of colorectal polyps, and future perspectives for the technology, including education. Recent studies have shown that the next-generation NBI system can detect significantly more colonic polyps than white light imaging, suggesting that NBI may become the modality of choice from the beginning of screening. The capillary pattern revealed by NBI, and the NBI International Colorectal Endoscopic classification are helpful for prediction of histology and for estimating the depth of invasion of colorectal cancer. However, NBI with magnifying colonoscopy is not superior to magnifying chromoendoscopy for estimation of invasion depth. Currently, therefore, chromoendoscopy should also be performed additionally if deep submucosal invasive cancer is suspected. If endoscopists become able to accurately estimate colorectal polyp pathology using NBI, this will allow adenomatous polyps to be resected and discarded; thus, reducing both the risk of polypectomy and costs. In order to achieve this goal, a suitable system for education and training in in vivo diagnostics will be necessary.
-
Citations
Citations to this article as recorded by- Near-focus mode for accurate operation during endoscopic submucosal tunneling procedure
Wei Peng, Huan Li, Yun Xu, Li Yan, Zhenzhen Tang, Xiaowei Tang, Xiangsheng Fu
Minimally Invasive Therapy & Allied Technologies.2022; 31(1): 99. CrossRef - Prediction of the histology of colorectal neoplasm in white light colonoscopic images using deep learning algorithms
Seong Ji Choi, Eun Sun Kim, Kihwan Choi
Scientific Reports.2021;[Epub] CrossRef - Narrow-Band Imaging: Clinical Application in Gastrointestinal Endoscopy
Sandra Barbeiro, Diogo Libânio, Rui Castro, Mário Dinis-Ribeiro, Pedro Pimentel-Nunes
GE - Portuguese Journal of Gastroenterology.2019; 26(1): 40. CrossRef - Maximizing the Effectiveness of Colonoscopy in the Prevention of Colorectal Cancer
John F. Sullivan, John A. Dumot
Surgical Oncology Clinics of North America.2018; 27(2): 367. CrossRef - Food properties and dietary habits in colorectal cancer prevention and development
Łukasz Pietrzyk
International Journal of Food Properties.2017; 20(10): 2323. CrossRef - Diagnostic performance of Japan NBI Expert Team classification for differentiation among noninvasive, superficially invasive, and deeply invasive colorectal neoplasia
Kyoku Sumimoto, Shinji Tanaka, Kenjiro Shigita, Nana Hayashi, Daiki Hirano, Yuzuru Tamaru, Yuki Ninomiya, Shiro Oka, Koji Arihiro, Fumio Shimamoto, Masaharu Yoshihara, Kazuaki Chayama
Gastrointestinal Endoscopy.2017; 86(4): 700. CrossRef - Global perspective on colonoscopy use for colorectal cancer screening: A multi-country survey of practicing colonoscopists
Céline Audibert, Anna Perlaky, Daniel Glass
Contemporary Clinical Trials Communications.2017; 7: 116. CrossRef - Advanced imaging and therapeutic endoscopy
Chaitanya Pant, Mojtaba S. Olyaee, Amit Rastogi
Techniques in Gastrointestinal Endoscopy.2017; 19(3): 151. CrossRef
- Near-focus mode for accurate operation during endoscopic submucosal tunneling procedure
- 18,154 View
- 480 Download
- 9 Web of Science
- 8 Crossref

- Diminutive and Small Colorectal Polyps: The Pathologist's Perspective
- Yun Kyung Kang
- Clin Endosc 2014;47(5):404-408. Published online September 30, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.5.404
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Recent progress in advanced endoscopic imaging and electronic chromoendoscopy allows the real-time endoscopic estimation of the histologic type of polyps, mainly for the differentiation of adenomas from hyperplastic polyps. Accordingly, a "resect-and-discard" strategy applied to diminutive colorectal polyps is now one of the emerging issues among gastroenterologists. The strategy has a practical advantage in terms of the potential cost savings. However, it has a number of limitations in the medical, academic, and legal aspects. The major pitfalls include the endoscopic investigation of colorectal polyps with a wide variety of histogenetic origins, including serrated polyps, and the lack of a standardized method for polyp size measurement. Another issue is the importance of the pathologic diagnosis for legal purposes and medical research. Moreover, it is not certain whether the implementation of the strategy has economic benefit in countries with an undervalued reimbursement system for pathologic examination. There is no doubt that a highly confident optical diagnosis of polyp type is a novel valuable tool. It can provide a more steady symbiosis between gastroenterologists and pathologists to allow a more evident diagnosis and management of patients with colorectal polyps.
-
Citations
Citations to this article as recorded by- Colorectal Polyp Image Detection and Classification through Grayscale Images and Deep Learning
Chen-Ming Hsu, Chien-Chang Hsu, Zhe-Ming Hsu, Feng-Yu Shih, Meng-Lin Chang, Tsung-Hsing Chen
Sensors.2021; 21(18): 5995. CrossRef - Virtual chromoendoscopy for the real-time assessment of colorectal polyps in vivo: a systematic review and economic evaluation
Joanna Picot, Micah Rose, Keith Cooper, Karen Pickett, Joanne Lord, Petra Harris, Sophie Whyte, Dankmar Böhning, Jonathan Shepherd
Health Technology Assessment.2017; 21(79): 1. CrossRef - Risk Factors of Advanced Adenoma in Small and Diminutive Colorectal Polyp
Yo Han Jeong, Kyeong Ok Kim, Chan Seo Park, Sung Bum Kim, Si Hyung Lee, Byung Ik Jang
Journal of Korean Medical Science.2016; 31(9): 1426. CrossRef - International Digestive Endoscopy Network 2014: Turnpike to the Future
Eun Young Kim, Kwang An Kwon, Il Ju Choi, Ji Kon Ryu, Ki Baik Hahm
Clinical Endoscopy.2014; 47(5): 371. CrossRef
- Colorectal Polyp Image Detection and Classification through Grayscale Images and Deep Learning
- 5,815 View
- 81 Download
- 4 Web of Science
- 4 Crossref

- Is Image-Enhanced Endoscopy Useful for the Diagnosis and Treatment of Gastrointestinal Tumor?
- Kyoung Oh Kim, Yang Suh Ku
- Clin Endosc 2013;46(3):248-250. Published online May 31, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.3.248
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Since the introduction of endoscopic submucosal dissection method for the treatment of early gastric cancer, endoscopic treatment of early gastric cancer has increased exponentially. Accordingly, early diagnosis of cancerous or precancerous lesion has become one of the most important missions for endoscopists. The desire to improve diagnostic capability of white light endoscopy led to the development of new imaging techniques called "image enhanced endoscopy." The usefulness of these image enhanced endoscopy has not been proven yet, although there are several studies reporting diagnostic superiority of these new imaging methods over white light endoscopy. Among these new imaging modalities, narrow band image (NBI) with magnification endoscopy has been most widely used and studied. This manuscript will be focused on the NBI with magnification endoscopy.
-
Citations
Citations to this article as recorded by- Comparison Trial between I-SCAN-Optical Enhancement and Chromoendoscopy for Evaluating the Horizontal Margins of Gastric Epithelial Neoplasms
Myeongseok Koh, Jong Yoon Lee, Song-Hee Han, Seong Woo Jeon, Su Jin Kim, Joo Young Cho, Seong Hwan Kim, Jae Young Jang, Gwang Ho Baik, Jin Seok Jang
Gut and Liver.2023; 17(2): 234. CrossRef - Comparison of the Diagnostic Usefulness of Conventional Magnification and Near-focus Methods with Narrow-band Imaging for Gastric Epithelial Tumors
Hee Yoon Jang, Su Jin Hong, Jae Pil Han, Se Kyung Park, Han Kyeol Yun, Bong Jin Ko
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2015; 15(1): 39. CrossRef - A comparison of NBI and WLI cystoscopy in detecting non-muscle-invasive bladder cancer: A prospective, randomized and multi-center study
Zhangqun Ye, Jia Hu, Xiaodong Song, Fan Li, Xuetao Zhao, Shan Chen, Xiaofeng Wang, Dalin He, Jinhai Fan, Dingwei Ye, Jinchun Xing, Tiejun Pan, Dongwen Wang
Scientific Reports.2015;[Epub] CrossRef - Interobserver Agreement in Using Magnifying Narrow Band Imaging System
Il Ju Choi
Clinical Endoscopy.2014; 47(1): 1. CrossRef - Efficacy of i-Scan Imaging for the Detection and Diagnosis of Early Gastric Carcinomas
Junichi Nishimura, Jun Nishikawa, Munetaka Nakamura, Atsushi Goto, Kouichi Hamabe, Shinichi Hashimoto, Takeshi Okamoto, Masato Suenaga, Yusuke Fujita, Yoshihiko Hamamoto, Isao Sakaida
Gastroenterology Research and Practice.2014; 2014: 1. CrossRef - Highlights of the 48th Seminar of Korean Society of Gastrointestinal Endoscopy
Kwang An Kwon, Il Ju Choi, Eun Young Kim, Seok Ho Dong, Ki Baik Hahm
Clinical Endoscopy.2013; 46(3): 203. CrossRef - Image-Enhanced Endoscopy for Diagnosis and Treatment of Gastrointestinal Tumor
Viroj Wiwanitkit
Clinical Endoscopy.2013; 46(4): 423. CrossRef
- Comparison Trial between I-SCAN-Optical Enhancement and Chromoendoscopy for Evaluating the Horizontal Margins of Gastric Epithelial Neoplasms
- 5,286 View
- 67 Download
- 7 Crossref

- Optical Diagnosis of Small Colorectal Polyp Histology with High-Definition Colonoscopy Using Narrow Band Imaging
- Amit Rastogi
- Clin Endosc 2013;46(2):120-129. Published online March 31, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.2.120
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Optical diagnosis of polyp histology can potentially result in enormous cost savings by way of the "resect and discard" strategy for diminutive polyps and the "do not resect" strategy for diminutive hyperplastic polyps in the distal colon. Narrow Band Imaging (NBI) highlights the surface mucosal and vascular pattern on polyps and has been shown to accurately characterize adenomatous and hyperplastic polyps by experts. However, the results have been a little discouraging amongst lesser experienced endoscopists. Studies have also shown that using the NBI diagnosis of diminutive polyp histology, experts can accurately define the future surveillance colonoscopy intervals. However nonexperts in academic or community setting have as yet failed to achieve the recommended thresholds. The subjectivity in assessment by endoscopists leads to the variable accuracy rates and can be circumvented by computer based automated tools. Although initial experience with a few computer based algorithms have shown accuracies comparable to experts, further refinement and validation will be required before these can be implemented in clinical practice. Incorporation of optical diagnosis of diminutive polyps into clinical practice is bound to face several hurdles. But the potential for enormous cost saving makes it an attractive strategy that can make colonoscopy more cost effective.
-
Citations
Citations to this article as recorded by- Solutions for submucosal injection: What to choose and how to do it
Rui Castro, Diogo Libânio, Inês Pita, Mário Dinis-Ribeiro
World Journal of Gastroenterology.2019; 25(7): 777. CrossRef - Optical Diagnosis of Colorectal Cancer
红梅 于
Medical Diagnosis.2019; 09(02): 52. CrossRef - Randomized Controlled Trial of Self-directed Versus In-Classroom Education of Narrow Band Imaging in Diagnosing Colorectal Polyps Using the NICE Criteria
James E. Allen, Prashanth Vennalaganti, Neil Gupta, Benjamin Hornung, Abhishek Choudhary, Mohammad Titi, Benjamin R. Alsop, Diego Lim, Prateek Sharma
Journal of Clinical Gastroenterology.2018; 52(5): 413. CrossRef - Correlation between microvascular characteristics and the expression of MVD, IGF-1 and STAT3 in the development of colonic polyps carcinogenesis
Hong Liu, Jing Wu, Xiang-Chun Liu, Nan Wei, Kui-Liang Liu, Yan-Hui Ma, Hong Chang, Quan Zhou
Experimental and Therapeutic Medicine.2017; 13(1): 49. CrossRef - Computer vision and augmented reality in gastrointestinal endoscopy: Figure 1.
Nadim Mahmud, Jonah Cohen, Kleovoulos Tsourides, Tyler M. Berzin
Gastroenterology Report.2015; 3(3): 179. CrossRef - New Paradigms in Polypectomy
Ana Ignjatovic Wilson, Brian P. Saunders
Gastrointestinal Endoscopy Clinics of North America.2015; 25(2): 287. CrossRef - Optical diagnosis of small colorectal polyps during colonoscopy: When to resect and discard?
Ana Wilson
Best Practice & Research Clinical Gastroenterology.2015; 29(4): 639. CrossRef - Tools for Polyp Histology Prediction
Shreyas Saligram, Amit Rastogi
Gastrointestinal Endoscopy Clinics of North America.2015; 25(2): 261. CrossRef - Advances in the removal of diminutive colorectal polyps
Silvia Paggi, Franco Radaelli, Alessandro Repici, Cesare Hassan
Expert Review of Gastroenterology & Hepatology.2015; 9(2): 237. CrossRef - Comparison of Narrow Band Imaging and Fujinon Intelligent Color Enhancement in Predicting Small Colorectal Polyp Histology
Hae Yeon Kang, Young Sun Kim, Seung Joo Kang, Goh Eun Chung, Ji Hyun Song, Sun Young Yang, Seon Hee Lim, Donghee Kim, Joo Sung Kim
Digestive Diseases and Sciences.2015; 60(9): 2777. CrossRef - Advanced colonoscopic imaging using endocytoscopy
Helmut Neumann, Shin‐Ei Kudo, Ralf Kiesslich, Markus F. Neurath
Digestive Endoscopy.2015; 27(2): 232. CrossRef - Small Colorectal Polyps
Rainer Schoefl, Alexander Ziachehabi, Friedrich Wewalka
Digestive Diseases.2015; 33(1): 38. CrossRef - Diminutive and Small Colorectal Polyps: The Pathologist's Perspective
Yun Kyung Kang
Clinical Endoscopy.2014; 47(5): 404. CrossRef - Highlights of the 48th Seminar of Korean Society of Gastrointestinal Endoscopy
Kwang An Kwon, Il Ju Choi, Eun Young Kim, Seok Ho Dong, Ki Baik Hahm
Clinical Endoscopy.2013; 46(3): 203. CrossRef
- Solutions for submucosal injection: What to choose and how to do it
- 11,992 View
- 114 Download
- 14 Crossref

- Introduction: What Are New Roles of Current Colonoscopy?
- Hyung Wook Kim
- Clin Endosc 2013;46(2):118-119. Published online March 31, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.2.118
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub The recent advances in endoscopic imaging technologies make great changes in the management of colorectal polyps. These changes include optical histologic diagnosis with high definition colonscopy, new management strategies such as resect and discard or do not resect, and differentiation of depth of submucosal invasion. In this focused review series, these new paradigms in management of colorectal polyps are discussed by three, world famous authors. First, Amit Rastogi explained optical diagnosis of small colorectal polyp with high definition colonoscopy using narrow band imaging. Second, Cesare Hassan explained new paradigms for colonoscopic management of diminutive colorectal polyps: resect and discard or do not resect. In the last, Shinji Tanaka described, in detail, endoscopic assessment of invasive colorectal cancer: slight vs. deep submucosal invasion. These focused review series introduce the new roles of current colonoscopy to readers and will help the readers to know how to use the new imaging technologies and paradigms in clinical practices.
-
Citations
Citations to this article as recorded by- Protein-Based Nanoplatform for Detection of Tumorigenic Polyps in the Colon Via Noninvasive Mucosal Routes
Chun-Chieh Chen, Mo A Baikoghli, R Holland Cheng
Pharmaceutical Patent Analyst.2021; 10(1): 13. CrossRef
- Protein-Based Nanoplatform for Detection of Tumorigenic Polyps in the Colon Via Noninvasive Mucosal Routes
- 4,435 View
- 31 Download
- 1 Crossref

- Differential Diagnosis of Inflammatory Bowel Disease: What Is the Role of Colonoscopy?
- Sung-Ae Jung
- Clin Endosc 2012;45(3):254-262. Published online August 22, 2012
- DOI: https://doi.org/10.5946/ce.2012.45.3.254
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Colonoscopy plays a crucial role in the diagnosis, treatment and follow-up monitoring of inflammatory bowel disease (IBD). Practitioners should be well informed of the colonoscopic findings of IBD to prevent the misdiagnosis, overtreatment or delayed treatment. Distinguishing between Crohn's disease and ulcerative colitis is essential in terms of pharmacological treatment, surgical decision-making, and prognosis. But there are still lesions with difficulty in differentiation that approximately 10% of the patients fall into the category of indeterminate colitis. Efforts are needed to carefully select treatment approach appropriate for each patient by providing a precise diagnosis on the extent and degree of lesions as well as to accurately delineate the lesions to assure that they are compared in subsequent rounds of follow-up monitoring in order to allow redetermination and adjustment of the treatment.
-
Citations
Citations to this article as recorded by- Factors Affecting Adherence to National Colorectal Cancer Screening: A 12-Year Longitudinal Study Using Multi-Institutional Pooled Data in Korea
Dae Sung Kim, Jeeyoung Hong, Kihyun Ryu, Sang Hyuk Lee, Hwanhyi Cho, Jehyeong Yu, Jieun Lee, Jong-Yeup Kim
Journal of Korean Medical Science.2024;[Epub] CrossRef - Acute Cytomegalovirus Colitis Presenting As Exacerbation of Ulcerative Colitis: A Case Report
Varun Daiya, Nishtha Manuja, Sourya Acharya, Sunil Kumar, Sharwari Jaiswal
Cureus.2024;[Epub] CrossRef - lncRNA H-19 and miR-200a implication and frequency of lncRNA H-19 rs2170425 SNP in ulcerative colitis and Crohn’s disease
Ebtsam H. Khalil, Olfat G. Shaker, Nabil A. Hasona
Comparative Clinical Pathology.2023; 32(4): 565. CrossRef - A Review of Colonoscopy in Intestinal Diseases
Seung Hong, Dong Baek
Diagnostics.2023; 13(7): 1262. CrossRef - Impact of Microbiome–Brain Communication on Neuroinflammation and Neurodegeneration
Iris Stolzer, Eveline Scherer, Patrick Süß, Veit Rothhammer, Beate Winner, Markus F. Neurath, Claudia Günther
International Journal of Molecular Sciences.2023; 24(19): 14925. CrossRef - Development and Validation of a Deep Neural Network for Accurate Identification of Endoscopic Images From Patients With Ulcerative Colitis and Crohn's Disease
Guangcong Ruan, Jing Qi, Yi Cheng, Rongbei Liu, Bingqiang Zhang, Min Zhi, Junrong Chen, Fang Xiao, Xiaochun Shen, Ling Fan, Qin Li, Ning Li, Zhujing Qiu, Zhifeng Xiao, Fenghua Xu, Linling Lv, Minjia Chen, Senhong Ying, Lu Chen, Yuting Tian, Guanhu Li, Zho
Frontiers in Medicine.2022;[Epub] CrossRef - Eosinophils in Inflammatory Bowel Disease
Rhiannon T Filippone, Lauren Sahakian, Vasso Apostolopoulos, Kulmira Nurgali
Inflammatory Bowel Diseases.2019; 25(7): 1140. CrossRef - Severe ischemic cytomegalovirus proctocolitis with multiple perforation
Reuban Toby D’cruz, Cheryl Chien-Li Lau, Thomas Paulraj Thamboo
Archives of Virology.2018; 163(7): 1927. CrossRef - Indicators of suboptimal biologic therapy over time in patients with ulcerative colitis and Crohn's disease in the United States
Haridarshan Patel, Trevor Lissoos, David T. Rubin, Shree Ram Singh
PLOS ONE.2017; 12(4): e0175099. CrossRef - Combining TNFSF15 and ASCA IgA can be used as a predictor for the stenosis/perforating phenotype of Crohn's disease
Chien‐Chih Tung, Jau‐Min Wong, Wen‐Chung Lee, Heng‐Hsiu Liu, Chin‐Hao Chang, Ming‐Chu Chang, Yu‐Ting Chang, Ming‐Jium Shieh, Cheng‐Yi Wang, Shu‐Chen Wei
Journal of Gastroenterology and Hepatology.2014; 29(4): 723. CrossRef - Highlights of the 48th Seminar of Korean Society of Gastrointestinal Endoscopy
Kwang An Kwon, Il Ju Choi, Eun Young Kim, Seok Ho Dong, Ki Baik Hahm
Clinical Endoscopy.2013; 46(3): 203. CrossRef
- Factors Affecting Adherence to National Colorectal Cancer Screening: A 12-Year Longitudinal Study Using Multi-Institutional Pooled Data in Korea
- 18,805 View
- 175 Download
- 11 Crossref

- Barrett's Esophagus - With Emphasis on Endoscopic Disgnosis
- Jun Haeng Lee, M.D.
- Korean J Gastrointest Endosc 2009;39(4):185-198. Published online October 30, 2009
-
 Abstract
Abstract
 PDF
PDF - Barrett's esophagus is a metaplastic change of the esophageal mucosa, such that the normal squamous epithelium is replaced by specialized columnar epithelium. During the last decades, there has been a significant change in the definition, endoscopic diagnosis, pathologic diagnosis, surveillance and management of Barrett's esophagus. Because of the rising prevalence of gastroesophgeal reflux disease in Korea, problems related to Barrett's esophagus are expected to be much more common in the near future. In this review, methods of endoscopic diagnosis of Barrett's esophagus are discussed in detail. Management strategies in the context of Korean epidemiology are also suggested. (Korean J Gastrointest Endosc 2009; 39:185-198)
- 2,212 View
- 12 Download

- Diagnostic Tips for Making the Diagnosis of Inflammatory Bowel Disease
- Dong Soo Han, M.D.
- Korean J Gastrointest Endosc 2009;38(4):181-187. Published online April 30, 2009
-
 Abstract
Abstract
 PDF
PDF - The incidence and prevalence of inflammatory bowel disease (IBD) in Korea and Asian countries is rapidly rising. There are no specific diagnostic tools for this malady and the diagnosis of IBD is based on the clinical, radiologic, endoscopic, pathologic findings. Endoscopy plays an important role for the diagnosis, follow-up, cancer surveillance and therapeutic interventions for IBD. This article reviews the utility of endoscopy for making the diagnosis of IBD and the key points of observation during the endoscopic procedures. (Korean J Gastrointest Endosc 2009;38:181-187)
- 1,748 View
- 11 Download


 KSGE
KSGE


 First
First Prev
Prev



